Influence of Hot-Etching Surface Treatment on Zirconia/Resin Shear Bond Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Zirconia Specimens
- Group 1: blank control;
- Group 2: airborne-particle-abrasion with 50 μm Al2O3 particles applied for 10 s at 0.25 MPa of air pressure; and
- Group 3: hot-etching treatment for 1 h. Specimens were placed in a reaction kettle (KH-200; Zhongkaiya, Yancheng, China) that was filled with hot-etching solution (800 mL of methanol, 200 mL of 37% HCl and 2 g of ferric chloride) [18]. The kettle was heated to a constant temperature in a 100 °C water bath with magnetic stirring (MS-400; Bante, Shanghai, China). A rotor was placed in the reaction kettle and rotated at 40 rev/s by the magnetic stirring apparatus.
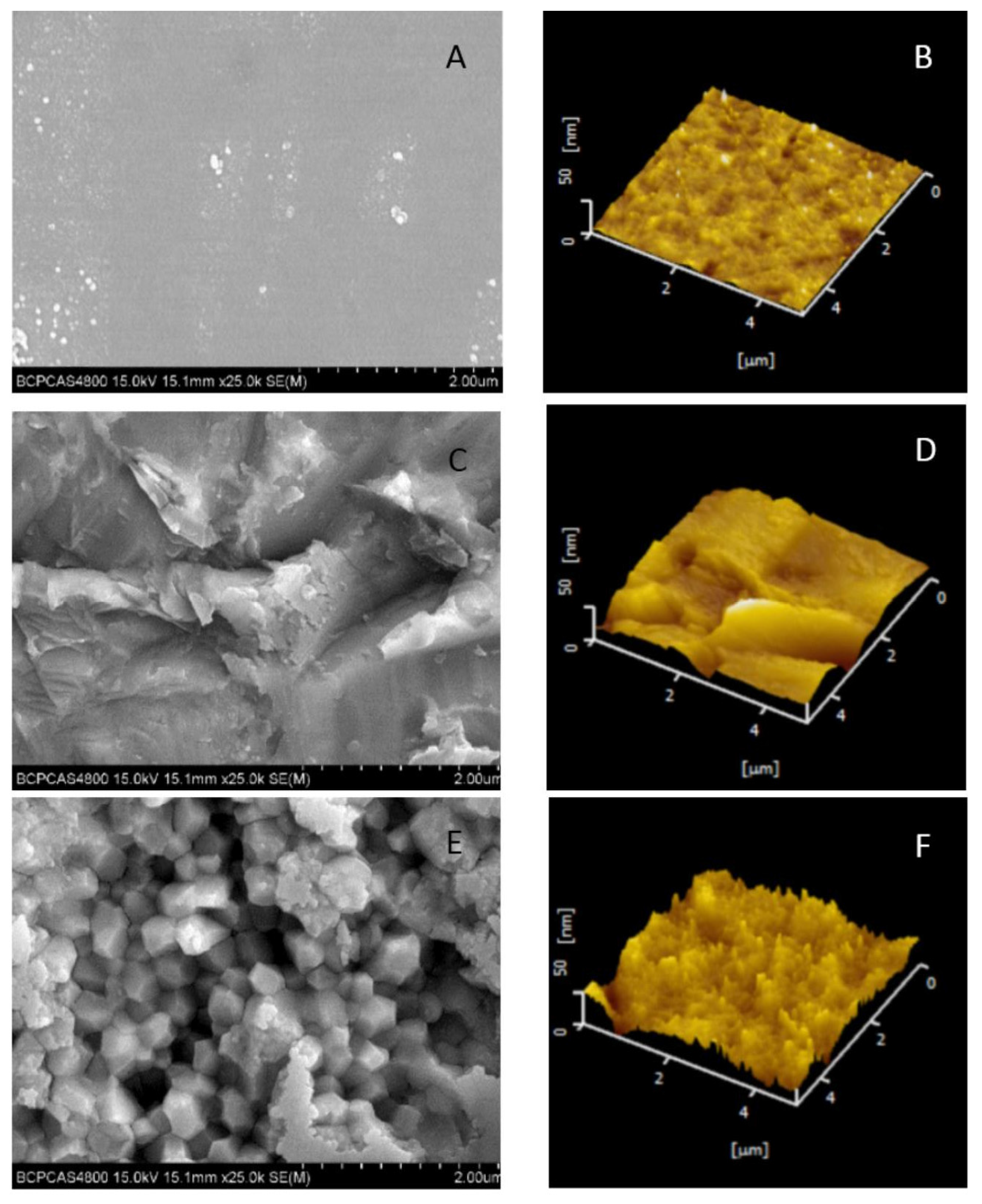
2.2. Evaluation by Scanning Electron Microscopy (SEM)
2.3. Evaluation by Atomic Force Microscopy (AFM)
2.4. X-ray Diffraction (XRD)
2.5. Preparation of an Isolated Tooth
2.6. Cementing Procedure and Thermocycling Test
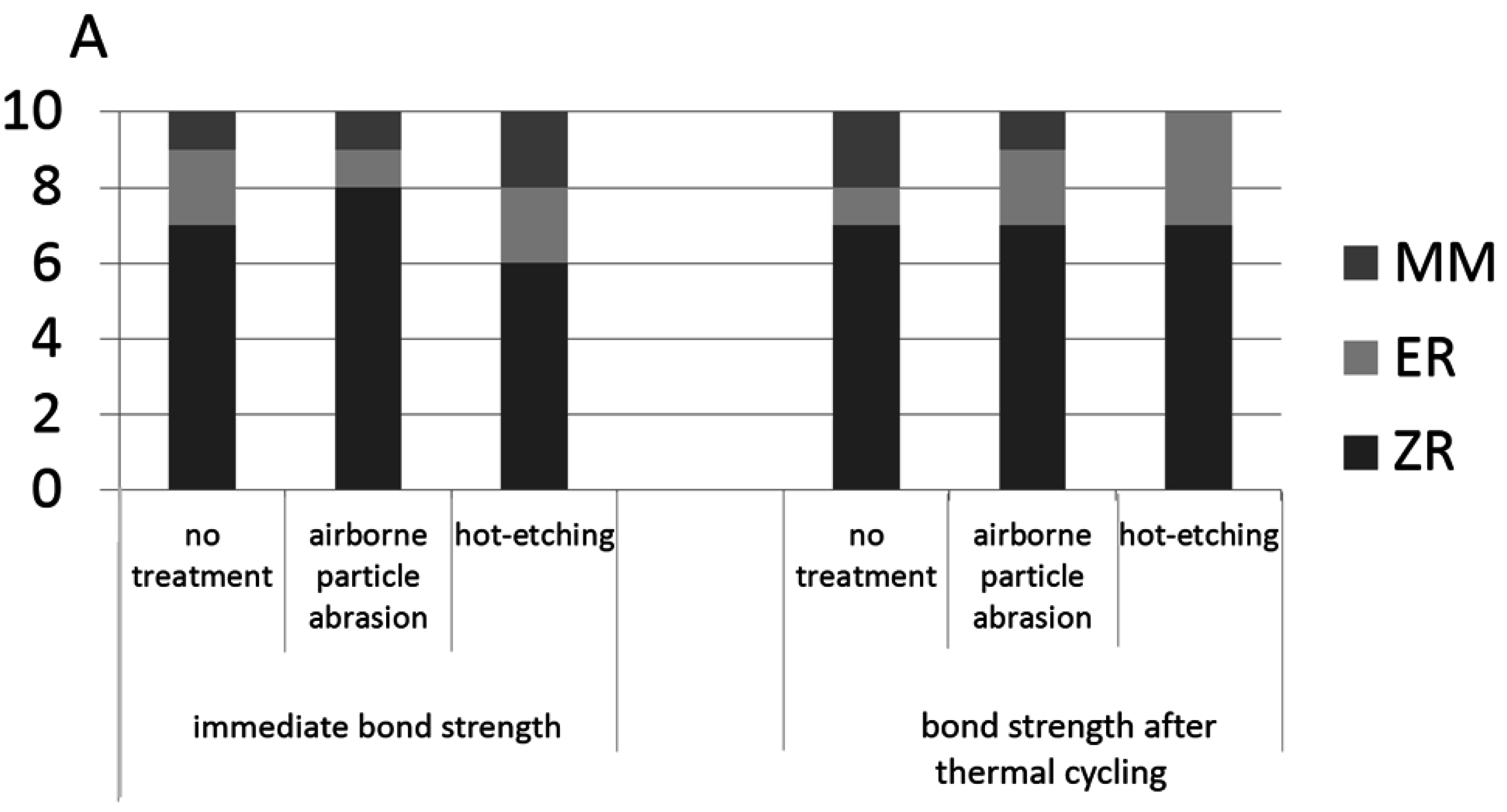
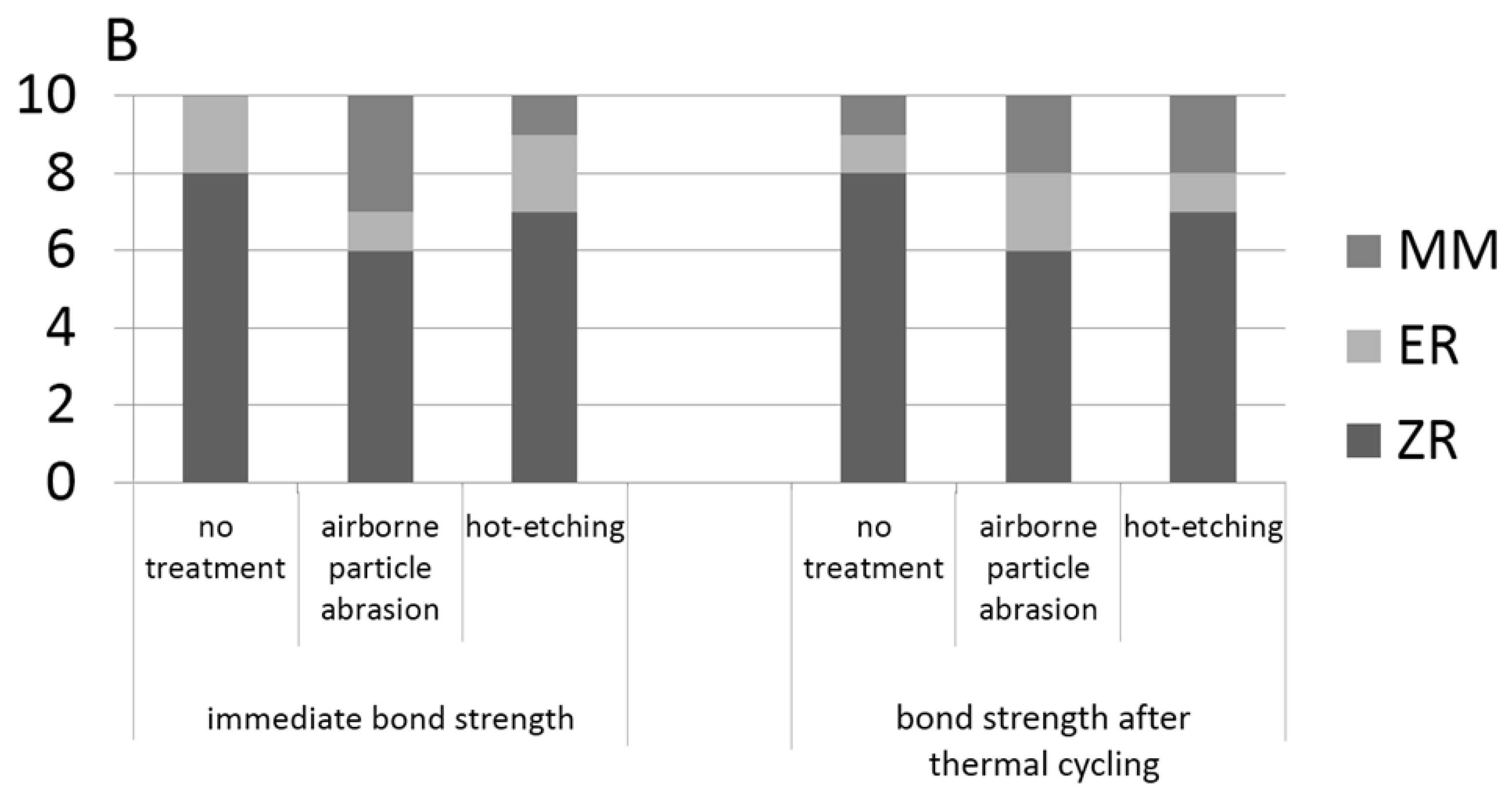
2.7. Shear Bond Strength Test and Fracture Mode Examination
2.8. Statistical Analysis
3. Results
| Surface Treantment | Surface Roughness | XM (%) |
|---|---|---|
| no treatemnt | 1.31 ± 0.96 a | 9.1 |
| airborne particle abrasion | 6.64 ± 2.07 b | 21.9 |
| hot-etching | 6.41 ± 0.49 b | 7.4 |
| Surface Treatments | Resin Cements | |||
|---|---|---|---|---|
| Superbond C & B | Panavia F2.0 | |||
| Immediate Bond Strengths | Bond Strengths after Thermal Cycling | Immediate Bond Strengths | Bond Strengths after Thermal Cycling | |
| no treatment | 23.37 ± 3.99 aAα | 9.61 ± 3.34 aAβ | 18.12 ± 4.73 bAα | 12.91 ± 3.33 aAβ |
| airborne particle abrasion | 26.06 ± 3.14 aAα | 15.8 ± 4.25 aBβ | 23.2 ± 5.47 aBα | 16.97 ± 2.9 aAβ |
| hot-etching | 34.43 ± 1.84 aBα | 29.25 ± 4.46 aCβ | 32.1 ± 7.73 aCα | 28.13 ± 6.53 aBα |


4. Discussion
4.1. Hot-Etching Technology
4.2. Surface Roughness and XRD Analysis
4.3. Shear Bond Strength Test
5. Conclusions
- Hot-etching technology could be performed in a simple reaction kettle;
- Hot-etching treatment produced significantly higher shear bond strength between zirconia and resin cement compared to no treatment and airborne-particle-abrasion treatment (p < 0.05); and
- Hot-etching treatment could provide a rougher zirconia surface while avoiding the detrimental tetragonal-to-monoclinic surface phase transition.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Sadan, A.; Kern, M. Resin-ceramic bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sahafi, A.; Peutzfeldt, A.; Asmussen, E.; Gotfredsen, K. Bond strength of resin cement to dentin and to surface-treated posts of titanium alloy, glass fiber, and zirconia. J. Adhes. Dent. 2003, 5, 153–162. [Google Scholar] [PubMed]
- Guazzato, M.; Proos, K.; Quach, L.; Swain, M. Strength, reliability and mode of fracture of bilayered porcelain/zirconia (Y-TZP) dental ceramics. Biomaterials 2004, 25, 5045–5052. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.Y.; Stoner, B.R.; Piascik, J.R.; Smith, R. Adhesion/cementation to zirconia and other non-silicate ceramics: Where are we now? Dent. Mater. 2011, 27, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Barloi, A.; Kern, M. Influence of air-abrasion on zirconia ceramic bonding using an adhesive composite resin. Dent. Mater. 2010, 26, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.M.; Gerdes, W.; Kern, M. Effect of different artificial aging conditions on ceramic-composite bond strength. Int. J. Prosthodont. 2002, 15, 267–272. [Google Scholar] [PubMed]
- Özcan, M.; Vallittu, P.K. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent. Mater. 2003, 19, 725–731. [Google Scholar] [CrossRef]
- Amaral, R.; Özcan, M.; Valandro, L.F.; Bottino, M.A. Microtensile bond strength of a resin cement to glass infiltrated zirconia-reinforced ceramic: The effect of surface conditioning. Dent. Mater. 2006, 22, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Wegner, S.M. Bonding to zirconia ceramic: Adhesion methods and their durability. Dent. Mater. 1998, 14, 64–71. [Google Scholar] [CrossRef]
- Kosmac, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. Strength and reliability of surface treated Y-TZP dental ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2000, 53, 304–313. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Selective infiltration-etching technique for a strong and durable bond of resin cements to zirconia-based materials. J. Prosthet. Dent. 2007, 98, 379–388. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, C.; Lv, P. Selective infiltrated etching surface treatment of zirconia using a modified glass agent. J. Adhes. Dent. 2014, 16, 553–557. [Google Scholar] [PubMed]
- Ferrari, M.; Cagidiaco, M.C.; Borracchini, A.; Bertelli, E. Evaluation of a chemical etching solution for nickel-chromium-beryllium and chromium-cobalt alloys. J. Prosthet. Dent. 1989, 62, 516–521. [Google Scholar] [CrossRef]
- Casucci, A.; Mazzitelli, C.; Monticelli, F.; Toledano, M.; Osorio, R.; Osorio, E.; Papacchini, F.; Ferrari, M. Morphological analysis of three zirconium oxide ceramics: Effect of surface treatments. Dent. Mater. 2010, 26, 751–760. [Google Scholar] [CrossRef] [PubMed]
- El-Korashy, D.; El-Refai, D. Mechanical properties and bonding potential of partially stabilized zirconia treated with different chemomechanical treatments. J. Adhes. Dent. 2014, 16, 365–376. [Google Scholar] [PubMed]
- Casucci, A.; Monticelli, F.; Goracci, C.; Mazzitelli, C.; Cantoro, A.; Papacchini, F.; Ferrari, M. Effect of surface pre-treatments on the zirconia ceramic-resin cement microtensile bond strength. Dent. Mater. 2011, 27, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Garvie, R.C.; Nicholson, P.S. Phase analysis in zirconia systems. J. Am. Ceram. Soc. 1972, 55, 303–305. [Google Scholar] [CrossRef]
- International Standards Organization. Dentistry—Polymer-Based Crown and Bridge Materials; ISO 10477; International Standards Organization (ISO): Geneva, Switzerland, 1996. [Google Scholar]
- International Standards Organization. Dental Materials—Testing of Adhesion to Tooth Structure, 2nd ed.; Technical Specification ISO/TS 11405; International Standards Organization (ISO): Geneva, Switzerland, 2003. [Google Scholar]
- Elsaka, S.E. Influence of surface treatments on the surface properties of different zirconia cores and adhesion of zirconia-veneering ceramic systems. Dent. Mater. 2013, 29, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Sadan, A.; Martin, J.; Lang, B. In vitro evaluation of shear bond strengths of resin to densely sintered high-purity zirconium-oxide ceramic after long-term storage and thermal cycling. J. Prosthet. Dent. 2004, 91, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Casucci, A.; Osorio, E.; Osorio, R.; Monticelli, F.; Toledano, M.; Mazzitelli, C.; Ferrari, M. Influence of different surface treatments on surface zirconia frameworks. J. Dent. 2009, 37, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Kosmac, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TPZ zirconia ceramic. Dent. Mater. 1999, 15, 426–433. [Google Scholar] [CrossRef]
- Borges, G.A.; Spohr, A.M.; de Goes, M.F.; Sobrinho, L.C.; Chan, D.C. Effect of etching and airborne particle abrasion on the microstructure of different dental ceramics. J. Prosthet. Dent. 2003, 89, 479–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R.; Malament, K.A.; van Thompson, P.; Rekow, E.D. Damage accumulation and fatigue life of particle-abraded ceramics. Int. J. Prosthodont. 2006, 19, 442–448. [Google Scholar] [PubMed]
- Chen, C.; Kleverlaan, C.J.; Feilzer, A.J. Effect of an experimental zirconia–silica coating technique on micro tensile bond strength of zirconia in different priming conditions. Dent. Mater. 2012, 28, e127–e134. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.Y.; Ha, S.R.; Lee, J.B.; Kim, S.H. Effect of sandblasting and various metal primers on the shear bond strength of resin cement to Y-TZP ceramic. Dent. Mater. 2010, 26, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Magne, P.; Paranhos, M.P.; Burnett, L.H., Jr. New zirconia primer improves bond strength of resin-based cements. Dent. Mater. 2010, 26, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Atsu, S.S.; Kilicarslan, M.A.; Kucukesmen, H.C.; Aka, P.S. Effect of zirconium-oxide ceramic surface treatments on the bond strength to adhesive resin. J. Prosthet. Dent. 2006, 95, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Dérand, P.; Dérand, T. Bond strength of luting cements to zirconium oxide ceramics. Int. J. Prosthodont. 2000, 13, 131–135. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, P.; Yang, X.; Jiang, T. Influence of Hot-Etching Surface Treatment on Zirconia/Resin Shear Bond Strength. Materials 2015, 8, 8087-8096. https://doi.org/10.3390/ma8125409
Lv P, Yang X, Jiang T. Influence of Hot-Etching Surface Treatment on Zirconia/Resin Shear Bond Strength. Materials. 2015; 8(12):8087-8096. https://doi.org/10.3390/ma8125409
Chicago/Turabian StyleLv, Pin, Xin Yang, and Ting Jiang. 2015. "Influence of Hot-Etching Surface Treatment on Zirconia/Resin Shear Bond Strength" Materials 8, no. 12: 8087-8096. https://doi.org/10.3390/ma8125409





