1-(Triethoxysilyl)buta-1,3-dienes—New Building Blocks for Stereoselective Synthesis of Unsymmetrical (E,E)-1,4-Disubstituted 1,3-dienes
Abstract
:1. Introduction
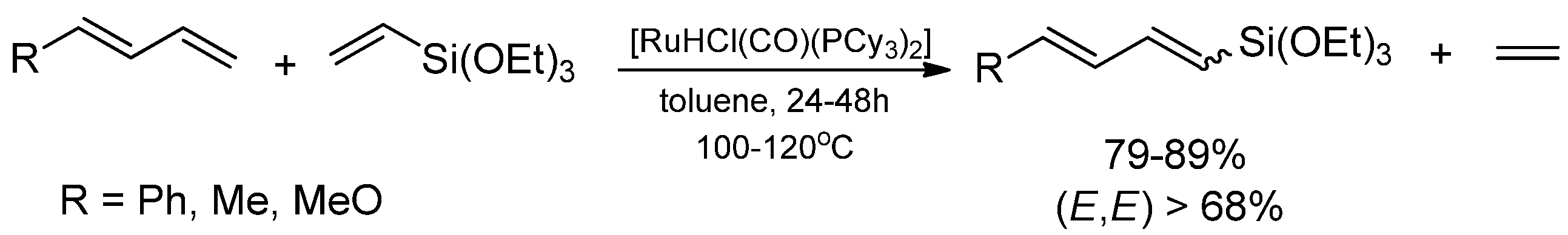
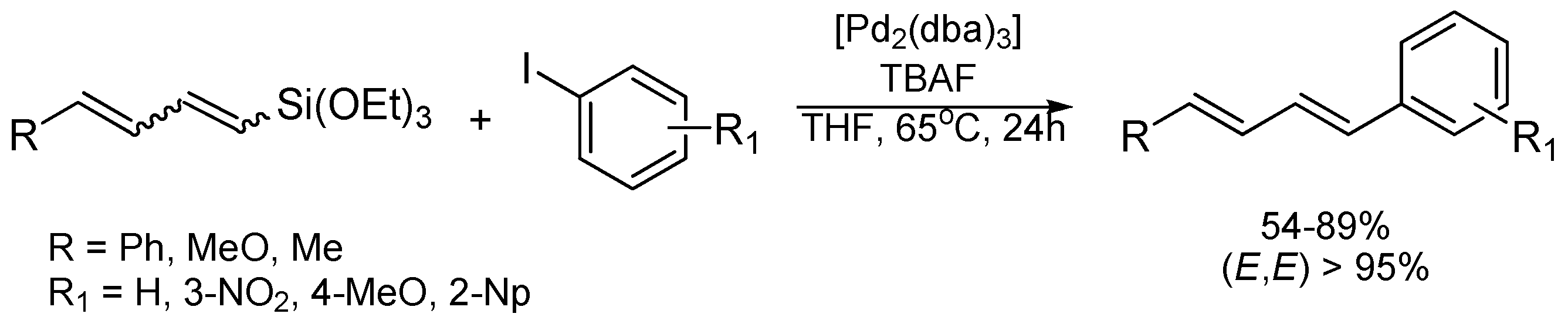
2. Results and Discussion
| Entry | R (Diene) | Aryl Iodide | Product | Isolated Yield [%] | Selectivity EE/EZ |
|---|---|---|---|---|---|
| 1 | Ph | PhI | 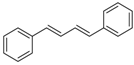 | 89 | 99:1 |
| 2 | Ph | 3-NO2C6H4I | 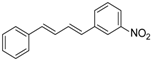 | 86 | >99 |
| 3 | Ph | 4-MeOC6H4I |  | 79 | >99 |
| 4 | Ph | 2-C10H7I |  | 55 | 99:1 |
| 5 | MeO | 4-MeOC6H4I | 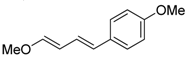 | 70 | 95:5 |
| 6 | MeO | PhI |  | 54 | 98:2 |
| 7 | Me | 4-MeOC6H4I | 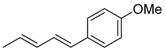 | 62 | 99:1 |
3. Experimental Section
3.1.General Procedure for the Synthesis of (E,E)-1,4-disubstituted buta-1,3-dienes (1–7)
3.1.1. (E,E)-1-methox-4-(4-methoxyphenyl)buta-1,3-diene (5); yellow oil; Yield: 0.13 g (70%)
3.1.2. (E,E)-1-Methoxy-4-(phenyl)Buta-1,3-diene (6); yellow oil; Yield: 0.035 g (54%)
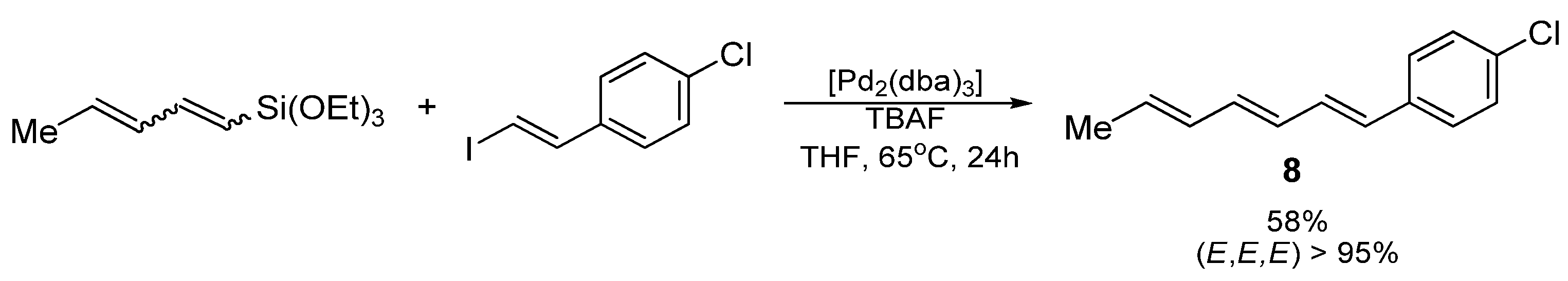
3.2. Synthesis of 1-(4-chlorophenyl)hepta-1,3,5-triene (8)
(E,E,E)-1-(4-chlorophenyl)hepta-1,3,5-triene (8); yellow oil; Yield: 0.048 g (58%)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [PubMed]
- Singh, A.K.; Darshi, M.; Kanvah, S. α,ω-Diphenylpolyenes cabable of exhibiting twisted intramolecular charge transfer fluorescence: A fluorescence and fluorescence probe study of nitro- and nitrocyano-substituted 1,4-diphenylbutadienes. J. Phys. Chem. A 2000, 104, 464–471. [Google Scholar] [CrossRef]
- Adachi, C.; Tsutsui, T.; Saito, S. Blue light emitting organic electroluminescent devices. Appl. Phys. Lett. 1990, 56, 799–801. [Google Scholar] [CrossRef]
- Mladenova, M.; Ventelon, L.; Blanchard-Desche, M. A convenient synthesis of push-pull polyenes designed for the elaboration of efficient nonlinear optical materials. Tetrahedron Lett. 1999, 40, 6923–6926. [Google Scholar] [CrossRef]
- Diemer, V.; Chaumeil, H.; Defoin, A.; Carré, Ch. Synthesis of alkoxynitrostilbenes as chromophores for nonlinear optical materials. Synthesis 2007, 21, 3333–3338. [Google Scholar]
- Cornil, J.; Guérinot, A.; Cossy, J. Linchpin dienes: Key building-blocks in the synthesis of polyenic frameworks. Org. Biomol. Chem. 2015, 13, 4129–4142. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Yamada, K.; Suginome, H.; Suzuki, A. Novel and convenient method for the stereo- and regiospecific synthesis of conjugated alkadienes and alkenynes via the palladium-catalyzed cross-coupling reaction of 1-alkenylboranes with bromoalkenes and bromoalkynes. J. Am. Chem. Soc. 1985, 107, 972–980. [Google Scholar] [CrossRef]
- Stille, J.K.; Groh, B.L. Stereospecific cross-coupling of vinyl halides with vinyl tin reagents catalyzed by palladium. J. Am. Chem. Soc. 1987, 109, 813–817. [Google Scholar] [CrossRef]
- Zeng, X.; Qian, M.; Hu, Q.; Negishi, E.-I. Highly stereoselective synthesis of (1E)-2-methyl-1,3-dienes by palladium-catalyzed trans-selective cross-coupling of 1,1-dibromo-1-alkenes with alkenylzinc reagents. Angew. Chem. Int. Ed. 2004, 43, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, Y.; Hiyama, T. Cross-coupling of organosilanes with organic halides mediated by a palladium catalyst and tris(diethylamino)sulfonium difluorotrimethylsilicate. J. Org. Chem. 1988, 53, 918–920. [Google Scholar] [CrossRef]
- Cai, M.; Ye, H.; Zhao, H.; Song, C. Stereoselective synthesis of 1,3-dienylstannanes by palladium catalyzed cross-coupling reactions. J. Organomet. Chem. 2003, 687, 462–465. [Google Scholar] [CrossRef]
- Cai, M.-Z.; Ye, X.-L.; Wang, P.-P. A facile stereoselective synthesis of (Z,E)-2-silyl-substituted 1,3-dienes via palladium-catalyzed cross-coupling reaction. Synthesis 2005, 16, 2654–2656. [Google Scholar] [CrossRef]
- Denmark, S.E.; Tymonko, S.A. Sequential cross-coupling of 1,4-bissilylbutadienes: synthesis of unsymmetrical 1,4-disubstituted 1,3-butadienes. J. Am. Chem. Soc. 2005, 127, 8004–8005. [Google Scholar] [CrossRef] [PubMed]
- Babudri, F.; Farinola, G.M.; Fiandanese, V.; Mazzone, L.; Naso, F. A straightforward route to polyenylsilanes by palladium- or nickel-catalyzed cross-coupling reactions. Tetrahedron 1998, 54, 1085–1094. [Google Scholar] [CrossRef]
- Ashe, A.J.; Mahmoud, S. 1,4-Dilithio-1,3-butadienes. Organometallics 1988, 7, 1878–1880. [Google Scholar] [CrossRef]
- Nozawa, D.; Takikawa, H.; Mori, K. Triterpenoid total synthesis. Part 5. Synthetic disproof of the triterpene structure proposed for naurol A, a cytotoxic metabolite of a Pacific sponge. J. Chem. Soc. Perkin Trans. 2000, 2043–2046. [Google Scholar]
- Fujii, S.; Chang, S.Y.; Burke, M.D. Total synthesis of synechoxanthin through iterative cross-coupling. Angew. Chem. Int. Ed. 2011, 50, 7862–7864. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.S.; Walczak, M.C. Tandem Stille/Suzuki−Miyaura coupling of a hetero-bis-metalated diene rapid, one-pot assembly of polyene systems. Org. Lett. 2005, 7, 2289–2291. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.S.; Walczak, M.C. Total synthesis of Gymnoconjugatins A and B. J. Org. Chem. 2006, 71, 9841–9844. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B., III; Foley, M.A.; Dong, S.; Orbin, A. (+)-Rimocidin synthetic studies: Construction of the C(1−27) aglycone skeleton. J. Org. Chem. 2009, 74, 5987–6001. [Google Scholar] [CrossRef] [PubMed]
- Babudri, F.; Farinola, G.M.; Naso, F.; Ragni, R.; Spina, G. A novel Stereoselective synthesis of symmetrical (1E,3E)-1,4-diarylbuta-1,3-dienes. Synthesis 2007, 19, 3088–3092. [Google Scholar]
- Ananikov, V.P.; Kashin, A.S.; Hazipov, O.V.; Beletskaya, I.P.; Starikova, Z.A. Highly selective catalytic synthesis of (E,E)-1,4-diiodobuta-1,3-diene via atom-efficient addition of acetylene and iodine: A versatile (E,E)-1,3-diene building block in cross-coupling reactions. Synlett 2011, 22, 2021–2025. [Google Scholar] [CrossRef]
- Hiyama, T. Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E.-I., Ed.; Wiley-Interscience: New York, NY, USA, 2002; pp. 285–309. [Google Scholar]
- Nakao, Y.; Hiyama, T. Silicon-based cross-coupling reaction: an environmentally benign version. Chem. Soc. Rev. 2011, 40, 4893–4901. [Google Scholar] [CrossRef] [PubMed]
- Sore, H.F.; Galloway, W.R.J.D.; Spring, D.R. Palladium-catalysed cross-coupling of organosilicon reagents. Chem. Soc. Rev. 2012, 41, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Prukała, W.; Majchrzak, M.; Pietraszuk, C.; Marciniec, B. Highly stereoselective synthesis of E-4-chlorostilbene and its derivatives via tandem cross-metathesis (or silylative coupling) and Hiyama coupling. J. Mol. Catal. A Chem. 2006, 254, 58–63. [Google Scholar] [CrossRef]
- Prukała, W.; Marciniec, B.; Majchrzak, M.; Kubicki, M. Highly stereoselective synthesis of para-substituted (E)-N-styrylcarbazoles via sequential silylative coupling–Hiyama coupling reaction. Tetrahedron 2007, 63, 1107–1115. [Google Scholar] [CrossRef]
- Prukala, W.; Majchrzak, M.; Posała, K.; Marciniec, B. A convenient one-pot synthesis of bis[(E)-4-halostyryl]arene derivatives. Synthesis 2008, 19, 3047–3052. [Google Scholar]
- Prukała, W.; Pawluć, P.; Posała, K.; Marciniec, B. A new stereoselective approach to (E)-poly(arylenevinylene)s. Synlett 2008, 1, 41–44. [Google Scholar]
- Pawluc, P.; Hreczycho, G.; Suchecki, A.; Kubicki, M.; Marciniec, B. Cyclic 1,1-bis(silyl)alkenes-new building blocks for the stereoselective synthesis of unsymmetrical (E)-stilbenes and (E,E)-1,4-diarylbuta-1,3-dienes. Tetrahedron 2009, 65, 5497–5502. [Google Scholar] [CrossRef]
- Pawluć, P.; Hreczycho, G.; Szudkowska, J.; Franczyk, A.; Marciniec, B. (Z)-1,2-bis(ethoxydimethylsilyl)arylethenes as new building blocks for organic synthesis. Appl. Organomet. Chem. 2010, 24, 853–857. [Google Scholar] [CrossRef]
- Marciniec, B.; Waehner, J.; Pawluć, P.; Kubicki, M. Highly stereoselective synthesis and application of functionalized tetravinylcyclotetrasiloxanes via catalytic reactions. J. Mol. Catal. A: Chem. 2007, 265, 25–31. [Google Scholar] [CrossRef]
- Denmark, S.E.; Fujimori, S. Total synthesis of RK-397. J. Am. Chem. Soc. 2005, 127, 8971–8973. [Google Scholar] [CrossRef] [PubMed]
- Szudkowska-Frątczak, J.; Marciniec, B.; Hreczycho, G.; Kubicki, M.; Pawluć, P. Ruthenium-catalyzed silylation of 1,3-butadienes with vinylsilanes. Org. Lett. 2015, 17, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Srimani, D.; Leitus, G.; Ben-David, Y.; Milstein, D. Direct catalytic olefination of alcohols with Sulfonem. Angew. Chem. In. Ed. 2014, 53, 11092–11095. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.L.; Kirst, H.A.; O’Neil, D.; Bridget, A.D.; Smith, C.K., II; Naylor, S.A. Synthesis and evaluation of a series of 1,4-diarylbutadienes for anticoccidial activity. Bioorg. Med. Chem. 2003, 11, 4083–4091. [Google Scholar] [CrossRef]
- Yamashita, M.; Hirano, K.; Satoh, T.; Miura, M. Synthesis of α,ω-Diarylbutadienes and Hexatrienes via Decarboxylative coupling of cinnamic acids with vinyl bromides under palladium catalysis. Org. Lett. 2010, 12, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.-J.; Li, H.-H.; Tian, S.-K. A highly tunable stereoselective olefination of semistabilized triphenylphosphonium ylides with N-sulfonyl imines. J. Am. Chem. Soc. 2010, 132, 5018–5020. [Google Scholar] [CrossRef] [PubMed]
- Mitsudo, T.; Fischetti, W.; Heck, R.F. Palladium-catalyzed syntheses of aryl polyenes. J. Org. Chem. 1984, 49, 1640–1646. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szudkowska-Frątczak, J.; Taczała, M.; Pawluć, P. 1-(Triethoxysilyl)buta-1,3-dienes—New Building Blocks for Stereoselective Synthesis of Unsymmetrical (E,E)-1,4-Disubstituted 1,3-dienes. Materials 2015, 8, 7250-7256. https://doi.org/10.3390/ma8115378
Szudkowska-Frątczak J, Taczała M, Pawluć P. 1-(Triethoxysilyl)buta-1,3-dienes—New Building Blocks for Stereoselective Synthesis of Unsymmetrical (E,E)-1,4-Disubstituted 1,3-dienes. Materials. 2015; 8(11):7250-7256. https://doi.org/10.3390/ma8115378
Chicago/Turabian StyleSzudkowska-Frątczak, Justyna, Mariusz Taczała, and Piotr Pawluć. 2015. "1-(Triethoxysilyl)buta-1,3-dienes—New Building Blocks for Stereoselective Synthesis of Unsymmetrical (E,E)-1,4-Disubstituted 1,3-dienes" Materials 8, no. 11: 7250-7256. https://doi.org/10.3390/ma8115378
APA StyleSzudkowska-Frątczak, J., Taczała, M., & Pawluć, P. (2015). 1-(Triethoxysilyl)buta-1,3-dienes—New Building Blocks for Stereoselective Synthesis of Unsymmetrical (E,E)-1,4-Disubstituted 1,3-dienes. Materials, 8(11), 7250-7256. https://doi.org/10.3390/ma8115378





