3.1. Thermal-Radiative Properties of the Coating Films
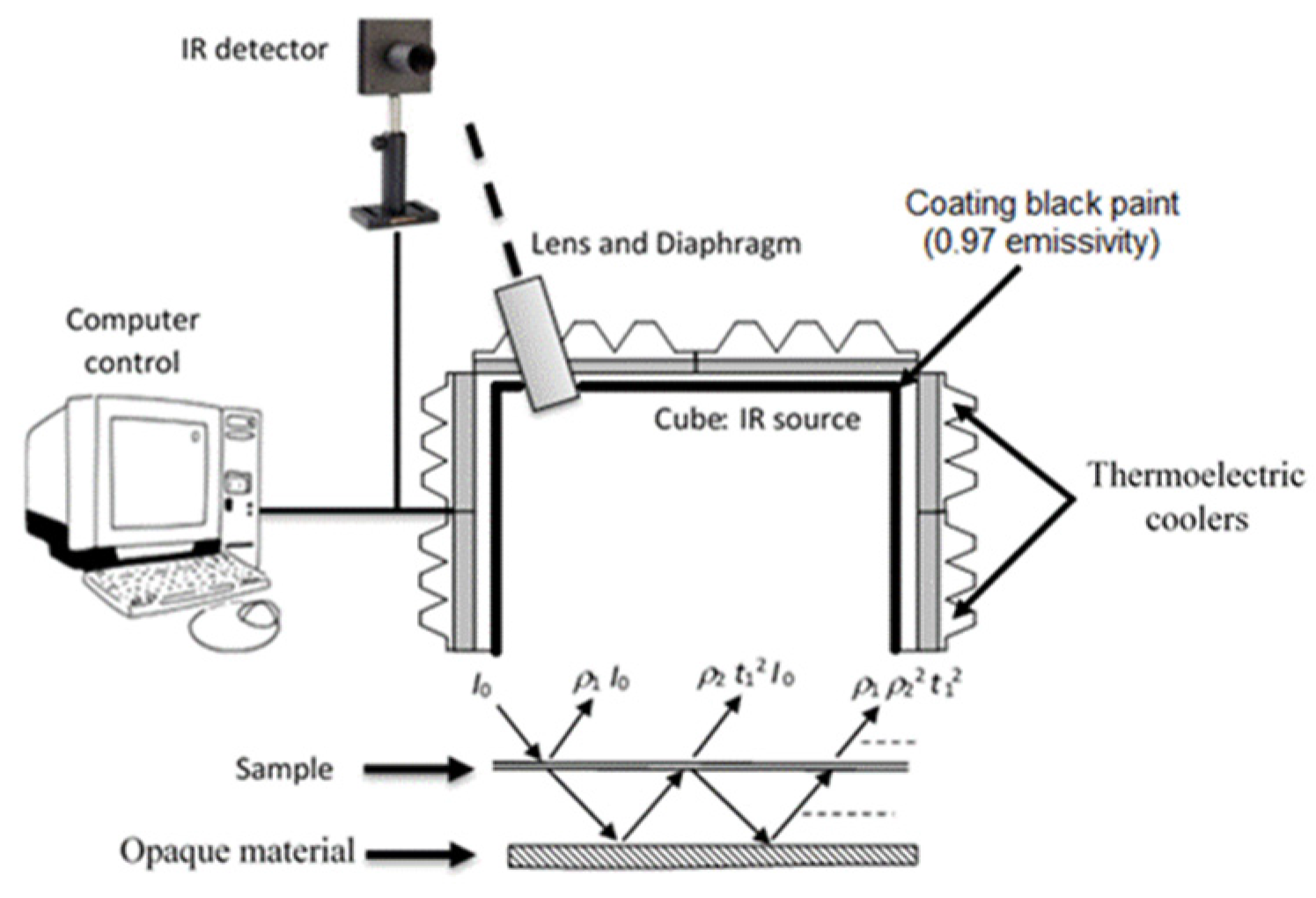
The results of emissivity, reflectivity and transmissivity measurements are presented in
Table 3. The procedure of semi-transparent materials (opaque material under sample) was applied for all samples except 5A20 (seems to be opaque).
Table 3.
Emissivity, reflectivity and transmissivity coefficients of coatings.
Table 3.
Emissivity, reflectivity and transmissivity coefficients of coatings.
| Coatings | ρ1 | t1 | ε1 |
|---|
| 1A20-65 | 0.231 ± 0.005 | 0.260 ± 0.012 | 0.509 ± 0.013 |
| 1A20-100 | 0.245 ± 0.006 | 0.192 ± 0.012 | 0.563 ± 0.013 |
| 3A20-100 | 0.349 ± 0.001 | 0.110 ± 0.003 | 0.541 ± 0.004 |
| 5A20-100 | 0.369 ± 0.006 | 0 | 0.631 ± 0.006 |
| 5A40-100 | 0.372 ± 0.009 | 0.132 ± 0.040 | 0.497 ± 0.041 |
| 5A60-100 | 0.370 ± 0.002 | 0.156 ± 0.006 | 0.475 ± 0.007 |
Figure 4 shows the variation of emissivity, reflectivity and transmissivity coefficients of A20 films as a function of aluminum content. We notice an increase of reflectivity when the aluminum concentration increases. The transmissivity tends to decreases substantially when the particles concentration increases until we obtain an opaque sample corresponding to 5% of A20 grade (
i.e., 3.5 wt % of aluminum). The evolution of the emissivity is less distinct; the sample 5A20 (3.5 wt % of Al) seems to exhibit a higher emissivity than 1A20 (0.7 wt % of Al) and 3A20 (2.1 wt % of Al) ones. In fact the sample 5A20 is an opaque material and in this case, the emissivity was calculated as ε = 1 − ρ. In fact, some polymers are highly transparent materials in the infrared range, and the emissivity of such composites strongly depends on the properties of the metallic particles themselves and the contact or porosity between particles [
13].
Figure 4.
Effect of A20 content on emissivity, reflectivity and transmissivity of coatings (error bars are lower than the data points size).
Figure 4.
Effect of A20 content on emissivity, reflectivity and transmissivity of coatings (error bars are lower than the data points size).
Yu
et al. have also observed a decrease of emissivity when increasing copper particle content in EPDM [
13]. Chen Hu
et al. have also shown that the emissivity of polysiloxane/aluminum opaque composites decreases with increasing aluminum content, at least up to 30 wt % of aluminum [
14]. In both cases, the metallic particle content is much higher than in our work and it may not be fully comparable. Just for example, if we accept to add the emissivity and transmissivity values as for the opaque materials, for the sample 1A20 and 3A20, we obtain also a decrease of the estimated value for all samples (1A20, 3A20 and 5A20) with the increasing of aluminum content.
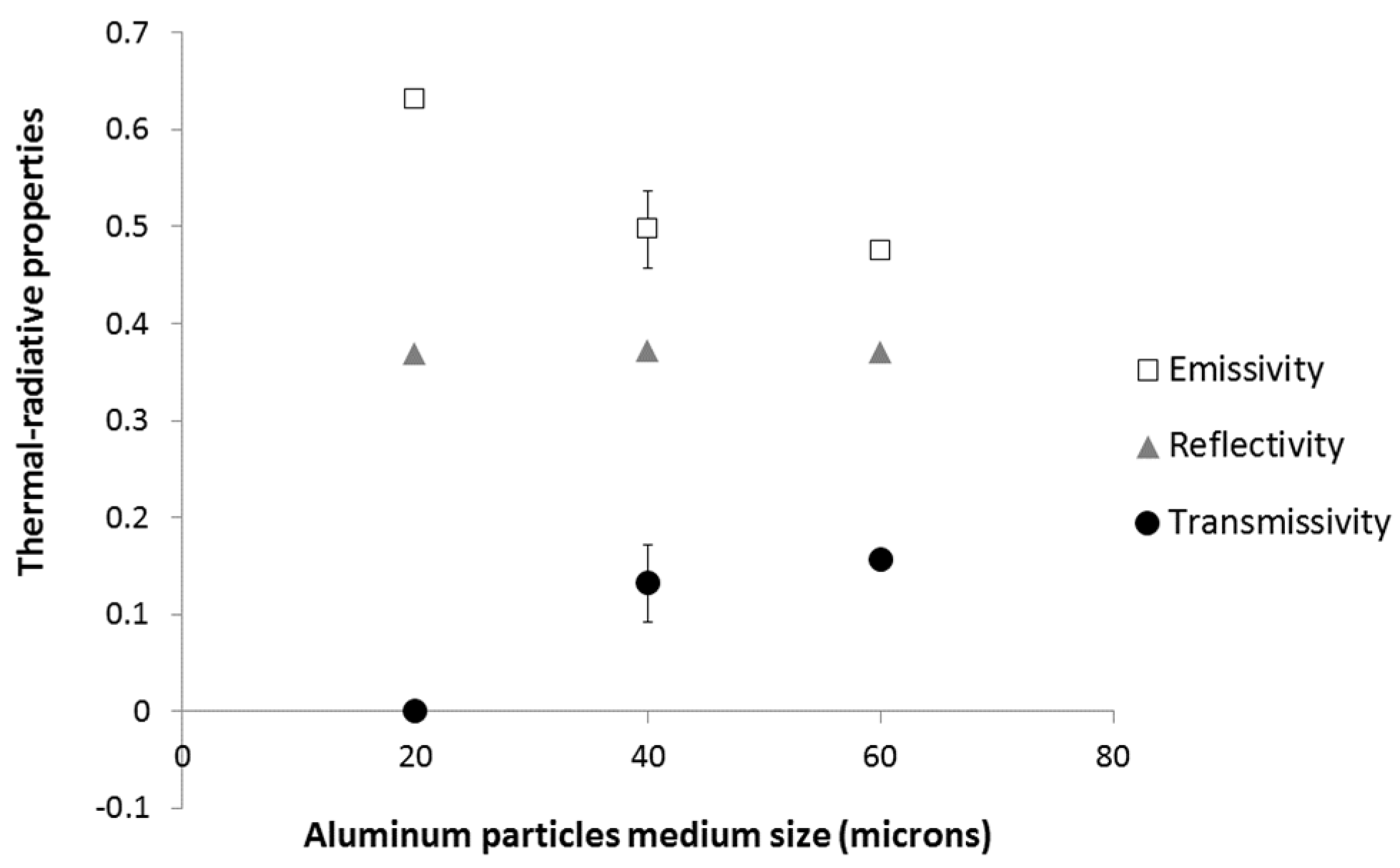
The effect of aluminum particles of medium size on emissivity, reflectivity and transmissivity is presented in
Figure 5. It is noted that the size of the aluminum particles has no effect on the reflectivity, but it significantly affects both emissivity and transmissivity properties. The increase of particle size at a constant weight concentration (3.7 wt % of Al) tends to increase the transmissivity, and to reduce the emissivity.
Figure 5.
Effect of aluminum particles medium size on emissivity, reflectivity and transmissivity of 5A20, 5A40 and 5A60 coatings.
Figure 5.
Effect of aluminum particles medium size on emissivity, reflectivity and transmissivity of 5A20, 5A40 and 5A60 coatings.
Finally, a characterization of two films (1A20) with the same filler content and different thicknesses (65 and 100 μm) was performed. The results highlight that an increase in the thickness of the film tends to increase the emissivity and the reflectivity and reduce the transmissivity. This effect was also observed by Chen Hu
et al. for polysiloxane filled with aluminum particles [
14].
3.2. Cone Calorimeter Results
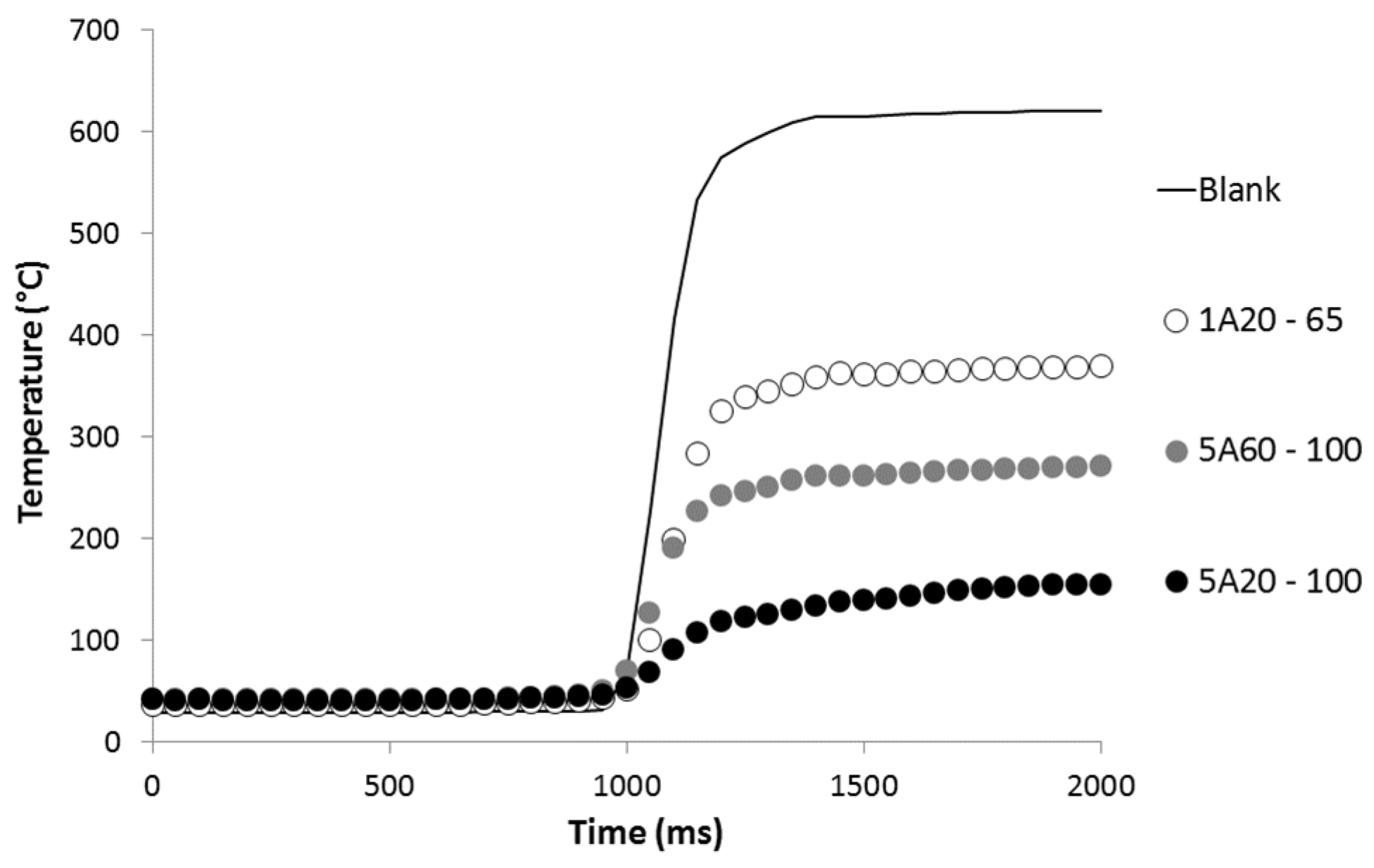
All formulations exhibit the same fire behavior at 35 kW/m2. The peak of heat release rate (pHRR) is around 700 kW/m2. The LDPE matrix is fully decomposed (residue yield is negligible) and the effective heat of combustion is close to 32 kJ/g. This last value is lower than the effective heat of complete combustion for polyethylene (40–42 kJ/g as measured in a pyrolysis-combustion flow calorimeter) leading to a combustion efficiency in the range 0.75–0.8.
Only time-to-ignition (TTI) is significantly modified by the different coatings at the top surface of the samples. TTI increases from 76 s for pure LDPE to 397 s for LDPE coated with 5A20-100 coating. Therefore, all the heat release rate curves are similar but shifted to different TTI according to the coating (see
Figure 6). As already reported by Schartel
et al. [
15], the coatings act as infrared mirrors delaying ignition, but do not change the behavior of the material once ignited. The maximum increase in TTI obtained in our study is in the same range (but slightly lower) than the increases observed by Schartel
et al. [
15]. In their study involving a three-layer coating, TTI increases from 58 to 537 s for PA66 and from 82 to 459 s for PC.
Figure 6.
Heat release rate curves obtained in a cone calorimeter test at 35 kW/m2 for various formulations.
Figure 6.
Heat release rate curves obtained in a cone calorimeter test at 35 kW/m2 for various formulations.
Considering 100 μm-thick films containing A20 particles, it is noteworthy that the effect of the coating is more significant at a low heat flux (
Figure 7). Indeed, the ratio between the TTI of LDPE coated with 5A20 containing film and TTI of pure LDPE is 5.2, 3.9 and 3.7 at 35, 50 and 75 kW/m
2, respectively. It must also be noted that at each heat flux, the TTI increases linearly when increasing aluminum particle content. The TTIs obtained with the 65 μm-thick film containing A20 particles are slightly lower than those measured with the 100 μm-thick film containing the same amount of A20 particles. For example, at 50 kW/m
2, the TTI is 90 s
versus 94 s for the thicker film.
Figure 7.
Time-to-ignition (s) versus A20 content (thickness 100 μm) at various heat fluxes.
Figure 7.
Time-to-ignition (s) versus A20 content (thickness 100 μm) at various heat fluxes.
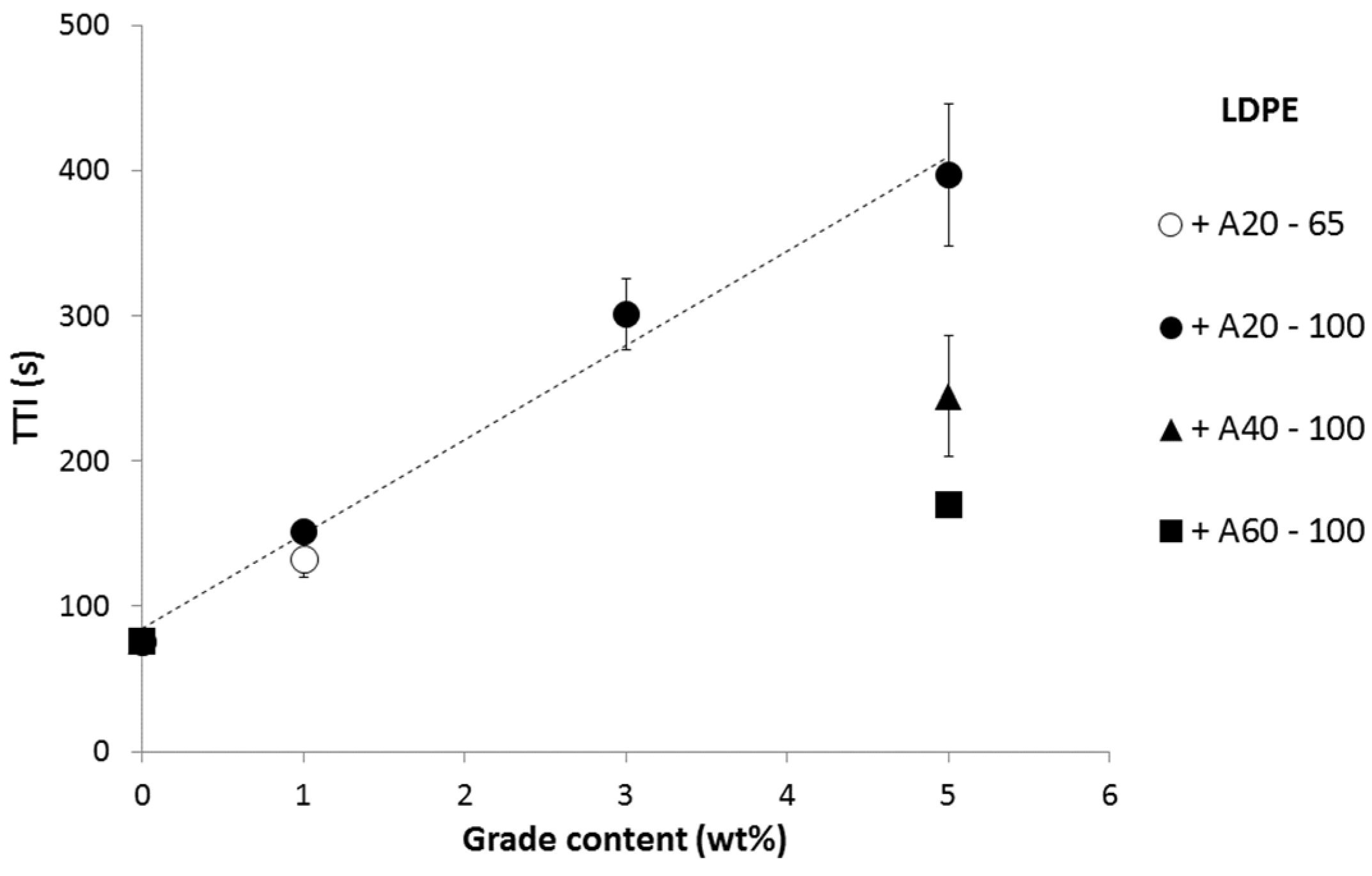
Figure 8 summarizes the TTI obtained at 35 kW/m
2 for various formulations. Besides the aluminum particle content, it appears that TTI also depends on the grade used. A20 is much more efficient than A40, and A60 is the least efficient grade. The thickness of the coating is another parameter affecting the time-to-ignition. LDPE coated with 65 and 100 μm-thick 1A20 coatings exhibits a TTI of 133 and 152 s, respectively, at 35 kW/m
2. This influence appears quite limited and further study is needed to confirm this preliminary result.
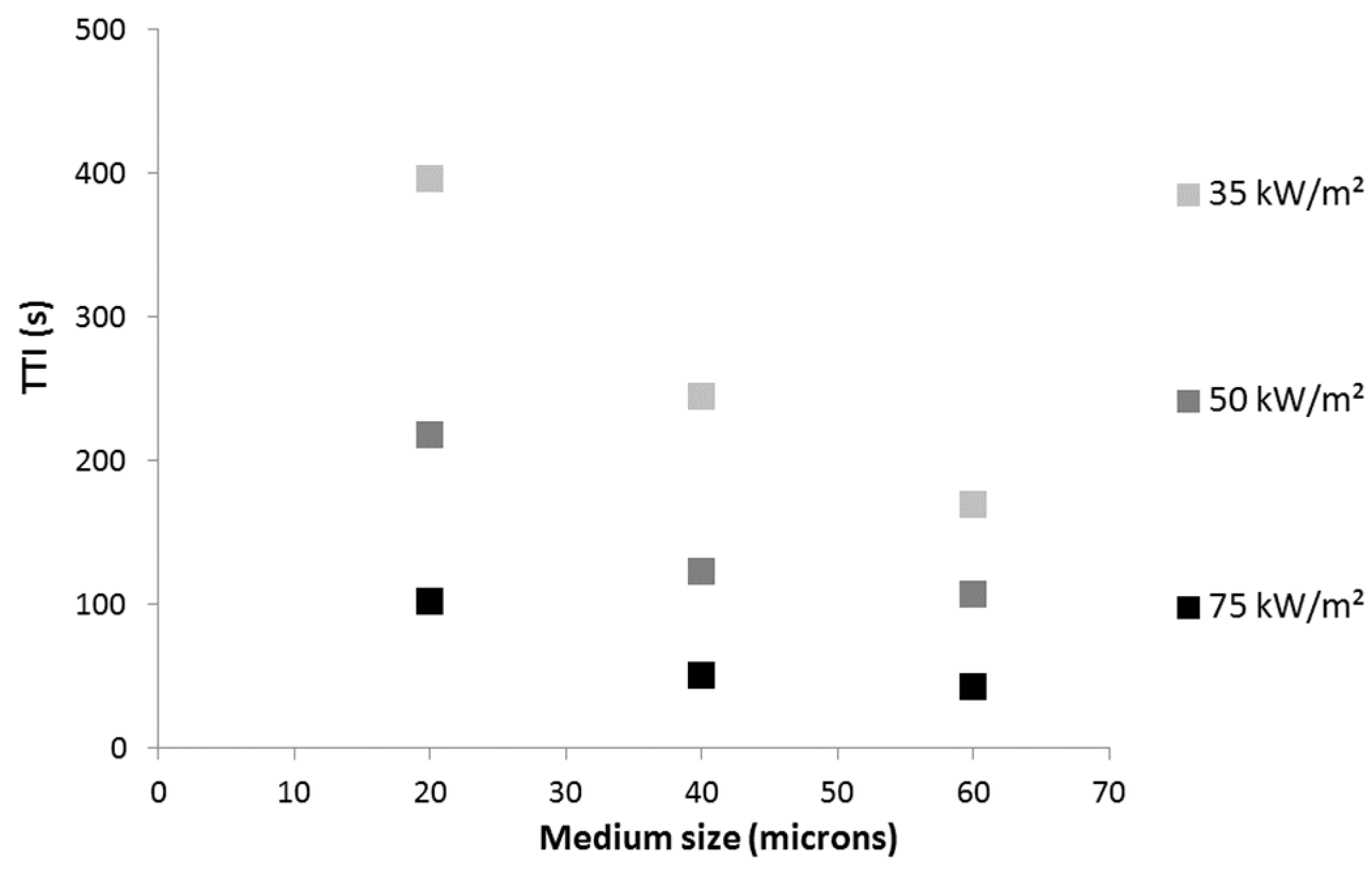
The efficiency of the various aluminum particles to delay ignition depends on their shape and size. Particles from A40 and A60 have the same shape (corn flakes) but A40 particles are smaller (medium size is around 40 μm
versus 60 μm for A60). Despite its higher absorptivity, 5A20 coating exhibits a much higher efficiency, which could be related to the smallest particle size (20 μm) or alternatively to their specific shape and to their leafing nature (
Figure 9). Leafing pigments may tend to float to the surface of the coating and to align parallel to the surface of the coating. The relative influence of these parameters (size, shape and leafing nature) is out of the scope of this article and needs further study. Our primary objective is to relate the efficiency of various grades to their thermal-radiative properties.
Figure 10 plots the TTI measured in a cone calorimeter test at 35 kW/m
2 versus the thermal-radiative properties of the coatings: reflectivity, transmissivity and emissivity. There is no simple relationship between TTI and one of these properties. This means that the ignition depends on a complex combination of these properties.
Figure 8.
Time-to-ignition (s) versus grade content in the coating at 35 kW/m2.
Figure 8.
Time-to-ignition (s) versus grade content in the coating at 35 kW/m2.
Figure 9.
Time-to-ignition (s) versus medium particle size for LDPE coated with 5A20, 5A40 and 5A60 coatings at various heat flux.
Figure 9.
Time-to-ignition (s) versus medium particle size for LDPE coated with 5A20, 5A40 and 5A60 coatings at various heat flux.
Figure 10.
Time-to-gnition (s) at 35 kW/m2 versus thermal-radiative properties of the coatings.
Figure 10.
Time-to-gnition (s) at 35 kW/m2 versus thermal-radiative properties of the coatings.
Figure 11 provides some evidences that TTI is not only dependent on the coating emissivity.
Figure 11A shows that the mass loss at ignition exceeds 1 g for some formulations. The coating corresponds to around 0.8–0.9 g of the total mass of the sample. This means that the ignition occurs when LDPE in the sheet (and not only in the coating) starts degrading and contributes to the fuel release. Then, the heating of the sheet is of primary importance. This heating depends not only on the reflectivity but also on the transmissivity of the coating.
Figure 11.
(A) Masse loss at time-to-ignition (TTI) for all formulations at 35 kW/m2 (the grey zone corresponds to the mass of low-density polyethylene (LDPE) in the coating); (B) Time to ignition in cone calorimeter at 35 kW/m2 versus heating rate measured using epiradiator (the dotted line is only a guideline for the eyes—labels correspond to the coating thermocompressed on the top surface of LDPE sheet).
Figure 11.
(A) Masse loss at time-to-ignition (TTI) for all formulations at 35 kW/m2 (the grey zone corresponds to the mass of low-density polyethylene (LDPE) in the coating); (B) Time to ignition in cone calorimeter at 35 kW/m2 versus heating rate measured using epiradiator (the dotted line is only a guideline for the eyes—labels correspond to the coating thermocompressed on the top surface of LDPE sheet).
Similarly,
Figure 11B plots the TTI measured in cone calorimeter at 35 kW/m
2 with the heating rate at the bottom surface of the coating as measured using an epiradiator. Keep in mind that this heating rate depends on many properties of the coating: thermal-radiative properties and also thermal conductivity. A good correlation can be found between the heating rate and the time-to-ignition, showing once again that ignition depends on several coating properties.
Two equations have been proposed to predict the time-to-ignition
versus the applied heat flux [
2]. In these equations, emissivity is not present explicitly. Actually, net heat flux must be considered rather than external heat flux. Net heat flux is equal to the product of external heat flux and emissivity. The modified equations (
i.e., when emissivity is taken into account explicitly) are given below. The first one corresponds to thermally thick materials and the second one to thermally thin materials:
with
k the thermal conductivity, ρ the density,
c the specific heat,
Tig the surface temperature at ignition,
T0 the room temperature (25 °C), ε the emissivity,
the applied heat flux and CHF the critical heat flux.
The dependence of TTI on heat flux is different according to the thermal behavior of the material. Then, a method to assess if the material is thermally thin or thick is to draw ()−1 or TTI−1 versus the heat flux. If the curve ()−1 versus heat flux is linear, the material is thermally thick. If the curve TTI−1 versus heat flux is linear, the material is thermally thin. Unfortunately, for all our materials, both curves seems to be linear. In that case, this method is of poor practical interest. To determine the thermal behavior of our materials, we need to know all their properties to calculate TTI using Equations (7) and (8).
We assume that the properties of LDPE are the same as reported by Lyon’s report [
1]: emissivity 0.92, density 925 kg/m
3, thermal conductivity 0.38 W·m
−1·K
−1, specific heat 1.55 kJ·kg
−1·K
−1, temperature at ignition 377 °C and CHF 13 kW/m
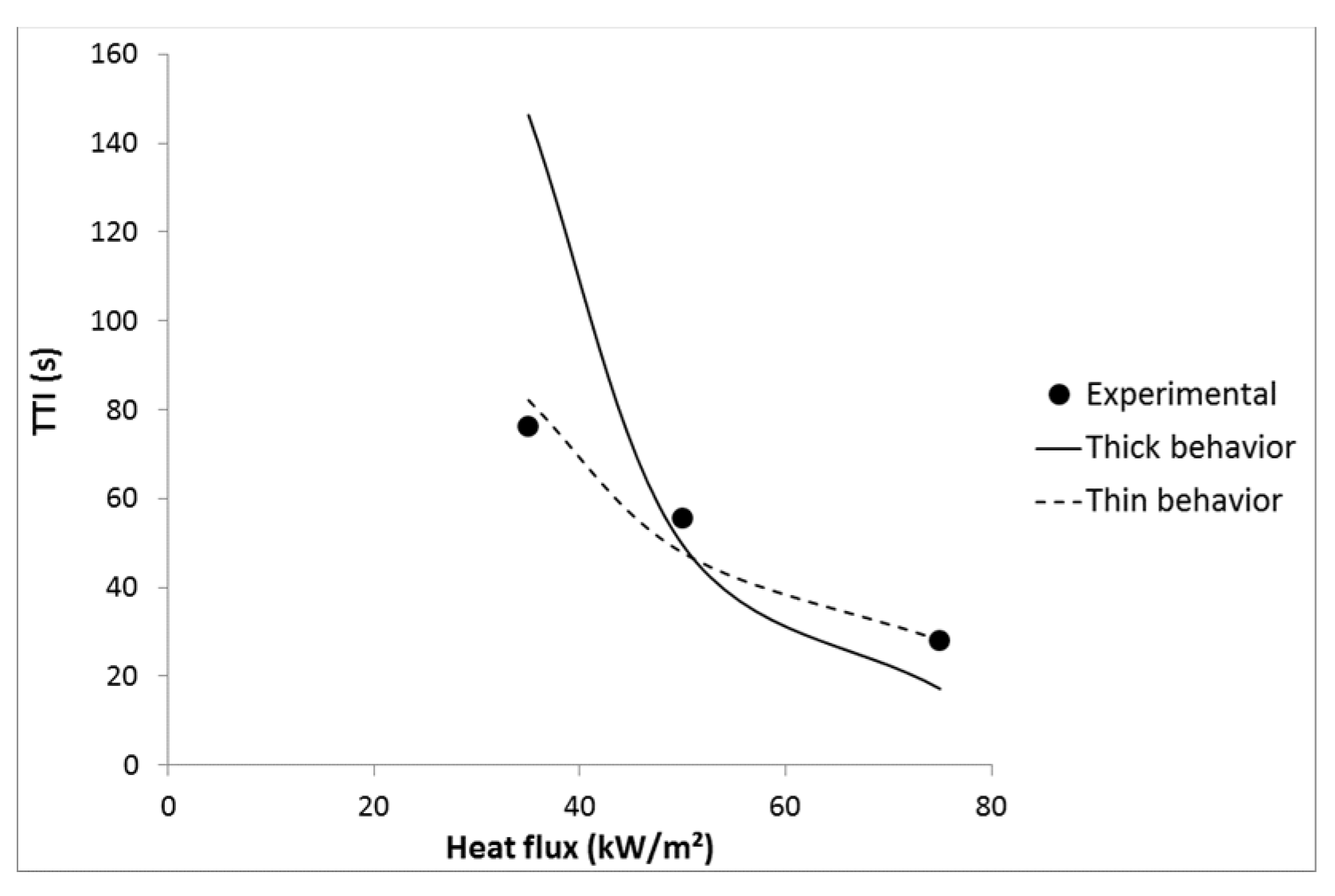
2. Using these values, time-to-ignition can be well predicted using Equation (8) corresponding to a thermally thin material (see
Figure 12). Indeed, pure LDPE does not strongly absorb in the infrared region. Linteris
et al. have measured the absorption coefficient of 11 thermoplastics and found that HDPE, PP and PS exhibit the lowest absorption coefficient. In the case of HDPE, approximately only 50% of the incident heat is absorbed in the first 500 μm [
21]. Therefore its behavior is thermally thin even at high heat flux.
Figure 12.
Experimental and predicted TTI versus heat flux for LDPE.
Figure 12.
Experimental and predicted TTI versus heat flux for LDPE.
It must be noted that Equations (7) and (8) do not take into account some phenomena. For example, thermophysical and thermal radiative properties are measured at ambient temperature. Their evolution at high temperature and when the material starts degrading is not known. Moreover, the absorption coefficient changes with the thickness as shown by Linteris
et al. [
21] even if this change is small for polyethylene. Finally, the absorption depends on the spectral distribution of the radiation which changes with the temperature of the heat source [
16,
21,
22,
24]. As a result, the absorptivity of the materials should change when heat flux changes. Despite these limitations, Equation (8) fits the experimental TTI of pure LDPE well.
For coated LDPE, all the properties listed above are maintained except the emissivity. Indeed, the presence of a very low aluminum amount into only a thin layer at the top of the surface can not seriously affect these properties. According to Rozenbaum
et al. the emissivity ε
1 can be expressed by Equation (6) for semi-transparent materials such as our coatings (transmissivity is not null) [
19]. Nevertheless, when these coatings are thermo-compressed onto an LDPE sheet, we assume that the materials are opaque and then emissivity should expressed by Equation 3 (transmissivity is null) [
19]. This is the reason why emissivity of the coated sheets (LDPE sheet + coating) is calculated by adding the emissivity and the transmissivity values of the corresponding coating alone.
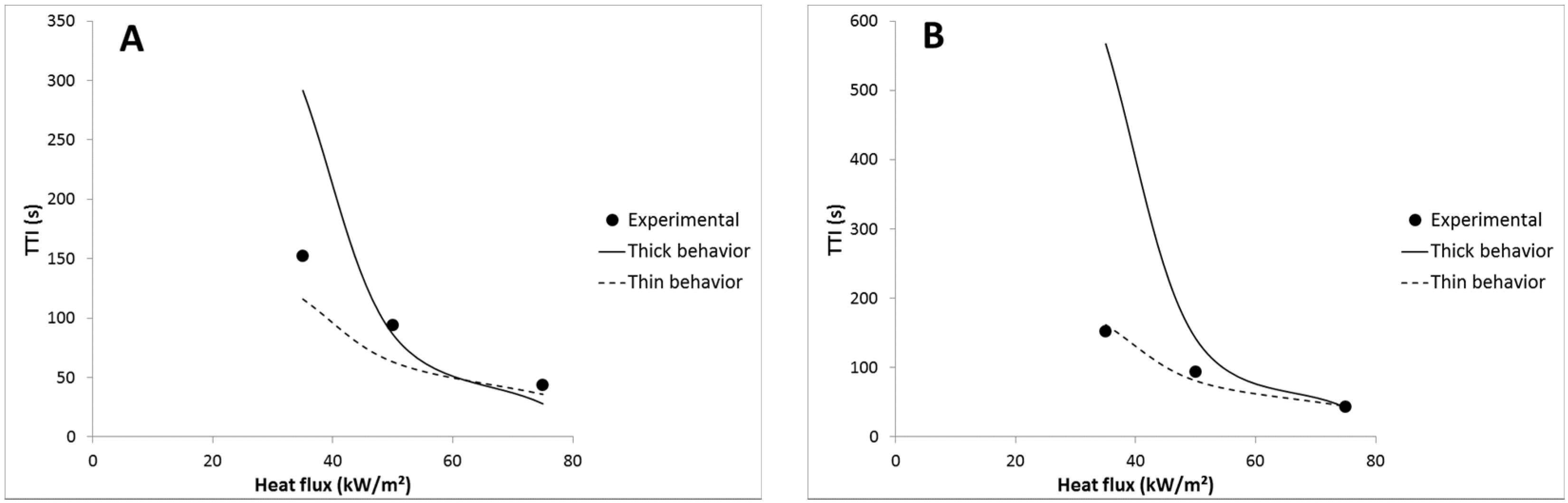
For most of the formulations (with 1A20, 5A40 and 5A60 coatings), the time-to-ignition can be predicted well considering a thermally thin behavior as for LDPE, but emissivity must be adjusted to a lower value than expected (example for LDPE coated with 1A20-100 coating in
Figure 13). In other words, the emissivity of the coated sheets calculated as the sum of the emissivity and the transmissivity of the coating is too high to match the experimental TTI using Equation (8). The emissivity used to fit properly the experimental TTI is called “fitted emissivity”. Its value is chosen to match at best the experimental TTI measured at the three heat fluxes (35, 50 and 75 kW/m
2).
Figure 13.
Experimental and predicted TTI versus heat flux for LDPE coated with 1A20-100 film: (A) emissivity = 0.76, (B) emissivity = 0.65.
Figure 13.
Experimental and predicted TTI versus heat flux for LDPE coated with 1A20-100 film: (A) emissivity = 0.76, (B) emissivity = 0.65.
To explain why a lower emissivity than expected must be considered, it must be noted that the decrease in emissivity is due to inert aluminum particles. While LDPE degradation occurs, the aluminum concentration in the coating increases. Then the emissivity may continuously decrease.
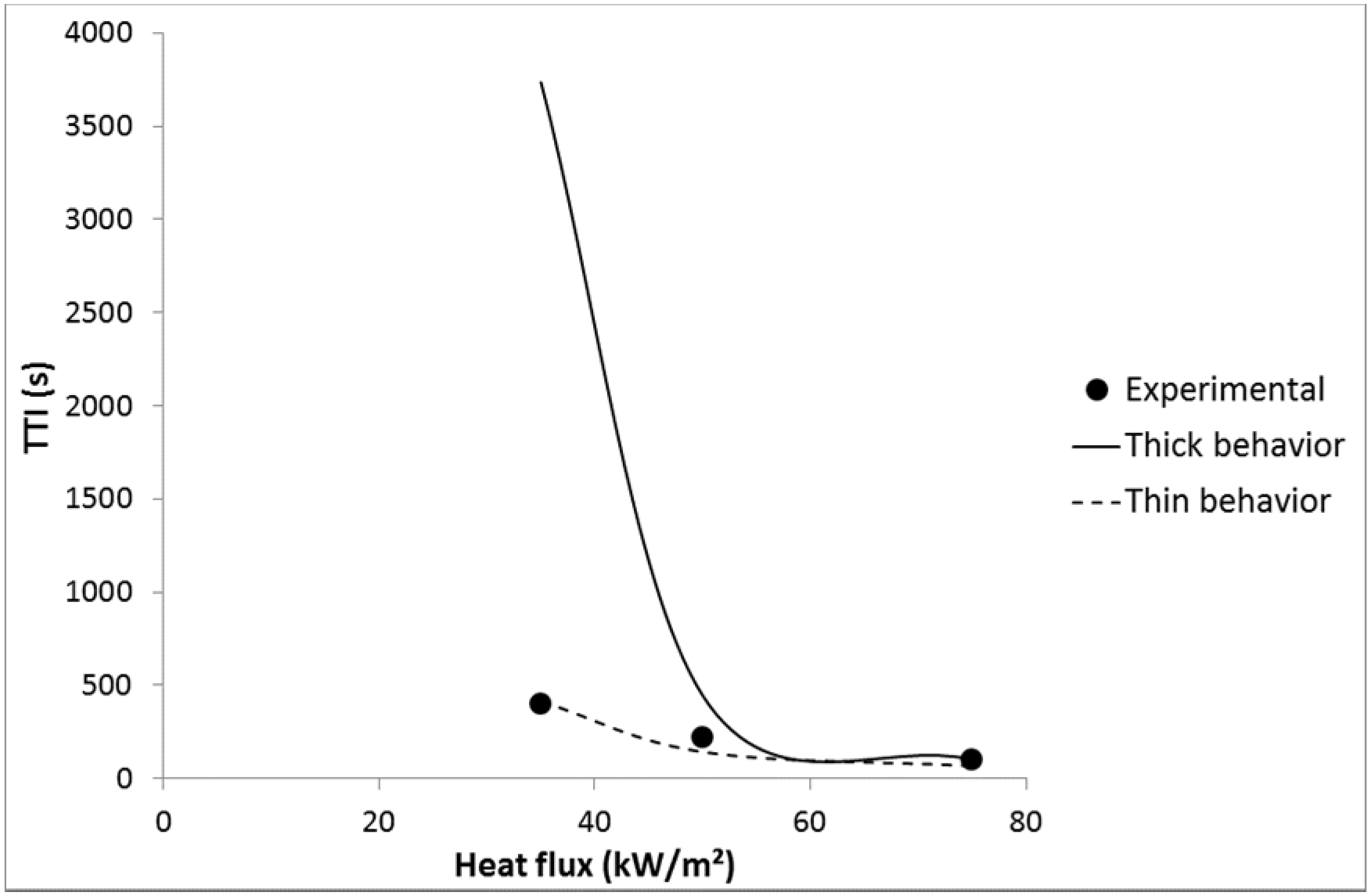
Nevertheless, for LDPE coated with 3A20 and 5A20 coatings, choosing a lower emissivity is not enough to fit well the experimental time-to-ignitions with a thermally thin behavior. For example, an emissivity of 0.48 allows matching the TTI at 35 kW/m
2 for LDPE coated with 5A20 coating, but the TTI at 75 kW/m
2 is then underestimated (69 s
versus 103 s). On the contrary, considering a thermally thick behavior at 75 kW/m
2 allows matching the experimental TTI (see
Figure 14). In other words, it is necessary to choose a lower emissivity and to consider a change of the thermal behavior from thin to thick when heat flux increases from 35 to 75 kW/m
2. At intermediate heat flux, the behavior appears hybrid. A thermally thick behavior at high heat flux allows a high time-to-ignition to be maintained. This change appears coherent with the thermal-radiative properties of the coating films. Indeed, 3A20 and 5A20 coatings exhibit the lowest transmissivity among all coatings. 5A20 coating is even the only fully opaque coating.
Figure 14.
Experimental and predicted TTI versus heat flux for LDPE coated with 5A20-100 coating.
Figure 14.
Experimental and predicted TTI versus heat flux for LDPE coated with 5A20-100 coating.
Table 4 summarizes the estimated and the fitted emissivities of the coated sheets. Recall that the estimated emissivity was calculated by adding the emissivity and the transmissivity values of the coating (measured in
Table 3). The difference between both values is the highest for the sheets coated with 3A20 and 5A20 films. While the corresponding coatings exhibit the lowest transmissivity, it can be assumed that a low transmissivity promotes a relatively fast degradation of LDPE in the coating and then a fast increase in aluminum particle concentration, leading to a low emissivity during burning.
Table 4.
Estimated emissivity and fitted emissivity of coated sheets
Table 4.
Estimated emissivity and fitted emissivity of coated sheets
| Sheet | Estimated Emissivity 1 − ρ1 | Fitted Emissivity ε2 | (1 − ρ1) − ε2 |
|---|
| LDPE | - | 0.92 [25] | - |
| 1A20-65 | 0.77 | 0.7 | 0.07 |
| 1A20-100 | 0.76 | 0.65 | 0.11 |
| 3A20-100 | 0.65 | 0.53 | 0.12 |
| 5A20-100 | 0.63 | 0.48 | 0.15 |
| 5A40-100 | 0.63 | 0.56 | 0.07 |
| 5A60-100 | 0.63 | 0.64 | −0.01 |
The highest efficiency of 3A20 and 5A20 coatings can be also related to the ability of the A20 particles to form a thin but cohesive aluminum film during the coating degradation.
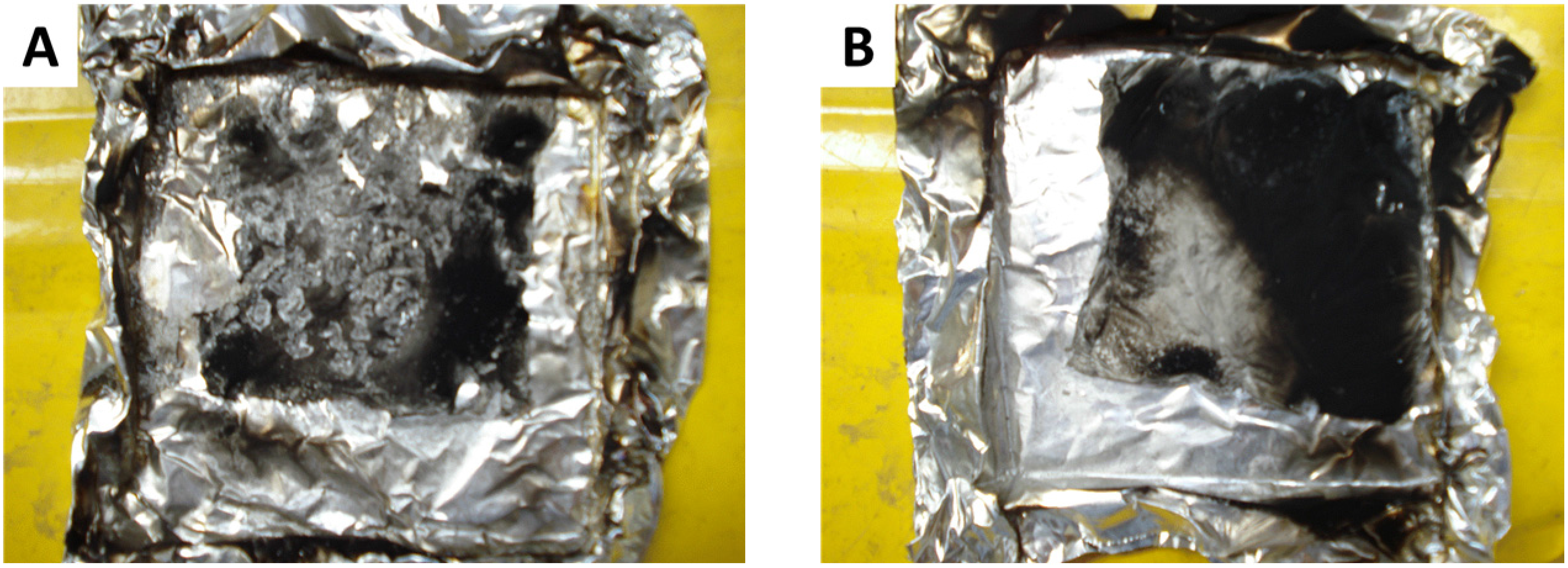
Figure 15 shows the residue of LDPE coated with 5A20 or 5A40 films after a cone calorimeter test at 35 kW/m
2. The residue from 5A20 is a very thin but cohesive aluminum film which can be removed without decomposing. It retracts during the test but covers the major part of the surface. Residue from LDPE coated with 3A20 film exhibits a similar aspect. The residues from LDPE coated with 5A40 films and all other films are in pieces and do not cover a significant fraction of the surface.
Figure 15.
Residues at the end of the cone calorimeter test. (A) LDPE coated with 5A40 coating. (B) LDPE coated with 5A20 coating.
Figure 15.
Residues at the end of the cone calorimeter test. (A) LDPE coated with 5A40 coating. (B) LDPE coated with 5A20 coating.
SEM observations of the residue from LDPE coated with 5A20 coating are shown in
Figure 16. It seems that particles overlap and are well-aligned parallel to the surface (probably due to the leafing effect). It can be suggested that this alignment favours a low transmissivity and facilitates the formation of a thin and cohesive aluminum film.
Figure 16.
Scanning electron microscope (SEM) observations of 5A20 film residue at two different scales. (A) Low magnification; (B) High magnification.
Figure 16.
Scanning electron microscope (SEM) observations of 5A20 film residue at two different scales. (A) Low magnification; (B) High magnification.