Advanced Chemical Reduction of Reduced Graphene Oxide and Its Photocatalytic Activity in Degrading Reactive Black 5
Abstract
:1. Introduction
| Properties | Anionic Azo Reactive Dye |
|---|---|
| Synonym name | Remazol Black B |
| Composition | ≥50% of dye content |
| Molecular formula | C26H21N5Na4O19S6 |
| Molecular weight | 991.82 g·mol−1 |
| Absorbance wavelength (λmax) | 597 nm |
| Molecular Structure |  |
| Authors/Year | Catalysts | Degradation Efficiency (%) | Conditions | Reference |
|---|---|---|---|---|
| Soltani et al. 2013 | BiFeO3 | 99% RB5 | Time: 50 min; Catalyst loading: 0.5 g·L−1; Irradiation: Natural Sunlight; pH 2.5 | [4] |
| Goharshadi et al. 2013 | ZnS | 95% RB5 | Time: 10 min; Catalyst loading: 0.15 g·L−1; Irradiation: UV light; pH 7 | [12] |
| Laohaprapanon et al. 2015 | ZnO | 85% RB5 | Time: 30 min; Catalyst loading: 1.25 g·L−1; Irradiation: UV light; pH 7 | [13] |
| Lucas et al. 2013 | TiO2 TiO2 P25 | 93% RB5 97% RB5 | Time: 60 min; Catalyst loading: 0.1 g·L−1; Irradiation: UV light; pH 5.7 | [11] |
2. Experimental Section
2.1. Chemicals and Materials
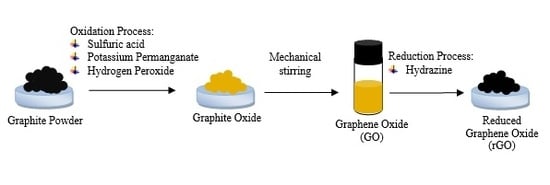
2.2. Synthesis of Reduced Graphene Oxide
2.3. Characterization
2.4. Photocatalytic Reaction
3. Results and Discussions
3.1. Characterization of Catalyst
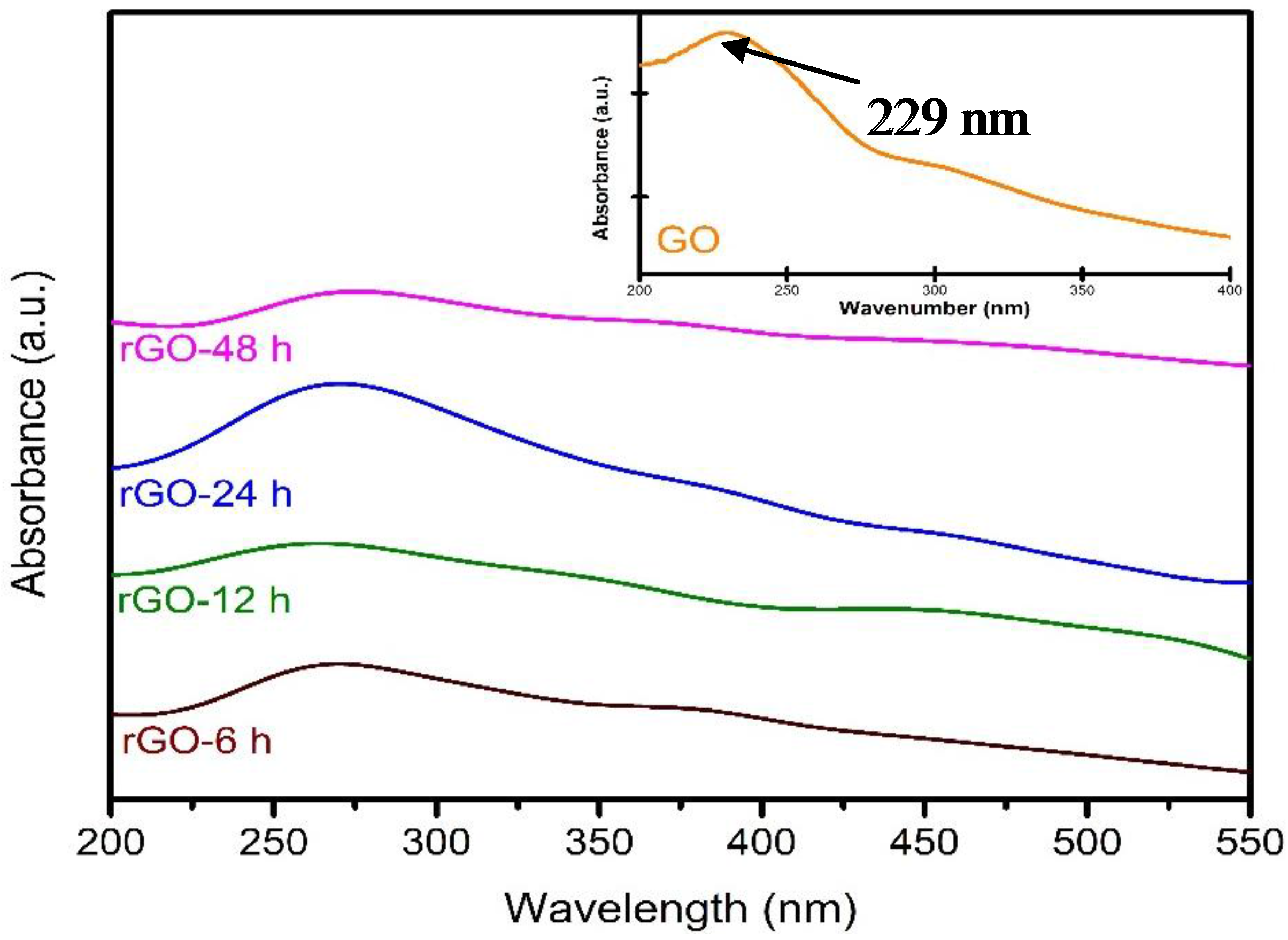
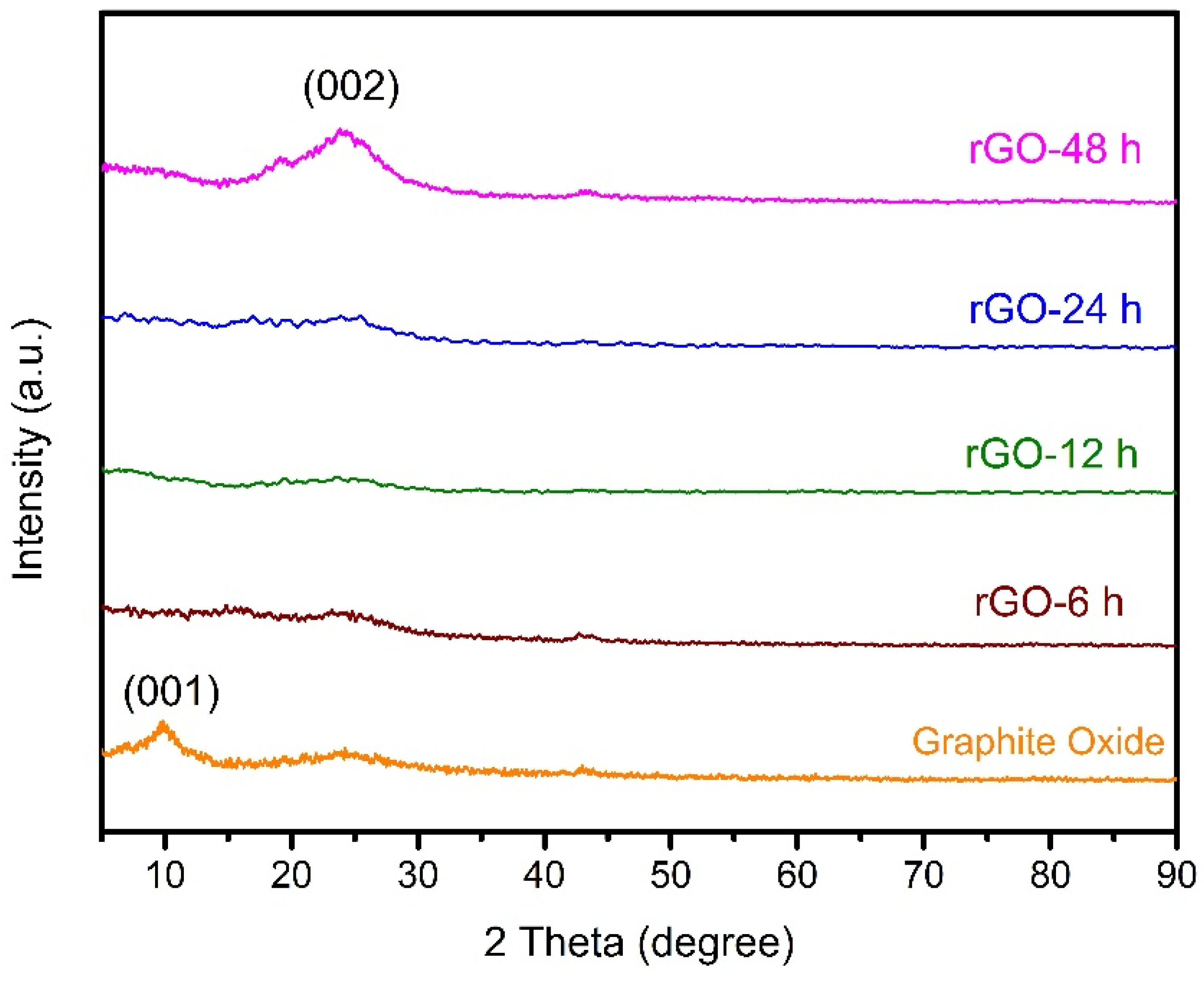

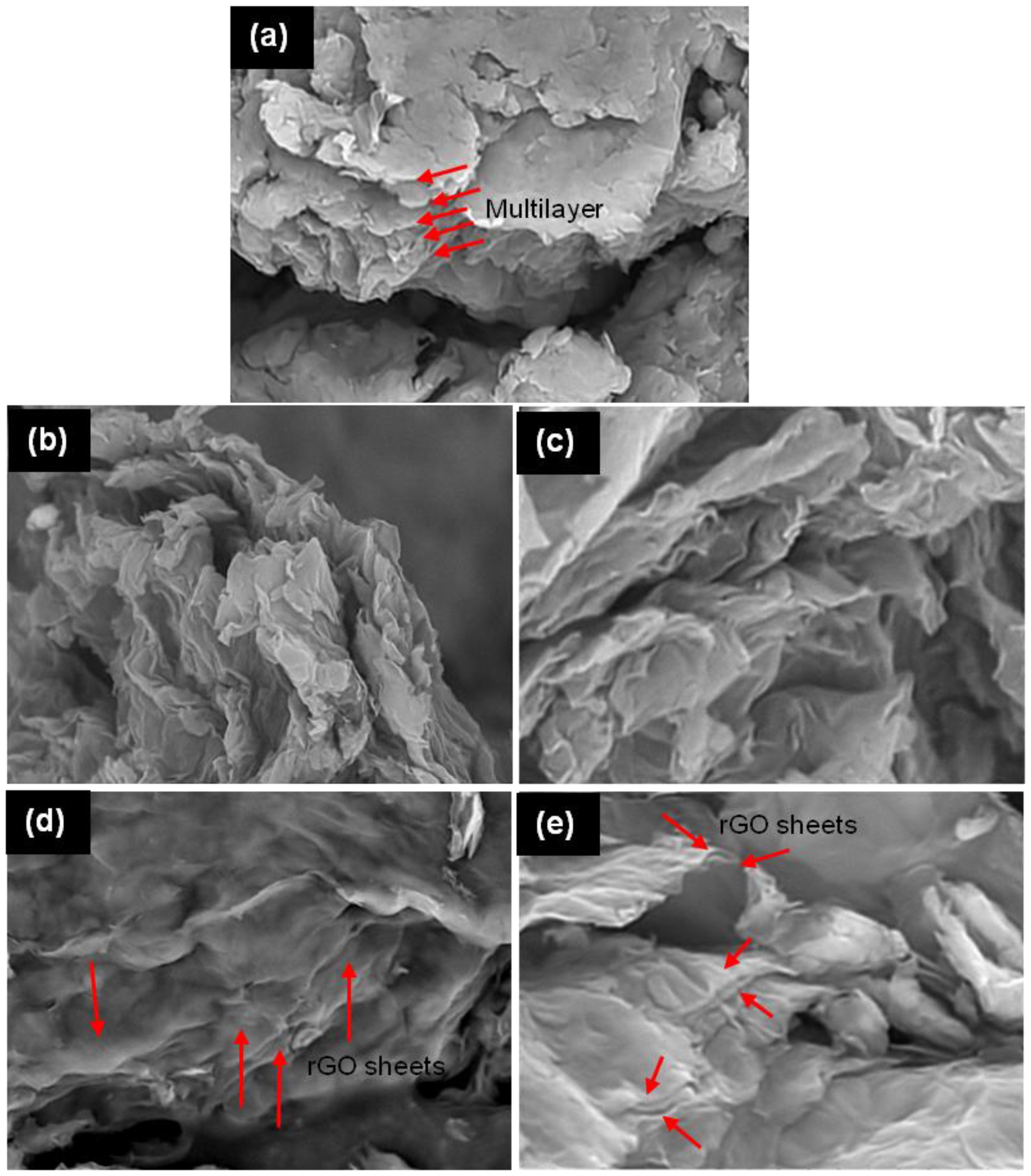
3.2. Reduction Time
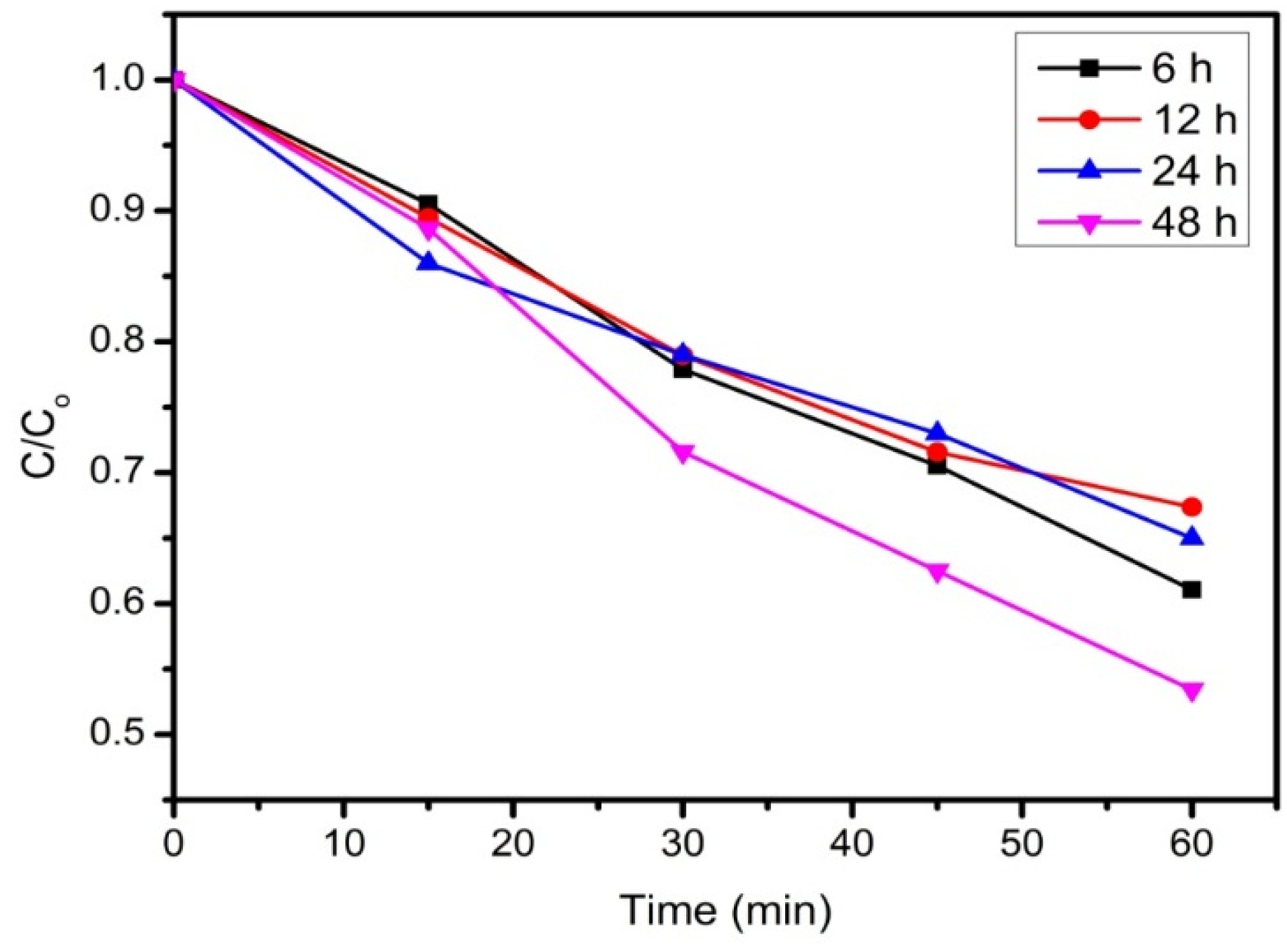
3.3. Effect of rGO Loading

3.4. Effect of RB5 Concentration and Its Kinetics

| Concentration of RB5 (mg/L) | Degradation efficiency (%) | R2 | Value of k (min−1) |
|---|---|---|---|
| 5 | 63 | 0.99684 | 0.01704 |
| 10 | 56 | 0.99188 | 0.01395 |
| 15 | 31 | 0.98723 | 0.00639 |
| 20 | 26 | 0.99768 | 0.00507 |
3.5. Effect of pH

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, K.M.; Abdul Hamid, S.B.; Lai, C.W. Multivariate analysis of photocatalytic-mineralization of Eriochrome Black T dye using ZnO catalyst and UV irradiation. Mater. Sci. Semicond. Process. 2015, 39, 40–48. [Google Scholar] [CrossRef]
- Mehra, M.; Sharma, T.R. Photocatalytic degradation of two commercial dyes in aqueous phase using photocatalyst TiO2. Adv. Appl. Sci. Res. 2012, 3, 849–853. [Google Scholar]
- Vinothkannan, M.; Karthikeyan, C.; Gnana kumar, G.; Kim, A.R.; Yoo, D.J. One-pot green synthesis of reduced graphene oxide (RGO)/Fe3O4 nanocomposites and its catalytic activity toward methylene blue dye degradation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Soltani, T.; Entezari, M.H. Solar photocatalytic degradation of RB5 by ferrite bismuth nanoparticles synthesized via ultrasound. Ultrason. Sonochem. 2013, 20, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Cho, Y.J.; Poh, P.E.; Jin, B. Evaluation of titanium dioxide photocatalytic technology for the treatment of reactive black 5 dye in synthetic and real greywater effluents. J. Clean. Prod. 2015, 89, 196–202. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Wu, Y.T.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Zhang, W.; Naidu, B.S.; Ou, J.Z.; O’Mullane, A.P.; Chrimes, A.F.; Carey, B.J.; Wang, Y.; Tang, S.Y.; Sivan, V.; Mitchell, A.; et al. Liquid metal/metal oxide frameworks with incorporated Ga2O3 for photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Kansal, S.; Kaur, N.; Singh, S. Photocatalytic degradation of two commercial reactive dyes in aqueous phase using nanophotocatalysts. Nanoscale Res. Lett. 2009, 4, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Yeong, J.J.; Tan, L.L.; Goh, B.T.; Yong, S.T.; Chai, S.P. Synergistic effect of graphene as a co-catalyst for enhanced daylight-induced photocatalytic activity of Zn0.5Cd0.5S synthesized via an improved one-pot co-precipitation-hydrothermal strategy. R. Soc. Chem. Adv. 2014, 4, 59676–59685. [Google Scholar] [CrossRef]
- Sahel, K.; Perol, N.; Chermette, H.; Bordes, C.; Derriche, Z.; Guillard, C. Photocatalytic decolourization of remazol black 5 (RB5) and procion red MX-5B-Isotherm of adsorption, kinetic of decolourization and mineralization. Appl. Catal. B Environ. 2007, 77, 100–109. [Google Scholar] [CrossRef]
- Lucas, M.S.; Tavares, P.B.; Peres, J.A.; Faria, J.L.; Rocha, M.; Pereira, C.; Freire, C. Photocatalytic degradation of reactive black 5 with TiO2-coated magnetic nanoparticles. Catal. Today 2013, 209, 116–121. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Hadadian, M.; Karimi, M.; Azizi-Toupkanloo, H. Photocatalytic degradation of reactive black 5 azo dye by zinc sulfide quantum dots prepared by a sonochemical method. Mater. Sci. Semicond. Process. 2013, 16, 1109–1116. [Google Scholar] [CrossRef]
- Laohaprapanon, S.; Matahum, J.; Tayo, L.; You, S.J. Photodegradation of reactive black 5 in a ZnO/UV slurry membrane reactor. J. Taiwan Inst. Chem. Eng. 2015, 49, 136–141. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family-A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y. Study of reduced graphene oxide preparation by Hummers’ method and related characterization. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Wang, D.T.; Li, X.; Chen, J.F.; Tao, X. Enhanced photoelectrocatalytic activity of reduced graphene oxide/TiO2 composite films for dye degradation. Chem. Eng. J. 2012, 198, 547–554. [Google Scholar] [CrossRef]
- Dong, S.Y.; Cui, Y.R.; Wang, Y.F.; Li, Y.K.; Hu, L.M.; Sun, J.Y.; Sun, J.H. Designing three-dimensional acicular sheaf shaped BiVO4/reduced graphene oxide composites for efficient sunlight-driven photocatalytic degradation of dye wastewater. Chem. Eng. J. 2014, 249, 102–110. [Google Scholar] [CrossRef]
- Liu, X.J.; Pan, L.K.; Lv, T.; Sun, Z.; Sun, C.Q. Visible light photocatalytic degradation of dyes by bismuth oxide-reduced graphene oxide composites prepared via microwaved-assisted method. J. Colloid Interface Sci. 2013, 408, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.J.; Xu, C.; Miao, S.D.; Sun, H.Q.; Wang, S.B. One-pot hydrothermal synthesis of Co(OH)2 nanoflakes on graphene sheets and their fast catalytic oxidation of phenol in liquid phase. J. Colloid Interface Sci. 2013, 40, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Kim, G.S.; Kim, S.J. Graphene nanosheets: Ultrasound assisted synthesis and characterization. Ultrason. Sonochem. 2013, 20, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Cai, X.; Shi, Q.S.; Liu, L.L.; Wan, D.L.; Tan, S.Z.; Ouyang, Y.S. Poly-L-lysine-modified reduced graphene oxide stabilizes the copper nanoparticles with higher water-solubility and long-term additively antibacterial activity. Colloids Surf. B Biointerfaces. 2013, 107, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Park, M.R.; Kwon, D.N.; Kim, J.H. Antibacterial activity of dithiothreitol reduced graphene oxide. J. Ind. Eng. Chem. 2013, 19, 1280–1288. [Google Scholar] [CrossRef]
- Satheesh, K.; Jayavel, R. Synthesis and electrochemical properties of reduced graphene oxide via chemical reduction using thiourea as a reducing agent. Mater. Lett. 2013, 113, 5–8. [Google Scholar] [CrossRef]
- Zainy, M.; Huang, N.M.; Kumar, S.V.; Lim, H.N.; Chia, C.H.; Harrison, L. Simple and scalable preparation of reduced graphene oxide-silver nanocomposites via rapid thermal treatment. Mater. Lett. 2012, 89, 180–183. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, T.J.; Wu, J.; Zhou, H.O. (001) Facet-exposed anatase-phase TiO2 nanotube hybrid reduced graphene oxide composite: Synthesis, characterization and application in photocatalytic degradation. Appl. Surf. Sci. 2013, 287, 359–368. [Google Scholar] [CrossRef]
- Wang, C.L.; Zhou, Y.; Sun, L.; Zhao, Q.; Zhang, X.; Wan, P.; Qiu, J.S. N/P codoped thermally reduced graphene for high-performance supercapacitor applications. J. Phys. Chem. C. 2013, 117, 14912–14919. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, L. Surface-enhanced Raman scattering studies of few-layer graphene on silver substrate with 514 nm excitation. J. Mol. Struct. 2011, 992, 48–51. [Google Scholar] [CrossRef]
- Bak, S.M.; Nam, K.W.; Lee, C.W.; Kim, K.H.; Jung, H.C.; Yang, X.Q.; Kim, K.B. Spinel LiMn2O4/reduced graphene oxide hybrid for high rate lithium ion batteries. J. Mater. Chem. 2011, 21, 17309–17315. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhang, D.C.; Yu, P.; Ma, Y.W. High performance supercapacitors based on reduced graphene oxide in aqueous and ionic liquid electrolytes. Carbon 2011, 49, 573–580. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Alfred, K.; Jia, Y.Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–565. [Google Scholar] [CrossRef]
- Petnikota, S.; Rotte, N.K.; Srikanth, V.V.S.S.; Kota, B.S.R.; Reddy, M.V.; Loh, K.P.; Chowdari, B.V.R. Electrochemical studies of few-layered graphene as an anode material for Li ion batteries. J. Solid State Electrochem. 2014, 18, 941–949. [Google Scholar] [CrossRef]
- Fu, C.J.; Zhao, G.G.; Zhang, H.J.; Li, S. Evaluation and characterization of reduced graphene oxide nanosheets as anode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2013, 8, 6269–6280. [Google Scholar]
- Mamba, G.; Mamo, M.A.; Mbianda, X.Y.; Mishra, A.K. Nd,N,S-TiO2 decorated on reduced graphene oxide for a visible light active photocatalyst for dye degradation: Comparison to its MWCNT/Nd,N,S-TiO2 analogue. Ind. Eng. Chem. Res. 2014, 53, 14329–14338. [Google Scholar] [CrossRef]
- Apollo, S.; Moyo, S.; Mabuoa, G.; Aoyi, O. Solar photodegradation of methyl orange and phenol using silica supported ZnO catalyst. Int. J. Innov. Manag. Technol. 2014, 5, 203–206. [Google Scholar] [CrossRef]
- Ong, S.A.; Min, O.M.; Ho, L.N.; Wong, Y.S. Comparative study on photocatalytic degradation of Mono Azo Dye Acid Orange 7 and Methyl Orange under solar light irradiation. Water Air Soil Pollut. 2012, 223, 5483–5493. [Google Scholar] [CrossRef]
- Ho, L.N.; Ong, S.A.; See, Y.L. Photocatalytic degradation of reactive black 5 by fish scale-loaded TiO2 composites. Water Air Soil Pollut. 2012, 223, 4437–4442. [Google Scholar] [CrossRef]
- Lee, K.M.; Abdul Hamid, S.B. Simple response surface methodology: Investigation on advance photocatalytic oxidation of 4-Chlorophenoxyacetic Acid using UV-active ZnO photocatalyst. Materials 2015, 8, 339–354. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir-Hinshelwood kinetics-A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Abulizi, A.; Yang, G.H.; Zhu, J.J. One-step simple sonochemical fabrication and photocatalytic properties of Cu2O-rGO composites. Ultrason Sonochem. 2014, 21, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Lucio, D.; Laurent, D.; Roger, G. Adsorption of remazol black B dye on activated carbon felt. Carbon Sci. Technol. 2008, 1/2, 66–71. [Google Scholar]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.P.P.; Lai, C.W.; Lee, K.M.; Hamid, S.B.A. Advanced Chemical Reduction of Reduced Graphene Oxide and Its Photocatalytic Activity in Degrading Reactive Black 5. Materials 2015, 8, 7118-7128. https://doi.org/10.3390/ma8105363
Wong CPP, Lai CW, Lee KM, Hamid SBA. Advanced Chemical Reduction of Reduced Graphene Oxide and Its Photocatalytic Activity in Degrading Reactive Black 5. Materials. 2015; 8(10):7118-7128. https://doi.org/10.3390/ma8105363
Chicago/Turabian StyleWong, Christelle Pau Ping, Chin Wei Lai, Kian Mun Lee, and Sharifah Bee Abd Hamid. 2015. "Advanced Chemical Reduction of Reduced Graphene Oxide and Its Photocatalytic Activity in Degrading Reactive Black 5" Materials 8, no. 10: 7118-7128. https://doi.org/10.3390/ma8105363