Fast Blue RR—Siloxane Derivatized Materials Indicate Wound Infection Due to a Deep Blue Color Development
Abstract
:1. Introduction
2. Results and Discussion
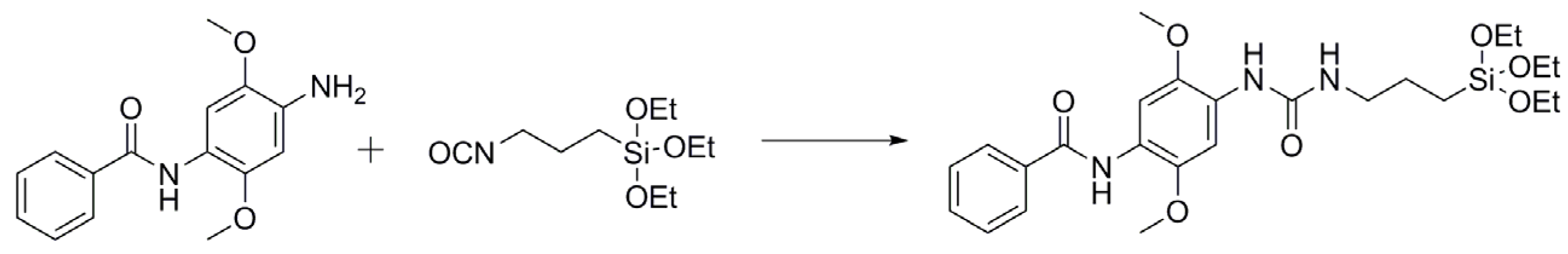

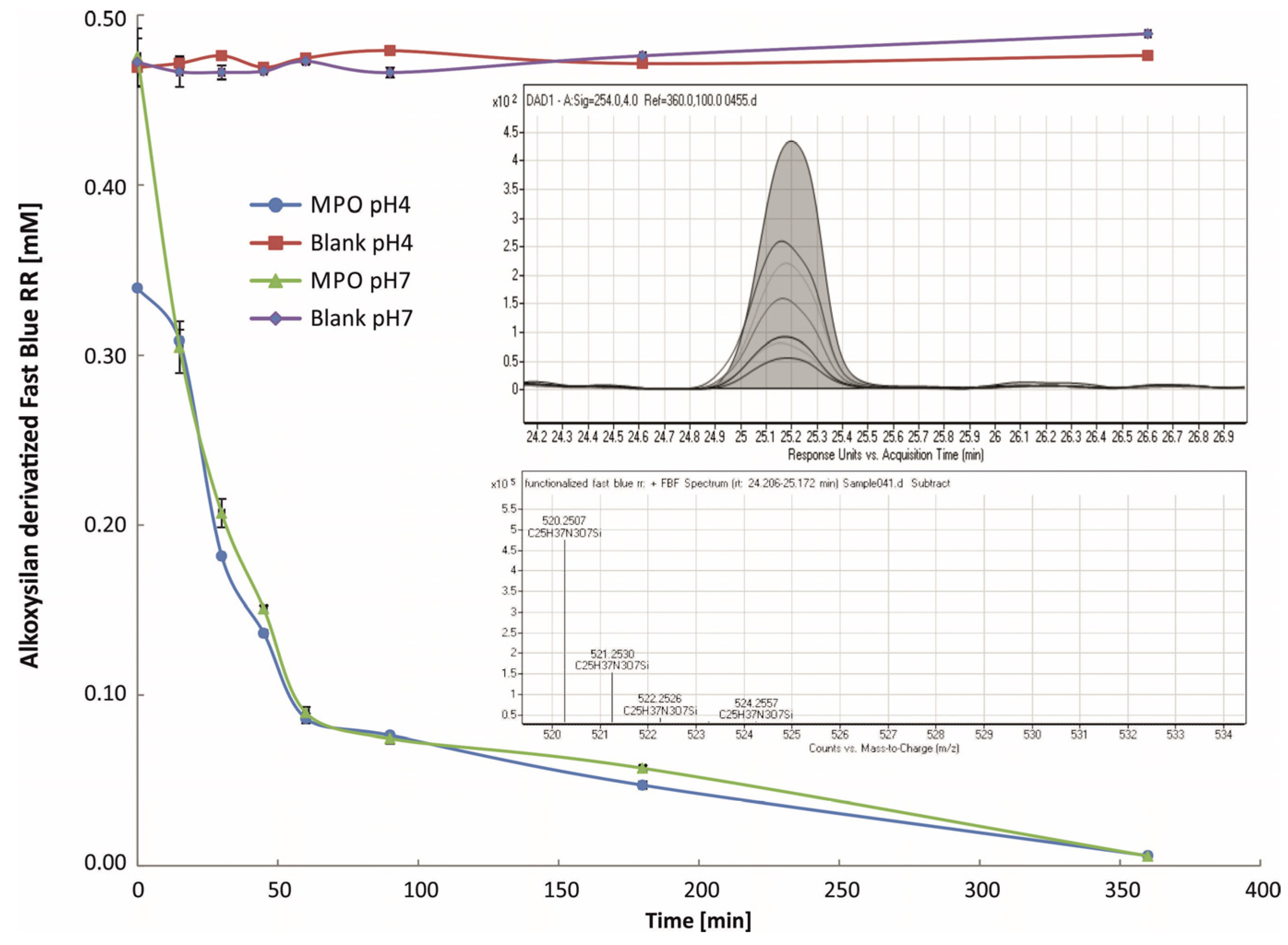
2.1. LC-ESI-TOF
3. Experimental Section
3.1. Wound Fluid Collection and Microbiological Determination
3.2. Functionalization of Fast Blue RR
3.3. Immobilization of Derivatized Fast Blue RR
3.4. Transformation of the Substrate
3.5. HPLC Sample Treatment
3.6. HPLC Measurement
3.6.1. LC
3.6.2. LC-ESI TOF
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Gardner, S.; Frantz, R.A.; Doebbeling, B.N. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen. 2001, 9, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.E.; Frantz, R.A.; Saltzman, C.L.; Hillis, S.L.; Park, H.; Scherubel, M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen. 2006, 14, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.E.; HIillis, S.L.; Frantz, R.A. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol. Res. Nurs. 2010, 11, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Tegl, G.; Schiffer, D.; Sigl, E.; Heinzle, A.; Guebitz, G.M. Biomarkers for infection: Enzymes, microbes, and metabolites. Appl. Microbiol. Biotechnol. 2015, 99, 4595–4614. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.; Wolcott, R.D. Wound infection and diagnostics in practice: What is emerging? Wounds Int. 2013, 4, 4–6. [Google Scholar]
- Schiffer, D.; Blokhuis-Arkes, M.; van der Palen, J.; Sigl, E.; Heinzle, A.; Guebitz, G.M. Assessment of infection in chronic wounds based on the monitoring of elastase, lysozyme and myeloperoxidase activities. Br. J. Dermatol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hajnsek, M.; Schiffer, D.; Harrich, D.; Koller, D.; Verient, V.; Palen, J.V.D.; Heinzle, A.; Binder, B.; Sigl, E.; Sinner, F.; et al. An electrochemical sensor for fast detection of wound infection based on myeloperoxidase activity. Sens. Actuators B Chem. 2015, 209, 265–274. [Google Scholar] [CrossRef]
- Hasmann, A.; Wehrschuetz-Sigl, E.; Marold, A.; Wiesbauer, H.; Schoeftner, R.; Gewessler, U.; Kandelbauer, A.; Schiffer, D.; Schneider, K.P.; Binder, B.; et al. Analysis of myeloperoxidase activity in wound fluids as a marker of infection. Ann. Clin. Biochem. 2013, 50, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.J.; Ashford, A.E. A simultaneous-coupling azo dye method for the quantitative assay of esterase using alpha-naphthyl acetate as substrate. Histochem. J. 1980, 12, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, D.A.; Vattuone, M.A.; Catalán, C.A.N. A new colorimetric method for determination of alkylresorcinols in ground and whole-cereal grains using the diazonium salt Fast Blue RR. Food Chem. 2009, 115, 1170–1174. [Google Scholar] [CrossRef]
- McGoldrick, C.A.; Jiang, Y.L.; Paromov, V.; Brannon, M.; Krishnan, K.; Stone, W.L. Identification of oxidized protein hydrolase as a potential prodrug target in prostate cancer. BMC Cancer 2014, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Kohnen, S.; Boudjeltia, K.Z.; van Antwerpen, P.; Bosseloir, A.; Niesten, A.; Gach, O.; Nys, M.; Deby-Dupont, G.; Serteyn, D. A new easy method for specific measurement of active myeloperoxidase in human biological fluids and tissue extracts. Talanta 2009, 80, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Divi, R.L.; Churchwell, M.I. Identification of the colored guaiacol oxidation product produced by peroxidases. Anal. Biochem. 1997, 250, 10–17. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiffer, D.; Tegl, G.; Vielnascher, R.; Weber, H.; Schoeftner, R.; Wiesbauer, H.; Sigl, E.; Heinzle, A.; Guebitz, G.M. Fast Blue RR—Siloxane Derivatized Materials Indicate Wound Infection Due to a Deep Blue Color Development. Materials 2015, 8, 6633-6639. https://doi.org/10.3390/ma8105329
Schiffer D, Tegl G, Vielnascher R, Weber H, Schoeftner R, Wiesbauer H, Sigl E, Heinzle A, Guebitz GM. Fast Blue RR—Siloxane Derivatized Materials Indicate Wound Infection Due to a Deep Blue Color Development. Materials. 2015; 8(10):6633-6639. https://doi.org/10.3390/ma8105329
Chicago/Turabian StyleSchiffer, Doris, Gregor Tegl, Robert Vielnascher, Hansjoerg Weber, Rainer Schoeftner, Herfried Wiesbauer, Eva Sigl, Andrea Heinzle, and Georg M. Guebitz. 2015. "Fast Blue RR—Siloxane Derivatized Materials Indicate Wound Infection Due to a Deep Blue Color Development" Materials 8, no. 10: 6633-6639. https://doi.org/10.3390/ma8105329
APA StyleSchiffer, D., Tegl, G., Vielnascher, R., Weber, H., Schoeftner, R., Wiesbauer, H., Sigl, E., Heinzle, A., & Guebitz, G. M. (2015). Fast Blue RR—Siloxane Derivatized Materials Indicate Wound Infection Due to a Deep Blue Color Development. Materials, 8(10), 6633-6639. https://doi.org/10.3390/ma8105329




