Modulating the Optoelectronic Properties of Silver Nanowires Films: Effect of Capping Agent and Deposition Technique
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Silver Nanowires
| Capping Agent | L/µm | FWHM/µm | D/nm Single AgNW | Percentage of Single AgNW (%) | D/nm AgNW Aggregates | Percentage of AgNW Aggregates (%) |
|---|---|---|---|---|---|---|
| PVP | 9.2 ± 0.3 | 9 ± 1 | 83 ± 2 | 28.6 | 155 ± 16 | 50.0 |
| C8 | 8.8 ± 0.7 | 12 ± 2 | 94 ± 6 | 38.4 | 160 ± 12 | 50.8 |
| C12 | 9.2 ± 0.9 | 14 ± 4 | 89 ± 7 | 35.8 | 157 ± 7 | 32.8 |
| C18 | 9.2 ± 0.5 | 12 ± 2 | 100 ± 2 | 11.3 | 160 ± 8 | 54.7 |
2.2. Silver Nanowire Films
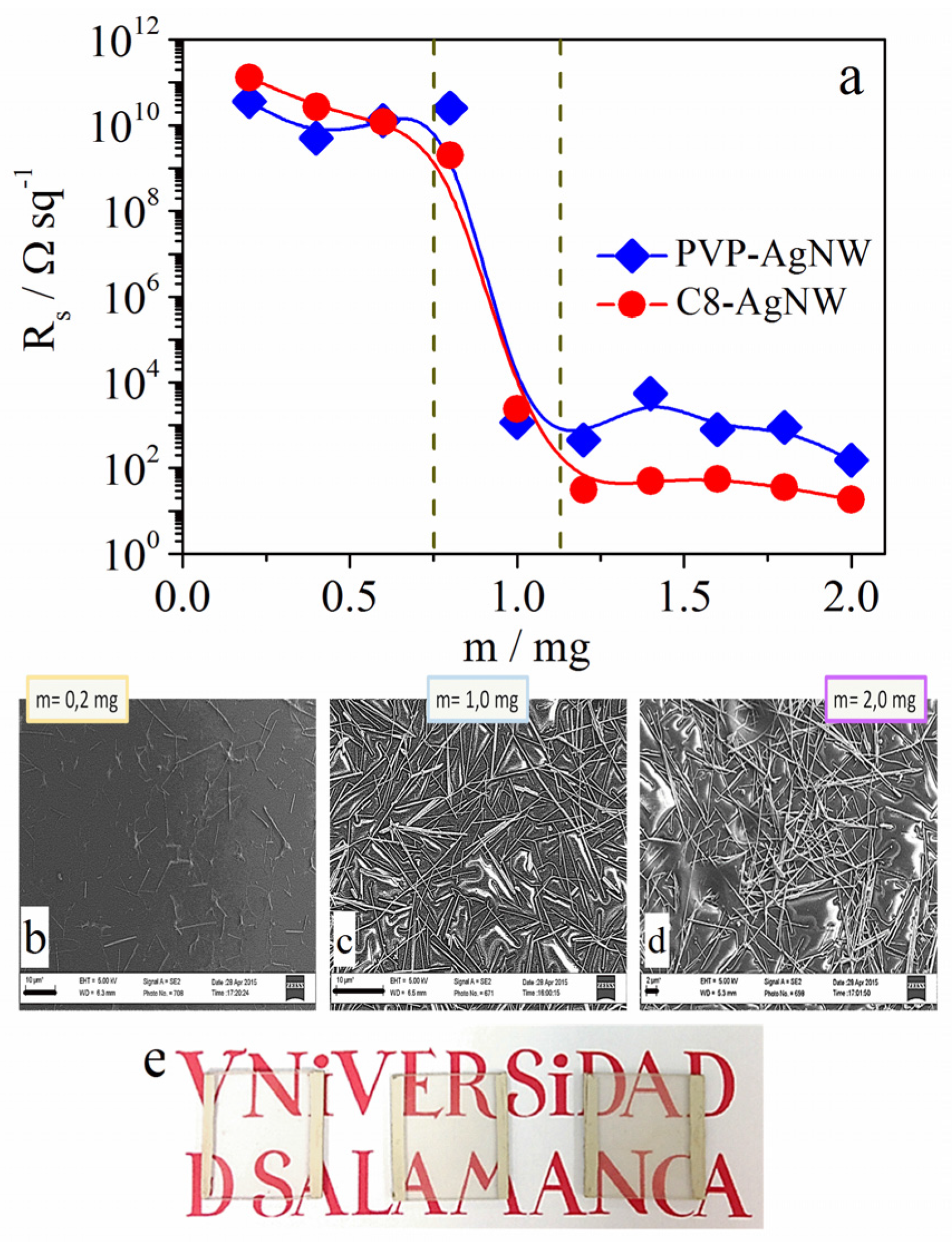
2.2.1. Spray-Coating Films
| Capping Agent | m/mg | Rs/Ω·sq−1 | T (%) |
|---|---|---|---|
| PVP | 2.25 | 192 | 63.2 |
| 3.00 | 22 | 52.8 | |
| C8 | 2.25 | 19 | 73.4 |
| 3.00 | 22 | 65.2 | |
| C12 | 2.25 | 1.5 × 105 | 76.4 |
| 3.00 | 8.5 × 105 | 73.0 | |
| C18 | 3.00 | Insulator | 56.0 |
| 4.50 | Insulator | 40.3 | |
| 6.00 | 2.0 × 104 | 28.3 |
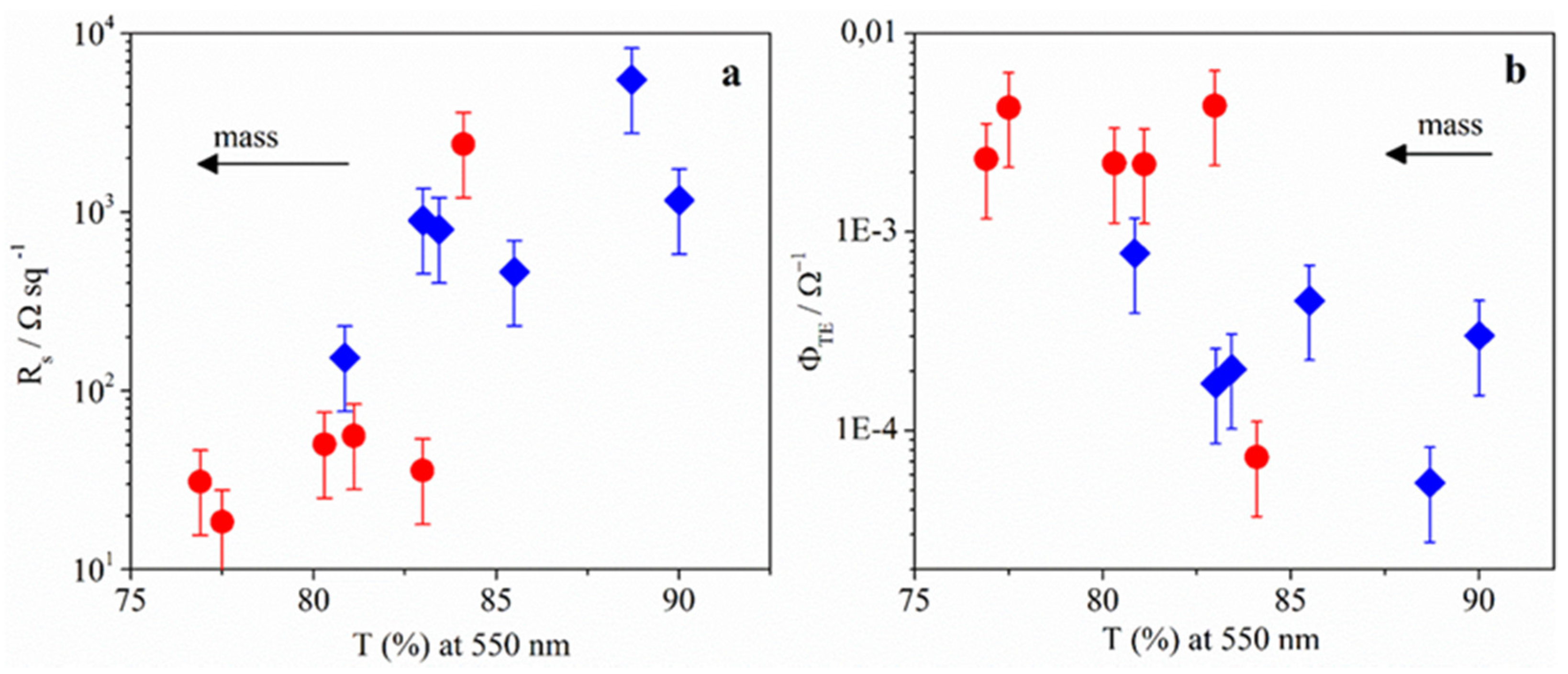
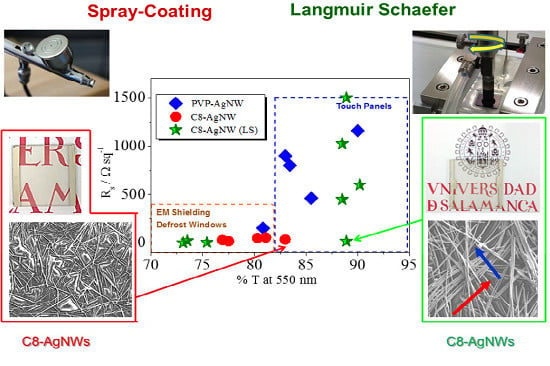
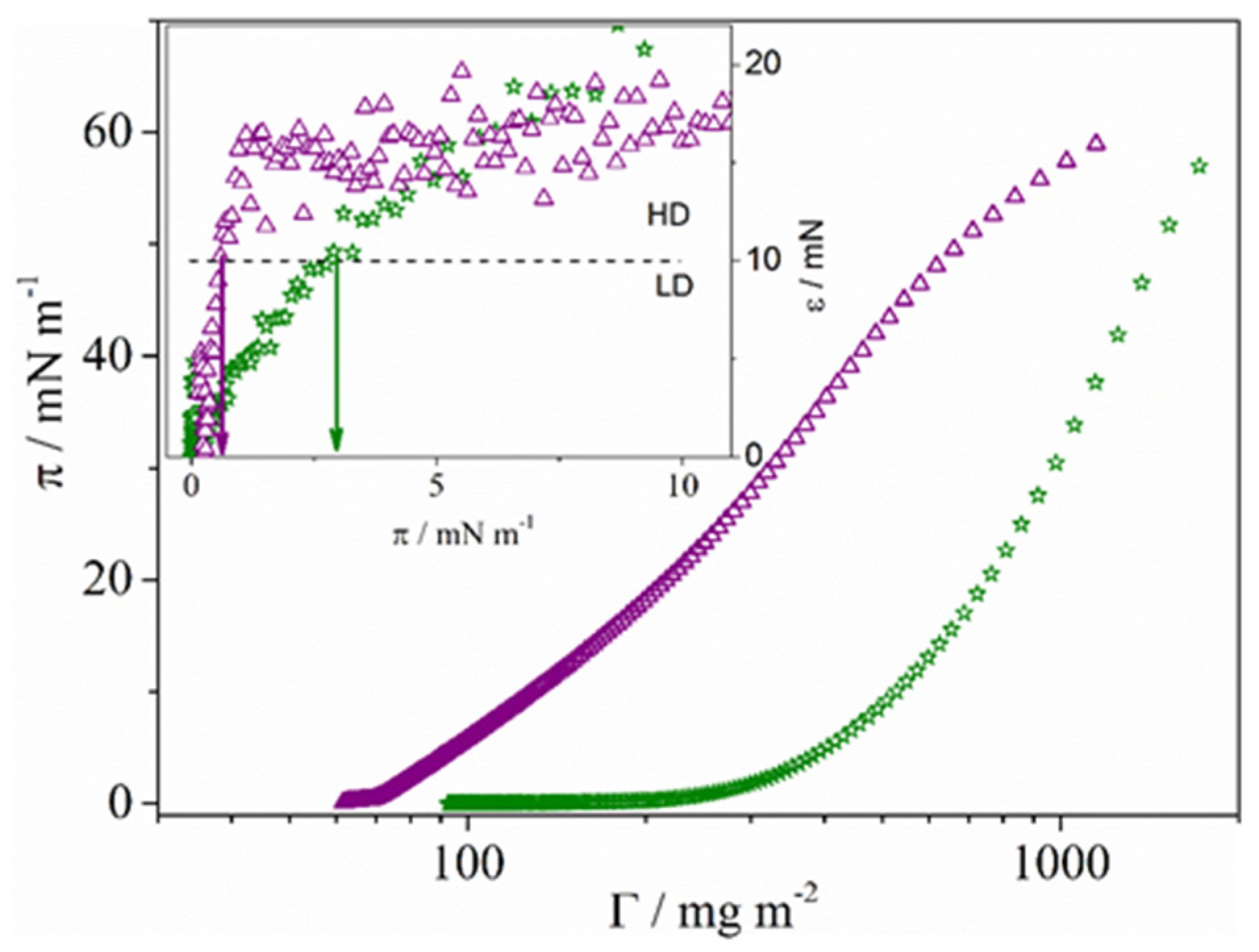
2.2.2. Langmuir-Schaefer Films
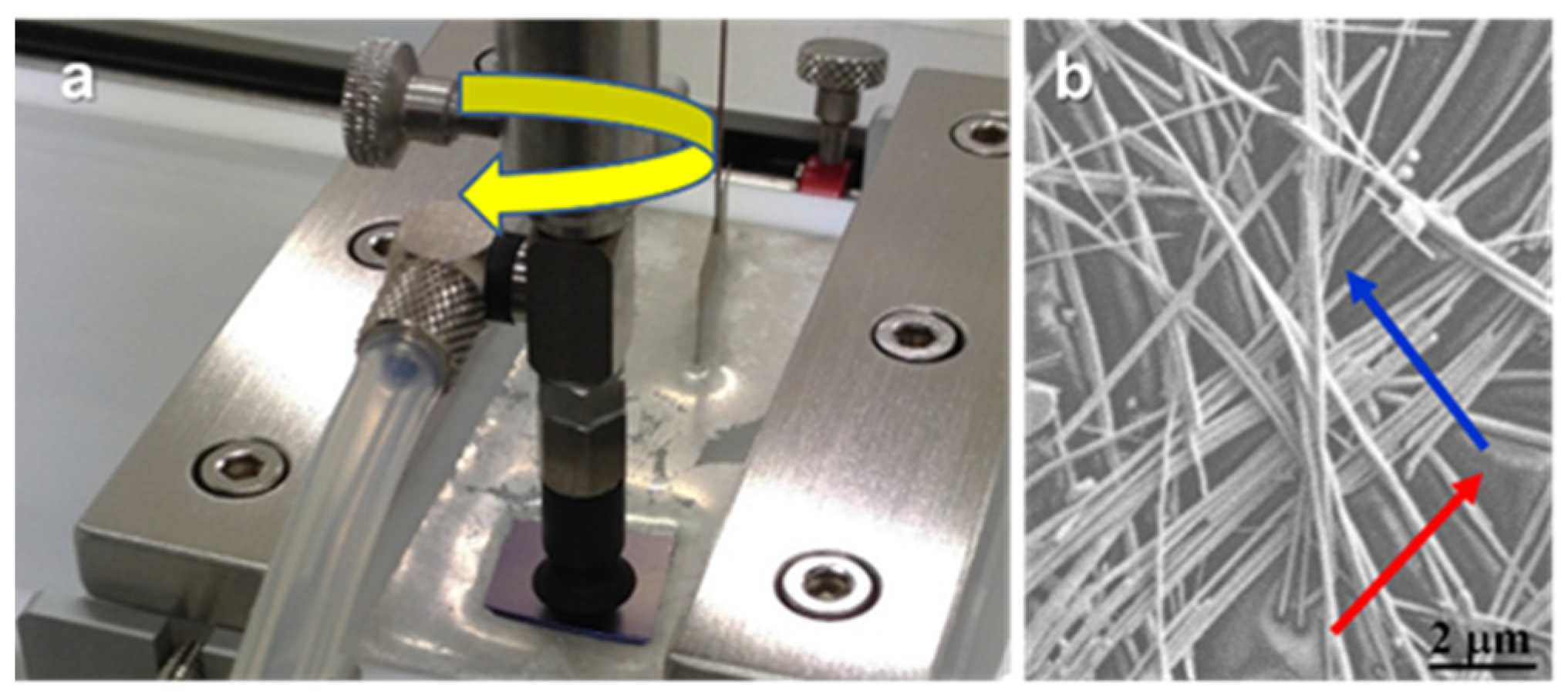
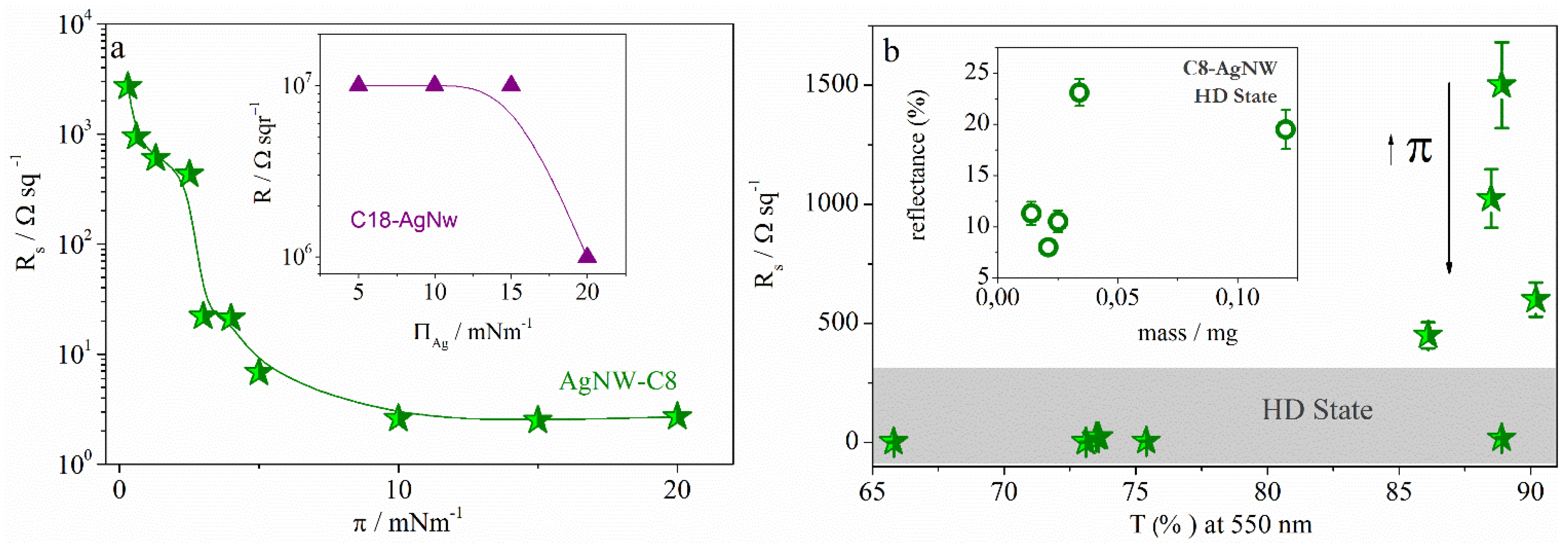

3. Experimental Section
3.1. Synthesis of Silver Nanowires
3.2. Experimental Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.-P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, H.; Lee, J.; Kwon, J.; Han, S.; Suh, Y.D.; Cho, H.; Shin, J.; Yeo, J.; Ko, S.H. Highly stretchable and transparent metal nanowire heater for wearable electronics applications. Adv. Mater. 2015, 27, 4744–4751. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Cameron, D.C. Investigation of annealing effects on sol-gel deposited indium tin oxide thin films in different atmospheres. Thin Solid Films 2002, 420, 76–82. [Google Scholar] [CrossRef]
- Park, S.-E.; Kim, S.; Lee, D.-Y.; Kim, E. Fabrication of silver nanowire transparent electrodes using electrohydrodynamic spray deposition for flexible organic solar cells. J. Mater. Chem. A 2013, 1, 14286–14293. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, conductive carbon nanotube films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Doherty, E.M.; De, S.; Lyonsa, P.E.; Shmeliova, A.; Nirmalraj, P.N.; Scardaci, V.; Joimel, J.; Blau, W.J.; Boland, J.J.; Coleman, J.N. The spatial uniformity and electromechanical stability of transparent, conductive films of single walled nanotubes. Carbon 2009, 47, 2466–2473. [Google Scholar] [CrossRef]
- Moon, I.K.; Kim, J.I.; Lee, H.; Hur, K.; Kim, W.C.; Lee, H. 2D graphene oxide nanosheets as an adhesive over-coating layer for flexible transparent conductive electrodes. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Yun, Y.S.; Kim, D.H.; Kim, B.; Park, H.H.; Jin, H.J. Transparent conducting films based on graphene oxide/silver nanowire hybrids with high flexibility. Synth. Metals 2012, 162, 1364–1368. [Google Scholar] [CrossRef]
- Madaria, A.; Kumar, A.; Ishikawa, F.; Zhou, C. Uniform, highly conductive, and patterned transparent films of a percolating silver nanowire network on rigid and flexible substrates using a dry transfer technique. Nano Res. 2010, 3, 564–573. [Google Scholar] [CrossRef]
- Song, T.-B.; Li, N. Emerging transparent conducting electrodes for organic light emitting diodes. Electronics 2014, 3, 190–204. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Serway, R.A. Principles of Physics, 2nd ed.; Saunder Colloge Pub.: Fort Worth, TX, USA, 1998. [Google Scholar]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-processed metal nanowire mesh transparent electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Canlier, A.; Kim, G.H.; Choi, J.; Park, M.; Han, S.M. Electrostatic spray deposition of high transparent silver nanowire electrode on flexible substrate. ACS Appl. Mater. Interfaces 2013, 5, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, P.; Lee, D.; Lee, S.S.; Ko, S.H. Large-scale synthesis and characterization of very long silver nanowires via successive multistep growth. Cryst. Growth Des. 2012, 12, 5598–5605. [Google Scholar] [CrossRef]
- Lee, J.; Lee, P.; Lee, H.; Lee, D.; Lee, S.S.; Ko, S.H. Very long ag nanowire synthesis and its application in a highly transparent, conductive and flexible metal electrode touch panel. Nanoscale 2012, 4, 6408–6414. [Google Scholar] [CrossRef] [PubMed]
- Leem, D.S.; Edwards, A.; Faist, M.; Nelson, J.; Bradley, D.D.; de Mello, J.C. Efficient organic solar cells with solution-processed silver nanowire electrodes. Adv. Mater. 2011, 23, 4371–4375. [Google Scholar] [CrossRef] [PubMed]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Huang, J.; Fan, R.; Connor, S.; Yang, P. One-step patterning of aligned nanowire arrays by programmed dip coating. Angew. Chem. Int. 2007, 46, 2414–2417. [Google Scholar] [CrossRef] [PubMed]
- Andrew, P.; Ilie, A. Functionalised silver nanowire structures. J. Phys. Conf. Ser. 2007, 61, 36–40. [Google Scholar] [CrossRef]
- Moran, C.H.; Rycenga, M.; Zhang, Q.; Xia, Y. Replacement of poly(vinyl pyrrolidone) by thiols: A systematic study of ag nanocube functionalization by surface-enhanced raman scattering. J. Phys. Chem. C 2011, 115, 21852–21857. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.; Jiang, G.; Zhou, J.; Chen, X.; Yu, H.; Yang, Q. Study on the synthesis of silver nanowires with adjustable diameters through the polyol process. Nanotechnology 2006, 17. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366. [Google Scholar] [CrossRef] [PubMed]
- Mutiso, R.M.; Sherrott, M.C.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Integrating simulations and experiments to predict sheet resistance and optical transmittance in nanowire films for transparent conductors. ACS Nano 2013, 7, 7654–7663. [Google Scholar] [CrossRef] [PubMed]
- Scardaci, V.; Coull, R.; Lyons, P.E.; Rickard, D.; Coleman, J.N. Spray deposition of highly transparent, low-resistance networks of silver nanowires over large areas. Small 2011, 7, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.S.; Chen, T.L.; Pruneri, V. Ultrathin Cu-Ti bilayer transparent conductors with enhanced figure-of-merit and stability. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Tao, A.R.; Huang, J.; Yang, P. Langmuir-blodgettry of nanocrystals and nanowires. Acc. Chem. Res. 2008, 41, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Diaz, D.; Velazquez, M.M.; Blanco de La Torre, S.; Perez-Pisonero, A.; Trujillano, R.; Garcia Fierro, J.L.; Claramunt, S.; Cirera, A. The role of oxidative debris on graphene oxide films. Chemphyschem 2013, 14, 4002–4009. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, R.S.; López-Díaz, D.; Velázquez, M.M. Graphene oxide thin films: Influence of chemical structure and deposition methodology. Langmuir 2015, 31, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Gaines, G.L.J. Insoluble Monolayers at Liquid-Gas Interfaces; Interscience: New York, NY, USA, 1966. [Google Scholar]
- Velázquez, M.M.; Ortega, F.; Monroy, F.; Rubio, R.G.; Pegiadou, S.; Pérez, L.; Infante, M.R. Langmuir monolayers of the zwitterionic surfactant hexadecyl 1-n-l-tryptophan glycerol ether. J. Colloid Interface Sci. 2005, 283, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves da Silva, A.M.; Romão, R.S.; Lucero Caro, A.; Rodrı́guez Patino, J.M. Memory effects on the interfacial characteristics of dioctadecyldimethylammonium bromide monolayers at the air–water interface. J. Colloid Interface Sci. 2004, 270, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Alejo, T.; Merchán, M.D.; Velázquez, M.M.; Pérez-Hernández, J.A. Polymer/surfactant assisted self-assembly of nanoparticles into langmuir–blodgett films. Mater. Chem. Phys. 2013, 138, 286–294. [Google Scholar] [CrossRef]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Mora, S.B.; Cloutier, S.G. Figures of merit for high-performance transparent electrodes using dip-coated silver nanowire networks. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform silver nanowires synthesis by reducing agno3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.; Sun, Y.; Xia, Y.; Yang, P. Langmuir-blodgett silver nanowire monolayers for molecular sensing using surface-enhanced raman spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Diaz, D.; Merino, C.; Velázquez, M.M. Modulating the Optoelectronic Properties of Silver Nanowires Films: Effect of Capping Agent and Deposition Technique. Materials 2015, 8, 7622-7633. https://doi.org/10.3390/ma8115405
Lopez-Diaz D, Merino C, Velázquez MM. Modulating the Optoelectronic Properties of Silver Nanowires Films: Effect of Capping Agent and Deposition Technique. Materials. 2015; 8(11):7622-7633. https://doi.org/10.3390/ma8115405
Chicago/Turabian StyleLopez-Diaz, D., C. Merino, and M. M. Velázquez. 2015. "Modulating the Optoelectronic Properties of Silver Nanowires Films: Effect of Capping Agent and Deposition Technique" Materials 8, no. 11: 7622-7633. https://doi.org/10.3390/ma8115405