Development of β Type Ti23Mo-45S5 Bioglass Nanocomposites for Dental Applications
Abstract
:1. Introduction
2. Experimental Details
2.1. Chemicals and Materials
- Ti23Mo nanocrystalline alloy is labeled as Ti23Mo.
- Ti23Mo-3 wt % 45S5 Bioglass nanocomposite is labeled as Ti23Mo 3BG.
- Ti23Mo-10 wt % 45S5 Bioglass nanocomposite is labeled as Ti23Mo 10BG.
2.2. Sample Preparation
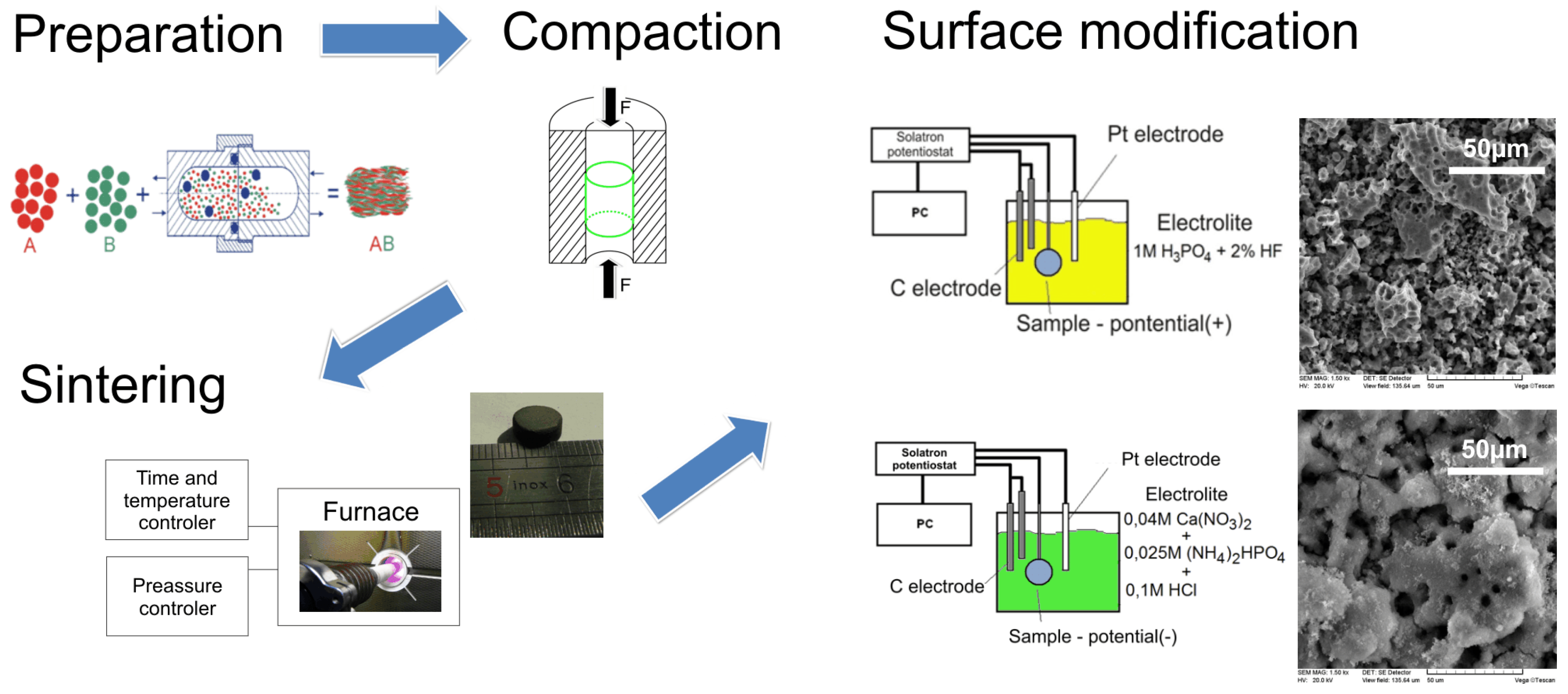
2.3. Material Characterization
2.4. In vitro Cytocompatibility
3. Results
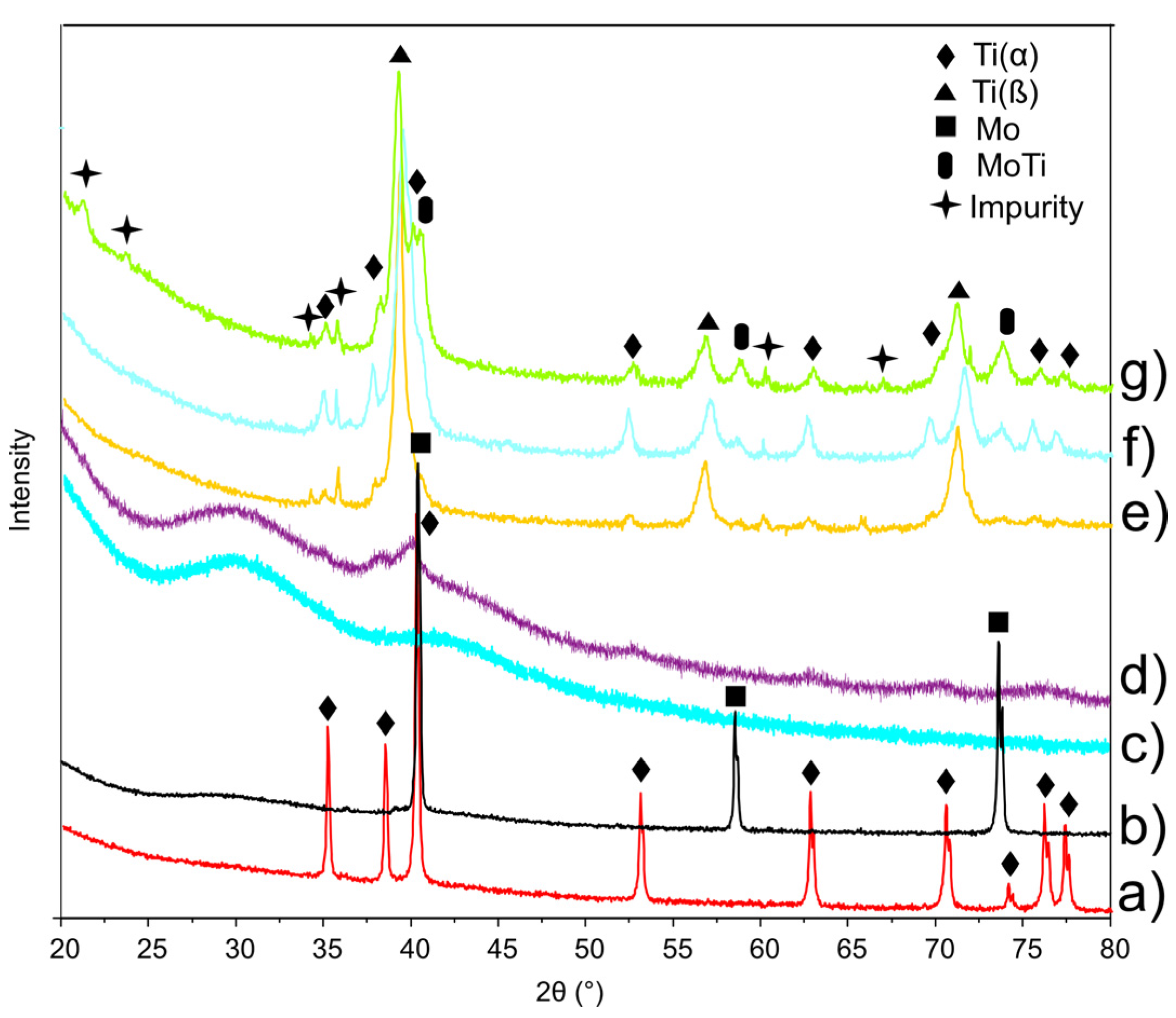
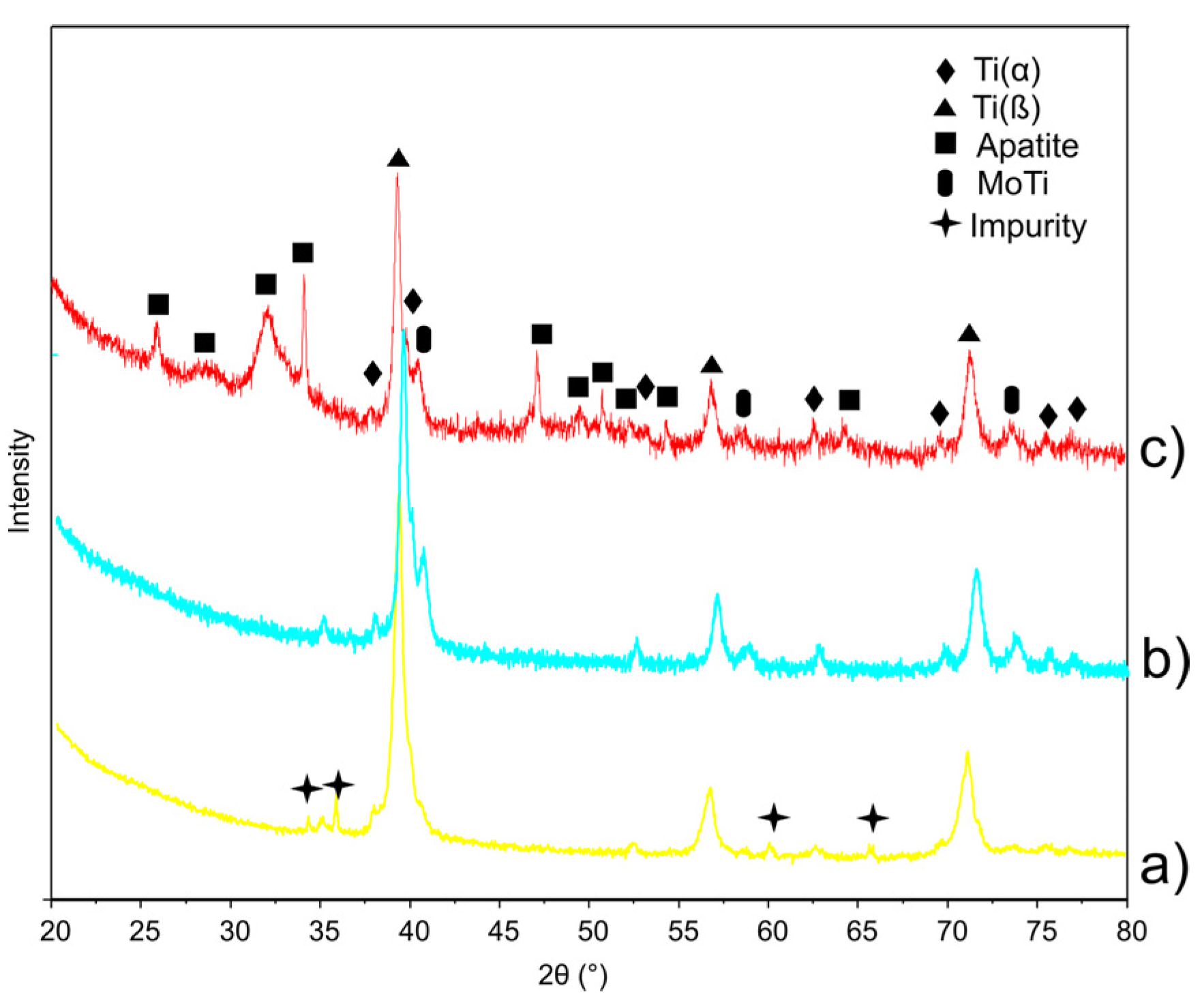
3.1. Structure Properties
| Sample | Phase | a (Å) | c (Å) | V (Å3) | d (nm) | ρth (g/cm3) | HV0.3/10 |
|---|---|---|---|---|---|---|---|
| Ti23Mo | - | 3.248 | - | 34.265 | 19.1 ± 2.1 | 6.701 | 350 |
| Ti23Mo 3BG | β | 3.234 | - | 33.834 | 16.2 ± 1.5 | 6.581 | 365 |
| Ti23Mo 10BG * | β | 3.249 | - | 34.296 | 15.4 ± 0.7 | - | - |
| α | 2.960 | 4.724 | 35.844 | - | 6.301 | 740 |

| Sample | Icorr (μA/cm2) | Ecorr (V) | Glycerol Contact Angle Θ (°) |
|---|---|---|---|
| Ti23Mo | 70.93 | −0.79 | 85.64 ± 0.72 |
| Ti23Mo 3BG | 64.44 | −0.79 | 41.41 ± 0.94 |
| Ti23Mo 3BG etched | 5.43 | −0.53 | 26.11 ± 1.62 |
| Ti23Mo 3BG etched and Ca-P deposited | 0.02 | −0.44 | 72.41 ± 0.56 |
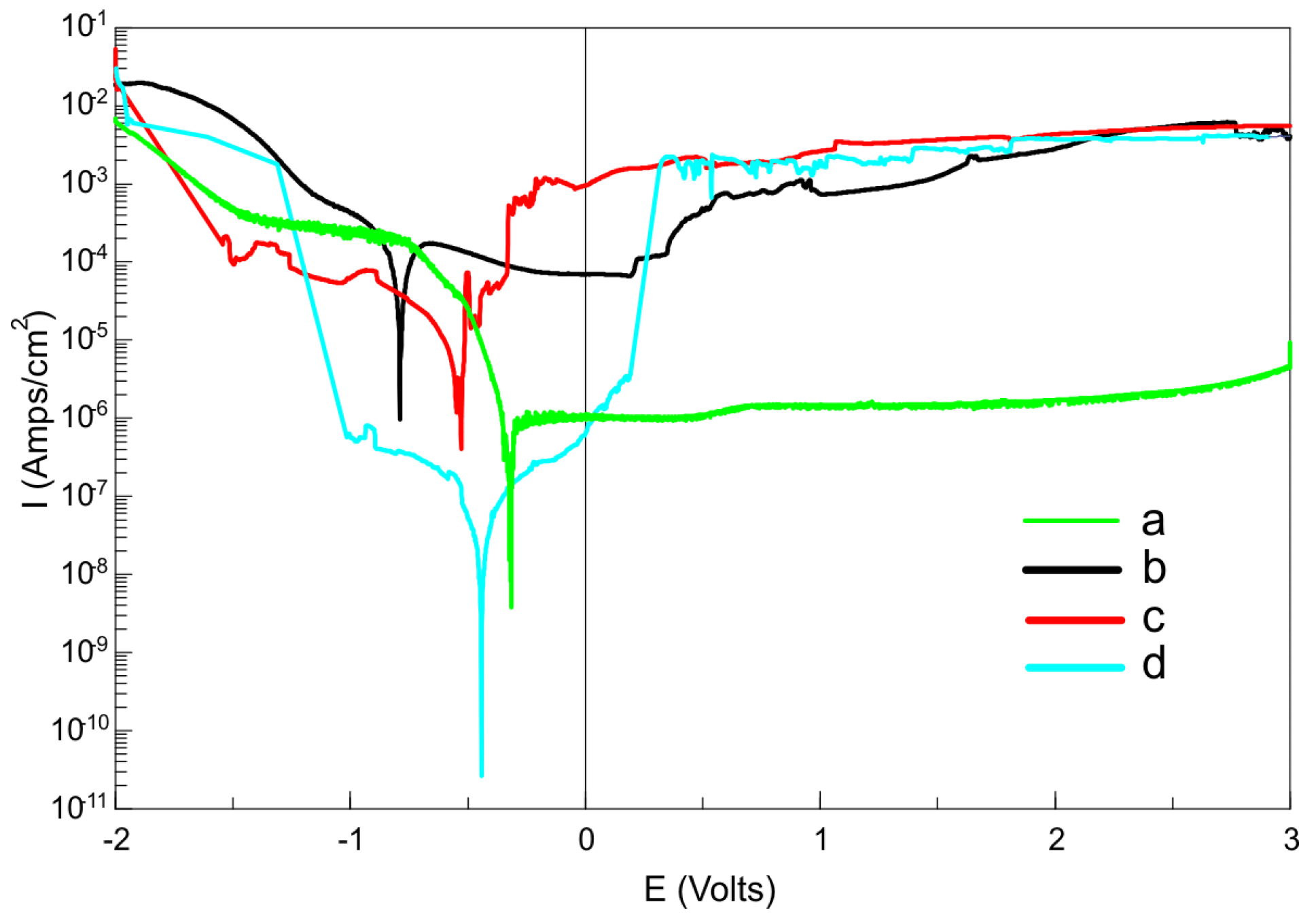
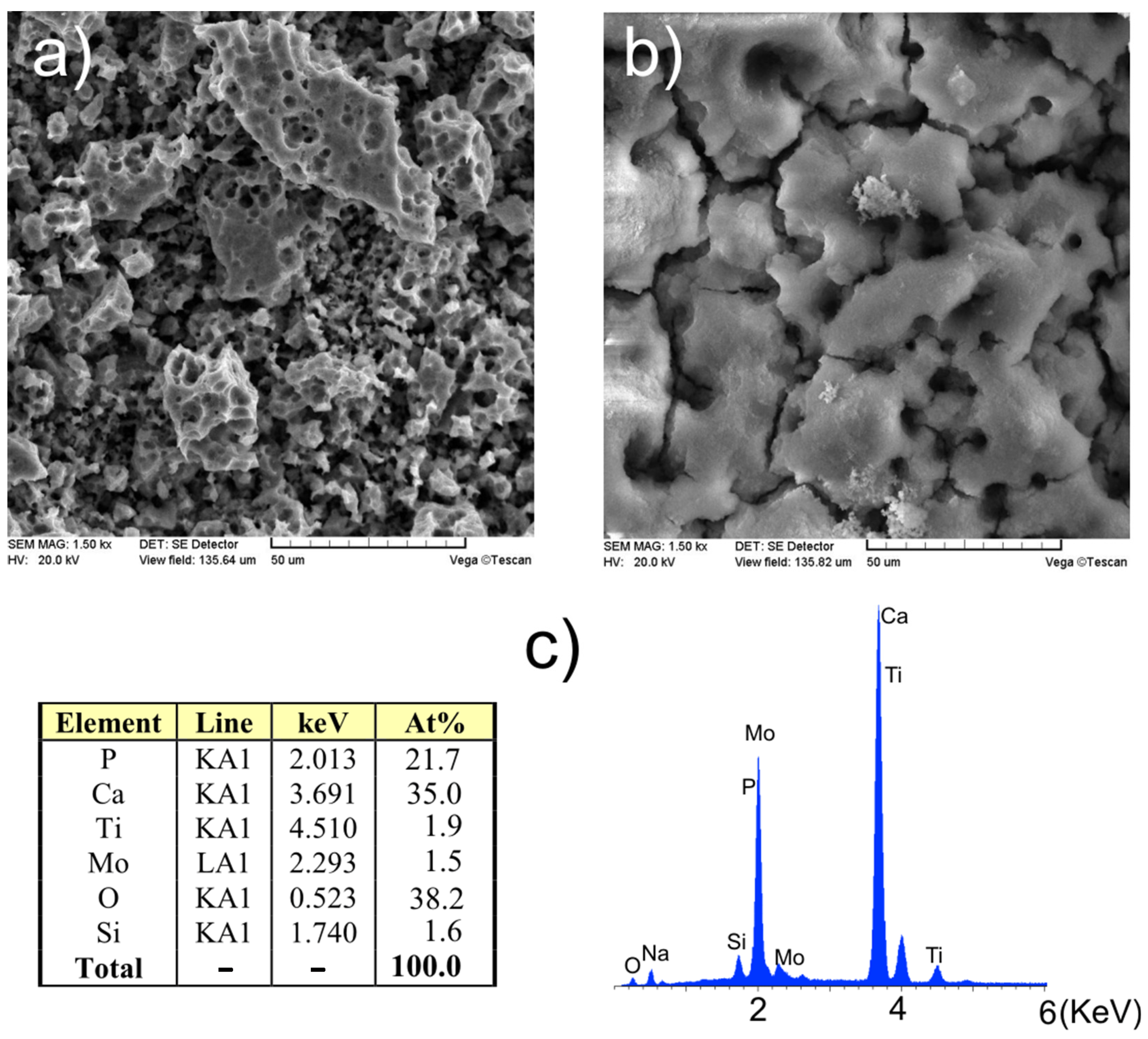
3.2. Surface Properties
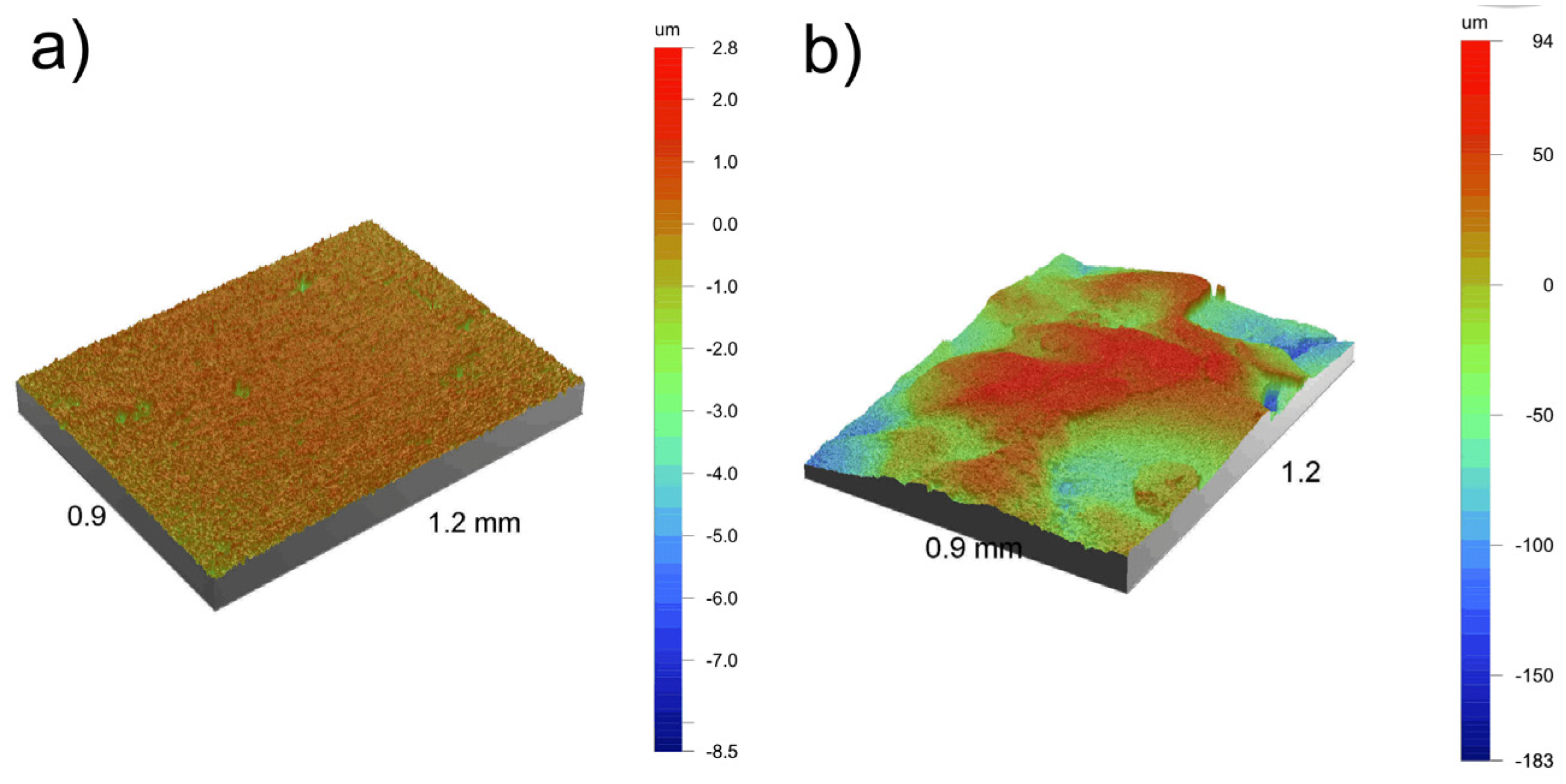
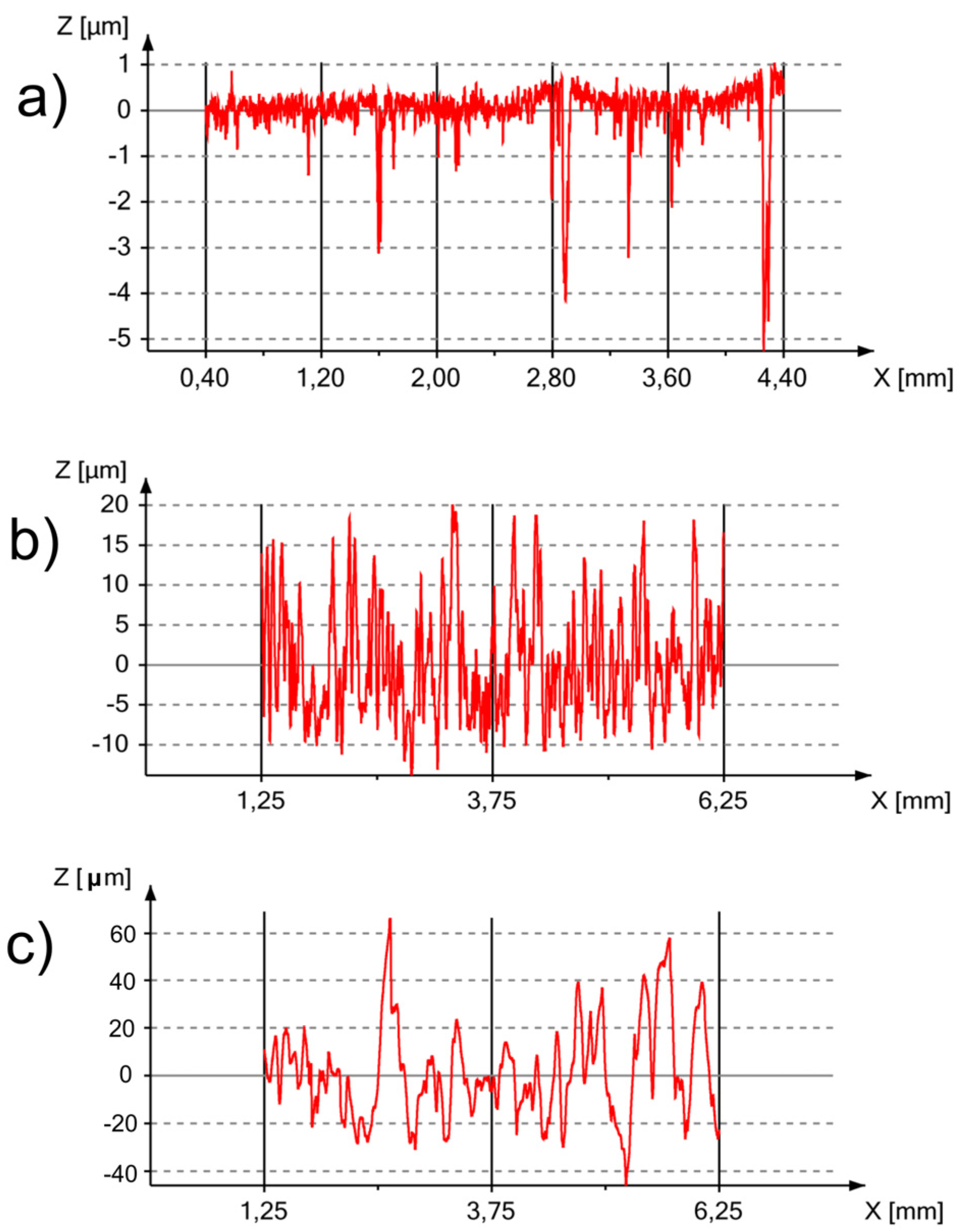
| Processing Route | Ra (μm) | Rt (μm) | Rz (μm) | Sdq (°) | Sdr (%) |
|---|---|---|---|---|---|
| before etching * | 0.29 | 11.29 | 8.24 | 8.97 | 1.22 |
| after etching | 6.42 | 42.47 | 35.54 | 74.37 | 341.72 |
| after etching with Ca-P deposition | 35.80 | 276.55 | 247.74 | 69.84 | 255.66 |
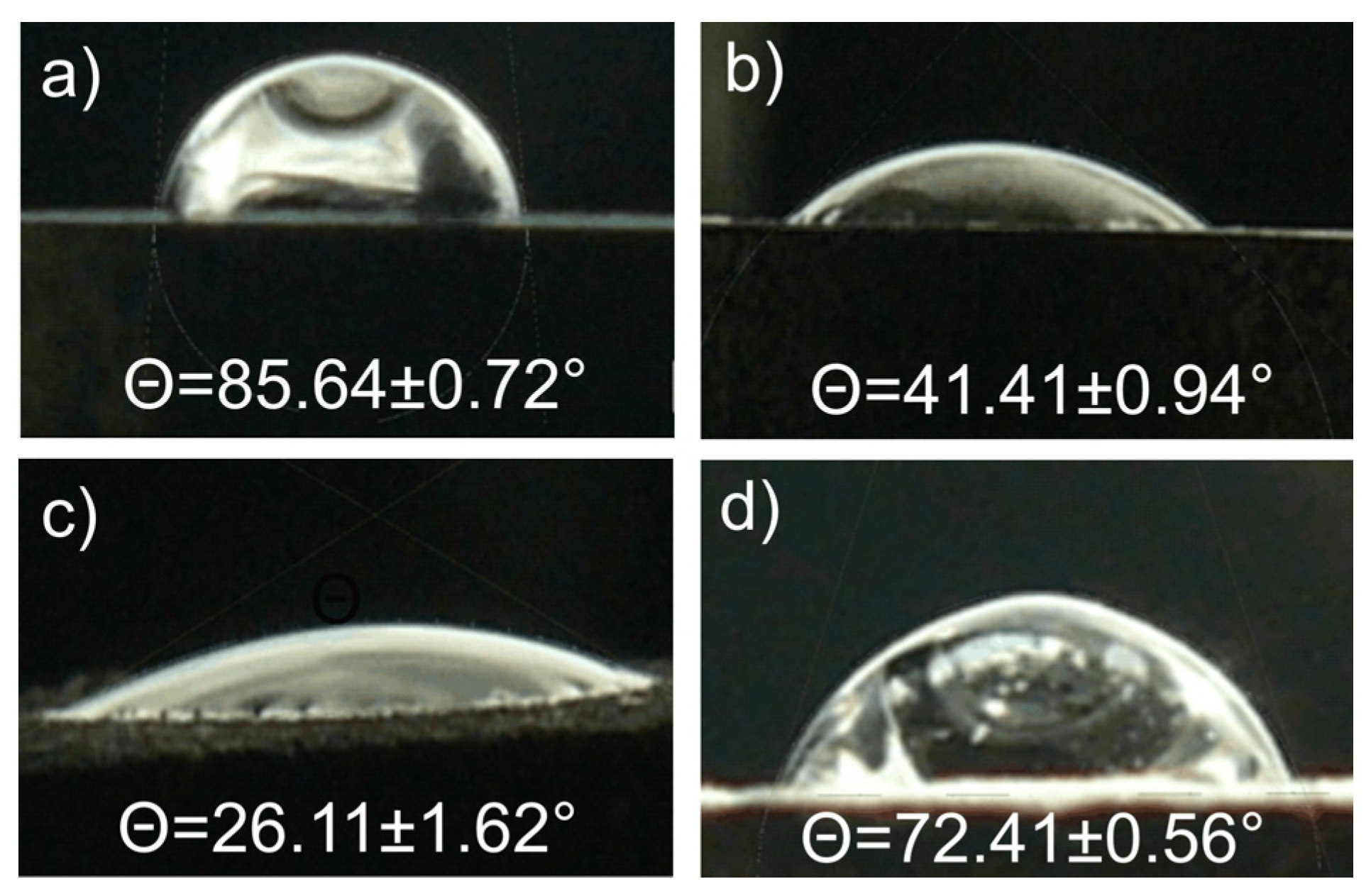
3.3. In vitro Cytocompatibility
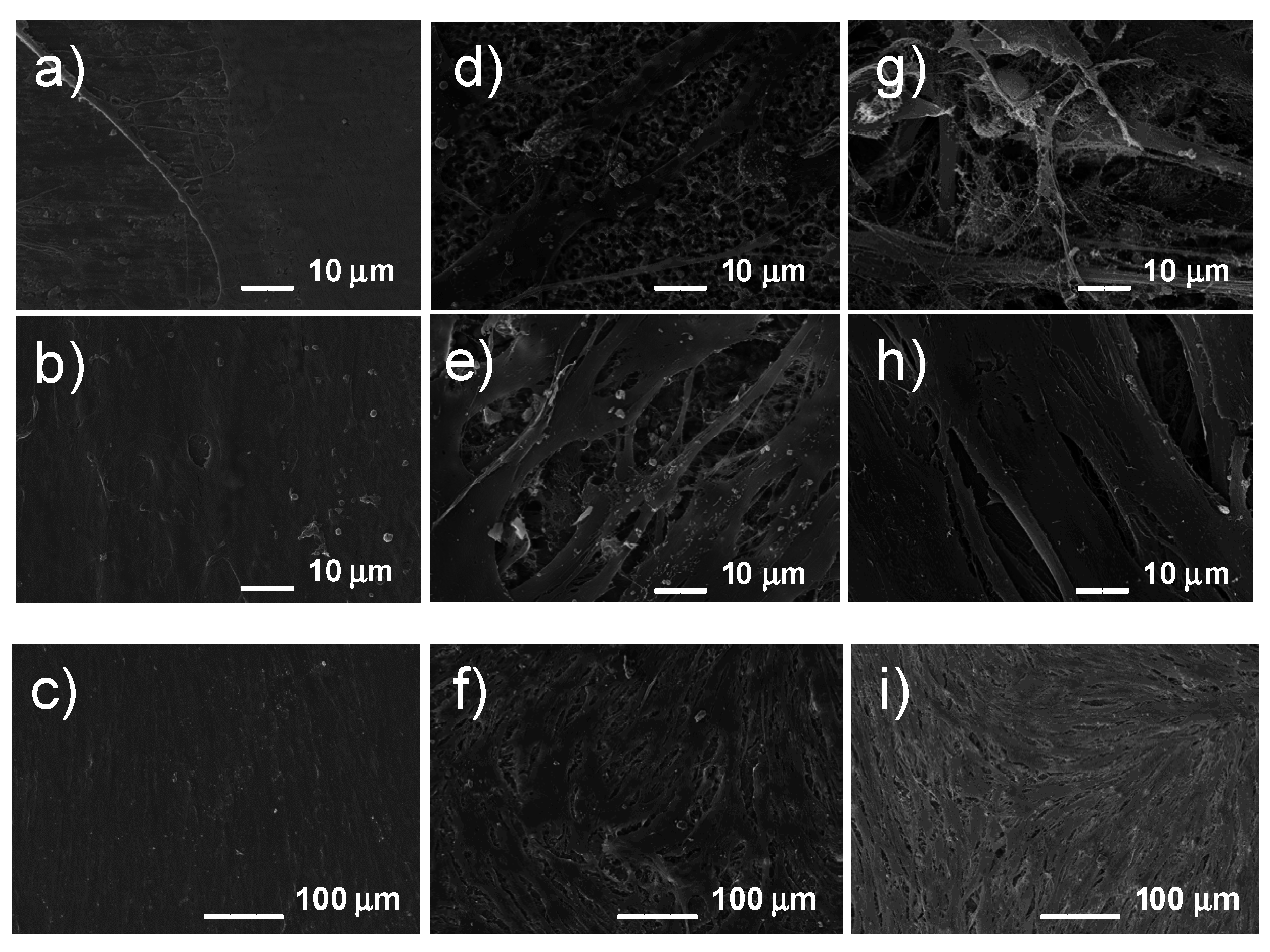
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Rack, H.J.; Qazi, J.J. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Kim, S.E.; Lim, J.H.; Lee, S.C.; Nam, S.C.; Kang, H.G.; Choi, J. Anodically nanostructured titanium oxides for implant applications. Electrochim. Acta 2008, 53, 4846–4851. [Google Scholar] [CrossRef]
- Okuno, O. Titanium alloys for dental applications. J. Jpn. Soc. Biomater. 1996, 14, 267–273. [Google Scholar]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new beta type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Yang, I.H.; Kim, S.Y.; Rubash, H.E.; Shanbhag, A.S. Fabrication of submicron titanium-alloy particles for biological response studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 1999, 48, 220–223. [Google Scholar] [CrossRef]
- Kobayashi, E.; Doi, H.; Takahashi, M.; Nakano, T.; Yoneyama, T.; Hamanaka, H. Castability and mechanical properties of Ti-6Al-7Nb alloy dental-cast. J. Jpn. Soc. Dent. Mater. Dev. 1995, 14, 406–413. [Google Scholar]
- Kobayashi, E.; Doi, H.; Yoneyama, T.; Hamanaka, H.; Matsumoto, S.; Kudaka, K. Evaluation of mechanical properties of dental casting Ti-Zr based alloys. J. Jpn. Soc. Dent. Mater. Dev. 1995, 14, 321–328. [Google Scholar]
- Doi, H.; Yoneyama, T.; Kobayashi, E.; Hamanaka, H. Mechanical properties and corrosion resistance of Ti-5Al-13Ta alloy castings. J. Jpn. Soc. Dent. Mater. Dev. 1998, 17, 247–252. [Google Scholar]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare foods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Ward, B.C.; Webster, T.J. Increased functions of osteoblasts on nanophase metals. Mater. Sci. Eng. C 2007, 27, 575–578. [Google Scholar] [CrossRef]
- Jurczyk, K.; Jurczyk, M. Applications of nanomaterials in dentistry. In Handbook of Clinical Nanomedicine: Nanoparticles, Imaging, Therapy, and Clinical Applications; Bawa, R., Audette, G.F., Rubinstein, I., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2015; Chapter 37. [Google Scholar]
- Jurczyk, K.; Adamek, G.; Kubicka, M.M.; Jakubowicz, J.; Jurczyk, M. Nanostructured titanium-10 wt % 45S5 Bioglass-Ag composite foams for medical applications. Materials 2015, 8, 1398–1412. [Google Scholar] [CrossRef]
- Abdeltawab, A.A.; Shoeib, M.A.; Mohamed, S.G. Electrophoretic deposition of hydroxyapatite coatings on titanium from dimethylformamide suspensions. Surf. Coat. Technol. 2013, 206, 43–50. [Google Scholar] [CrossRef]
- Jurczyk, K.; Niespodziana, K.; Jurczyk, M.U.; Jakubowicz, J.; Jurczyk, M. Titanium-10 wt % 45S5 Bioglass nanocomposite for biomedical applications. Mater. Chem. Phys. 2011, 131, 540–546. [Google Scholar] [CrossRef]
- Miklaszewski, A.; Jurczyk, M.U.; Jurczyk, K.; Jurczyk, M. Plasma surface modification of titanium by TiB precipitation for biomedical applications. Surf. Coat. Technol. 2011, 206, 330–337. [Google Scholar] [CrossRef]
- Ning, C.Q.; Zhou, Y. On the microstructure of biocomposites sintered from Ti, HA and bioactive glass. Biomaterials 2004, 5, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Romeis, S.; Hoppe, A.; Detsch, R.; Boccaccini, A.R.; Schmidt, J.; Peukert, W. Top-down processing of submicron 45S5 Bioglass® for enhanced in vitro bioactivity and biocompatibility. Procedia Eng. 2015, 102, 534–541. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, B.C.; Chang, E.; Wu, B.C. Bond degradation at the plasma-sprayed HA coating/Ti-6AI-4V alloy interface: An in vitro study. J. Mater. Sci. Mater. Med. 1995, 6, 258–265. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Yoshimura, M.; Inoue, A. Synthesis of Ti-based glassy alloy/hydroxyapatite composite by spark plasma sintering. Mater. Trans. 2008, 49, 502–505. [Google Scholar] [CrossRef]
- Niespodziana, K.; Jurczyk, K.; Jakubowicz, J.; Jurczyk, M. Fabrication and properties of titanium-hydroxyapatite nanocomposites. Mater. Chem. Phys. 2010, 123, 160–165. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Jurczyk, M.U.; Rubis, B.; Banaszak, A.; Kolecka, A.; Paszel, A.; Jurczyk, K.; Murias, M.; Sikora, J.; Jurczyk, M. In vitro biocompatibility of Ti-45S5 Bioglass nanocomposites and their scaffolds. J. Biomed. Mater. Res. 2014, 102A, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.S. Mechanical alloying. Sci. Am. 1976, 234, 40–46. [Google Scholar] [CrossRef]
- Suryanarayna, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Webster, T.B.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Guehennec, L.; Souedan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C. International strategy for nanotechnology research and development. J. Nanopart. Res. 2001, 3, 353–360. [Google Scholar] [CrossRef]
- Khang, D.; Lu, J.; Yao, C.; Haberstroh, K.M.; Webster, T.J. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials 2008, 29, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Swaim, J.S.; Yao, C.; Haberstroh, K.M.; Nauman, E.A.; Webster, T.J. Increased osteoblast cell density on nanostructured PLGA-coated nanostructured titanium for orthopedic applications. Int. J. Nanomed. 2007, 2, 493–499. [Google Scholar]
- Jakubowicz, J.; Adamek, G.; Jurczyk, M.U.; Jurczyk, M. 3D topography study of the biofunctionalized nanocrystalline Ti-6Zr-4Nb/Ca-P. Mater. Charact. 2012, 70, 55–62. [Google Scholar] [CrossRef]
- Estrin, Y.; Kasper, C.; Diederichs, S.; Lapovok, R. Accelerated growth of preosteoblastic cells on ultrafine grained titanium. J. Biomed. Mater. Res. A 2008, 90, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Pye, A.D.; Lockhart, D.E.A.; Dawson, M.P.; Murray, A.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- Tulinski, M.; Jurczyk, M. Nanostructured nickel-free austenitic stainless steel composites with different content of hydroxyapatite. Appl. Surf. Sci. 2012, 260, 80–83. [Google Scholar] [CrossRef]
- Jurczyk, M. (Ed.) Bionanomaterials for Dental Applications; Pan Stanford Publishing: Singapore, 2012.
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of Low Rigidity β-type Titanium Alloy for Biomedical Applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Wlodarski, W.; Kandasamy, S.; Kalantar-Zadeh, K. Fabrication of nanostructured TiO2 by nnodization: A comparison between electrolytes and substrates. Sens. Actuators B 2008, 130, 25–31. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: The oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Cao, W.P.; Hench, L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering—Review. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef]
- Lefebvre, L.; Chevalier, J.; Gremillard, L.; Zenati, R.; Thollet, G.; Bernache-Assolant, D.; Govin, A. Structural transformations of bioactive glass 45S5 with thermal treatments. Acta Mater. 2007, 55, 3305–3313. [Google Scholar] [CrossRef] [Green Version]
- Masaki, C.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C. Effects of implant surface microtopography on osteoblast gene expression. Clin. Oral Implant. Res. 2005, 16, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Nishimura, I. Genes differentially expressed in titanium implant healing. J. Dent. Res. 2006, 85, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Webster, T.J. Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int. J. Nanomed. 2008, 3, 477–485. [Google Scholar]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J. Biomed. Mater. Res. A 2008, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Variola, F.; Vetrone, F.; Richert, L.; Jedrzejowski, P.; Yi, J.H.; Zalzal, S.; Clair, S.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: An overview of strategies, fabrication methods, and challenges. Small 2009, 5, 996–1006. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurczyk, K.; Miklaszewski, A.; Jurczyk, M.U.; Jurczyk, M. Development of β Type Ti23Mo-45S5 Bioglass Nanocomposites for Dental Applications. Materials 2015, 8, 8032-8046. https://doi.org/10.3390/ma8125441
Jurczyk K, Miklaszewski A, Jurczyk MU, Jurczyk M. Development of β Type Ti23Mo-45S5 Bioglass Nanocomposites for Dental Applications. Materials. 2015; 8(12):8032-8046. https://doi.org/10.3390/ma8125441
Chicago/Turabian StyleJurczyk, Karolina, Andrzej Miklaszewski, Mieczyslawa U. Jurczyk, and Mieczyslaw Jurczyk. 2015. "Development of β Type Ti23Mo-45S5 Bioglass Nanocomposites for Dental Applications" Materials 8, no. 12: 8032-8046. https://doi.org/10.3390/ma8125441








