The Effects of Zr Doping on the Optical, Electrical and Microstructural Properties of Thin ZnO Films Deposited by Atomic Layer Deposition
Abstract
:1. Introduction
2. Experimental Section
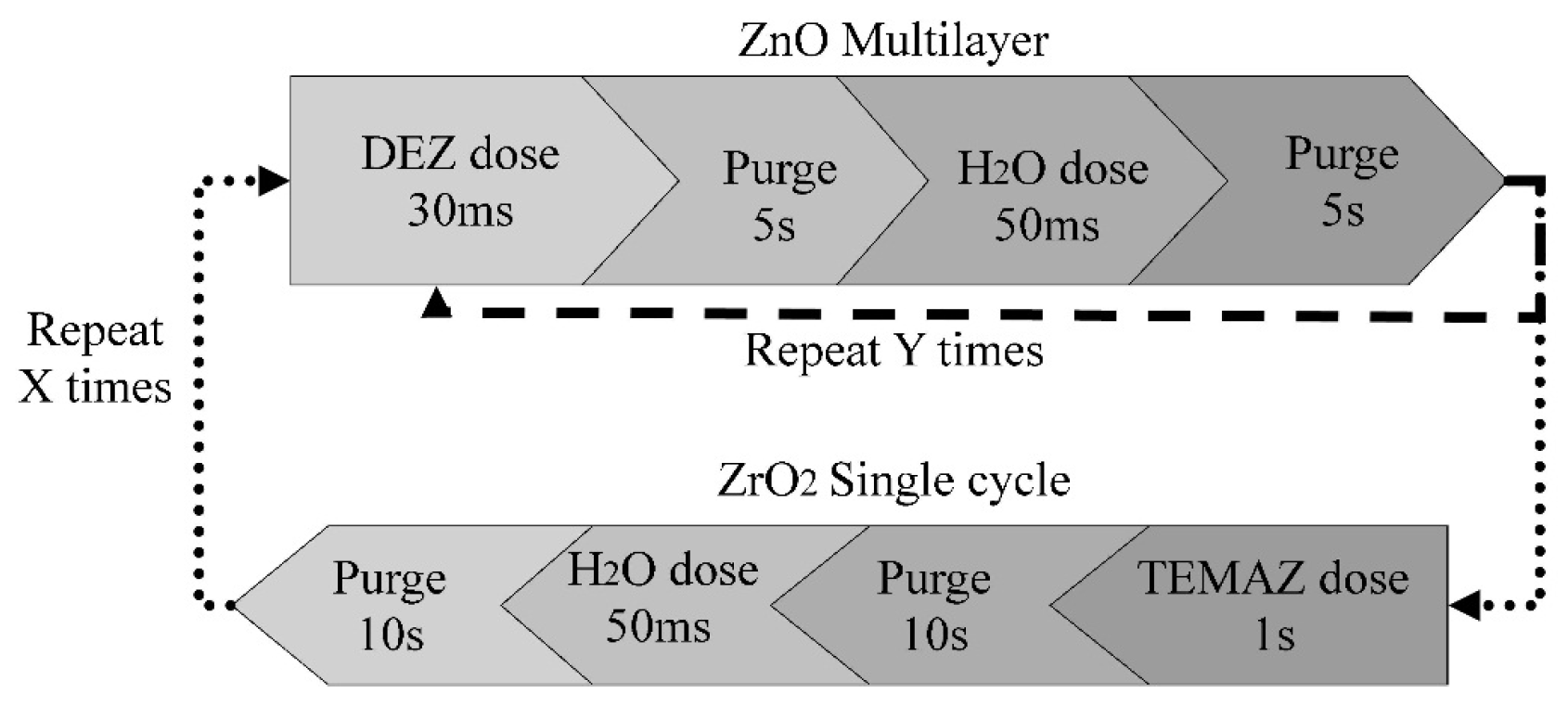
3. Results and Discussion
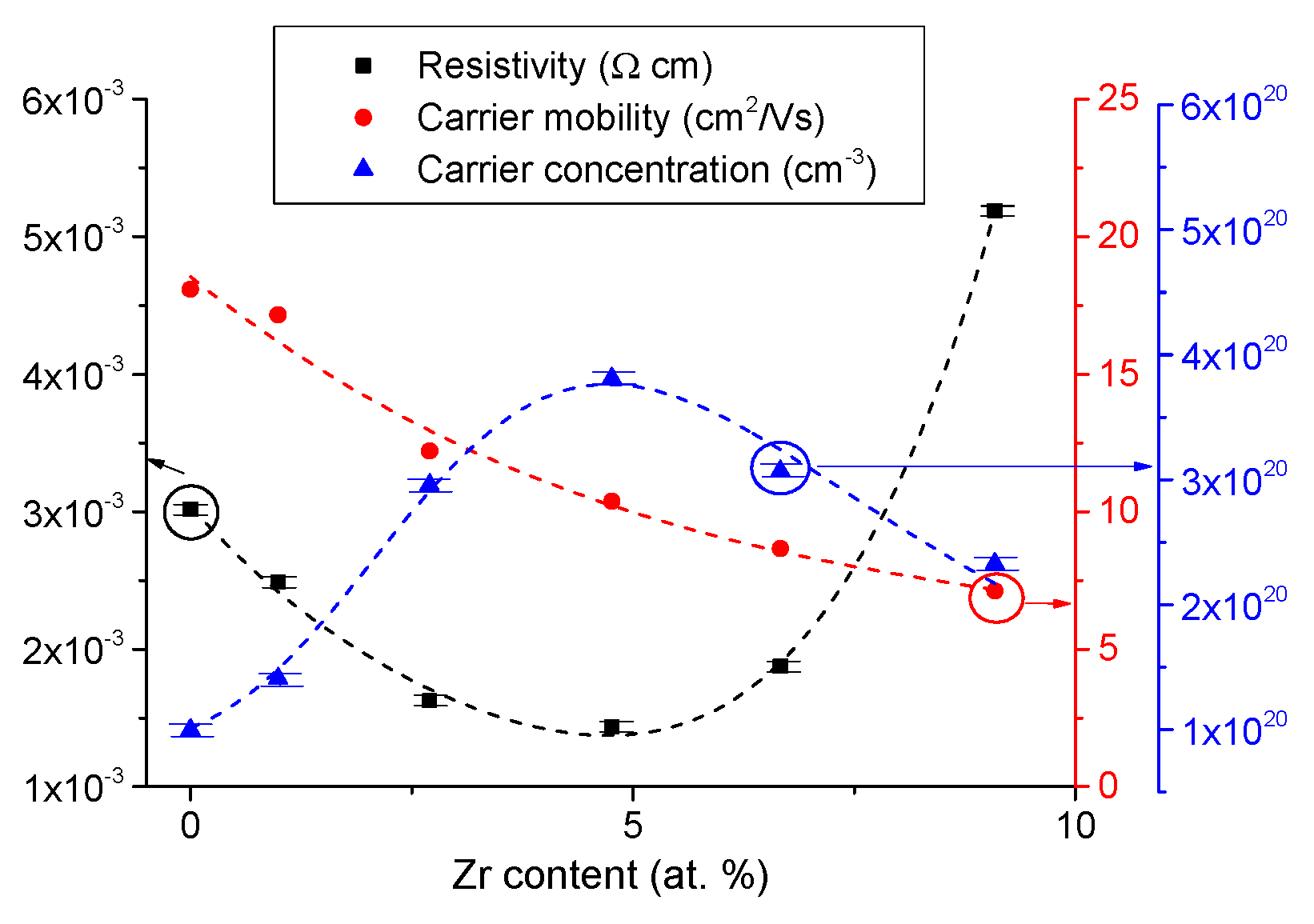
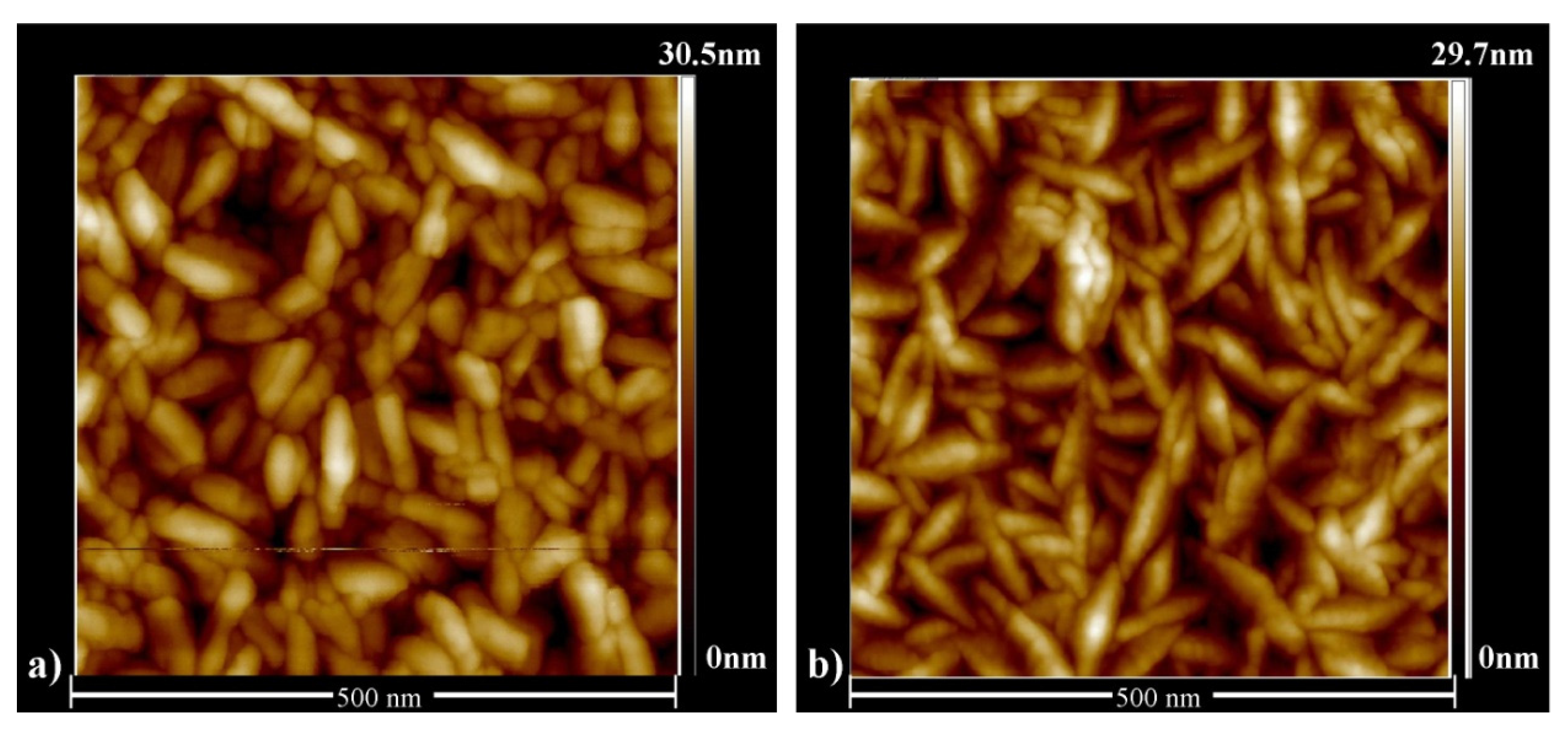
3.1. Effect of Doping and Film Thickness on the Microstructure and Electrical Properties.

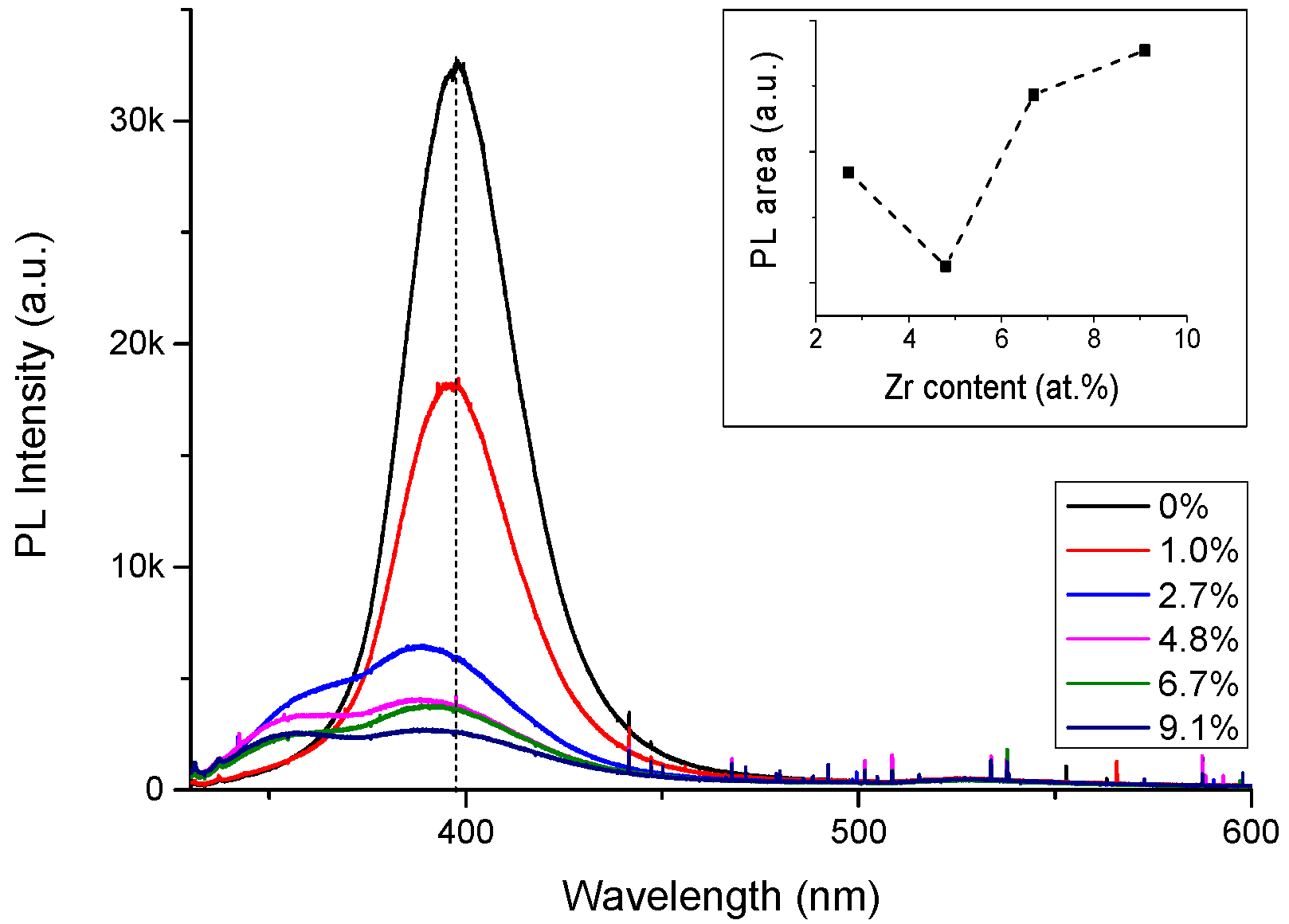
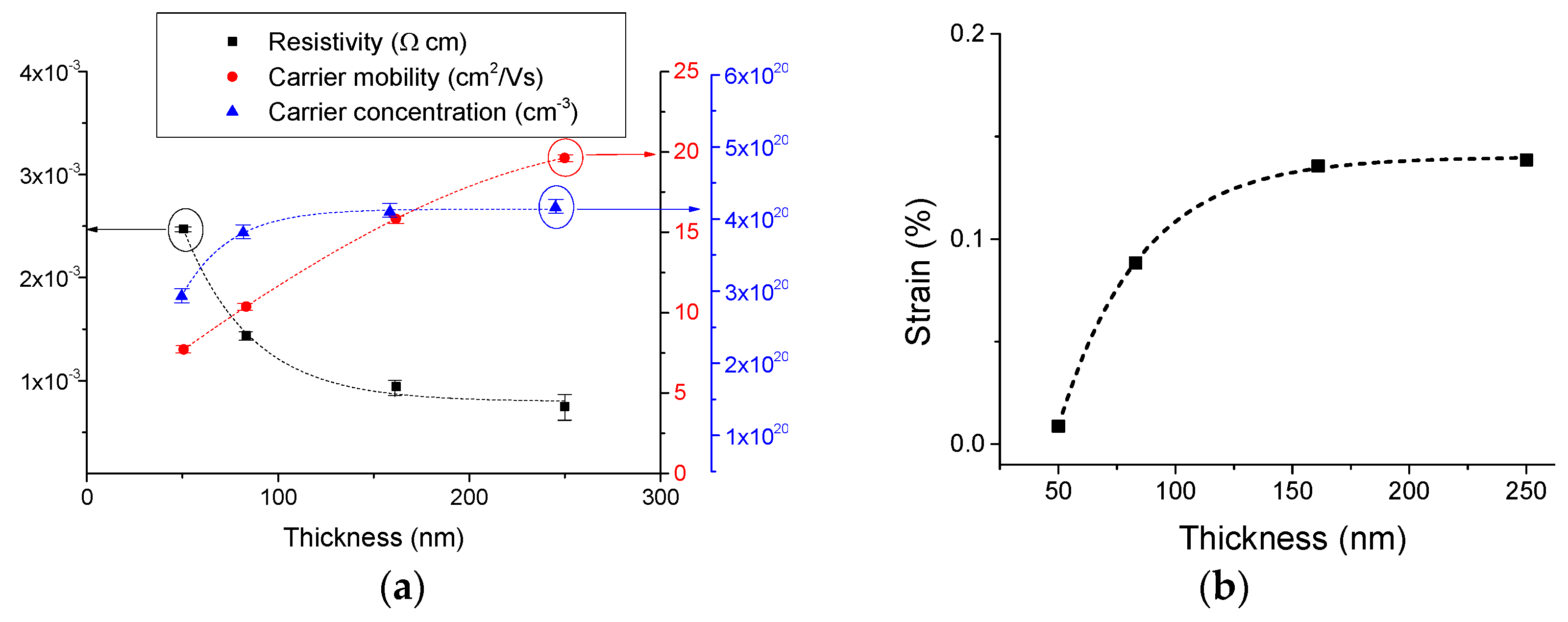
3.2. Effect of Doping on the Optical Properties.
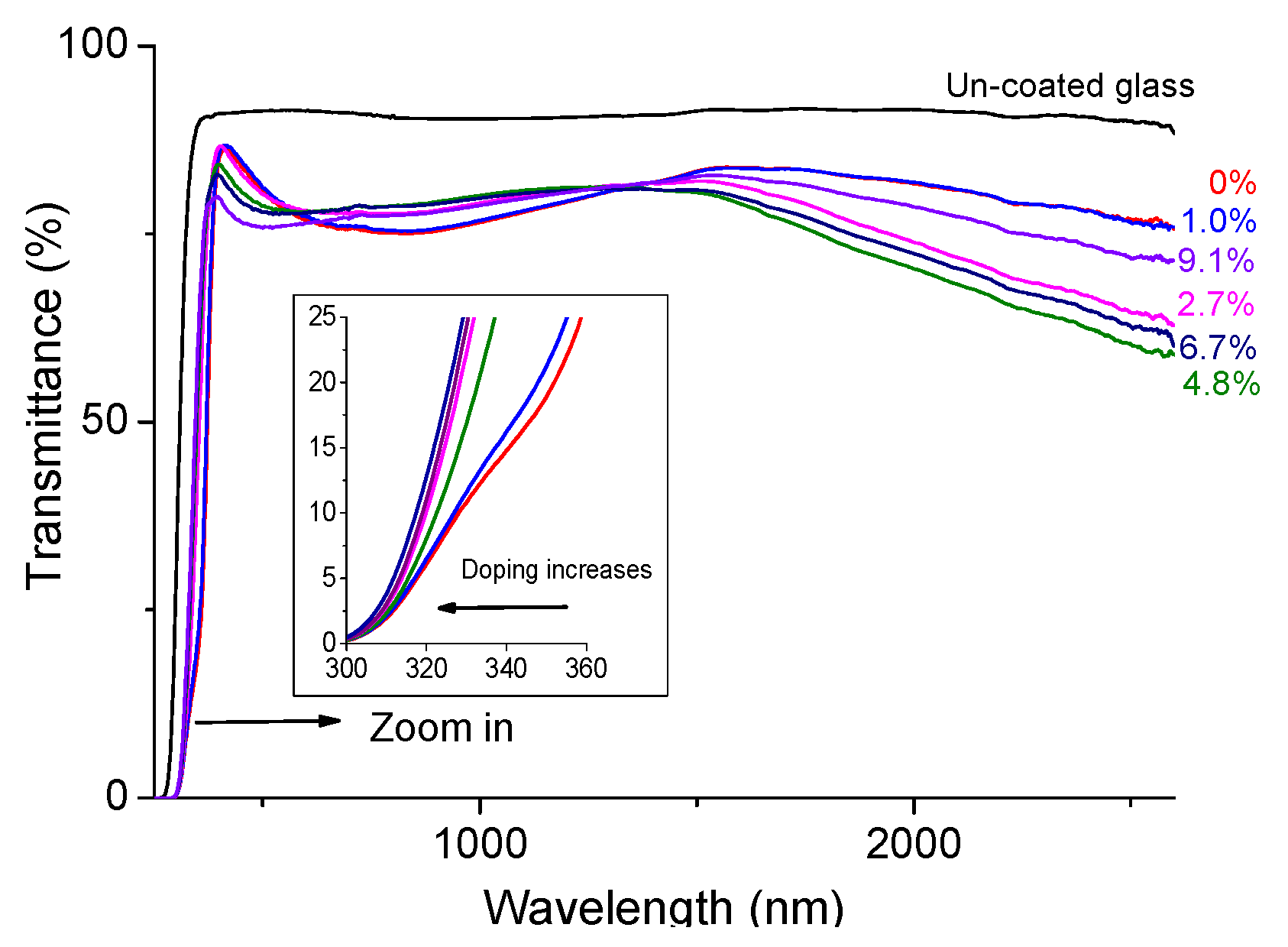
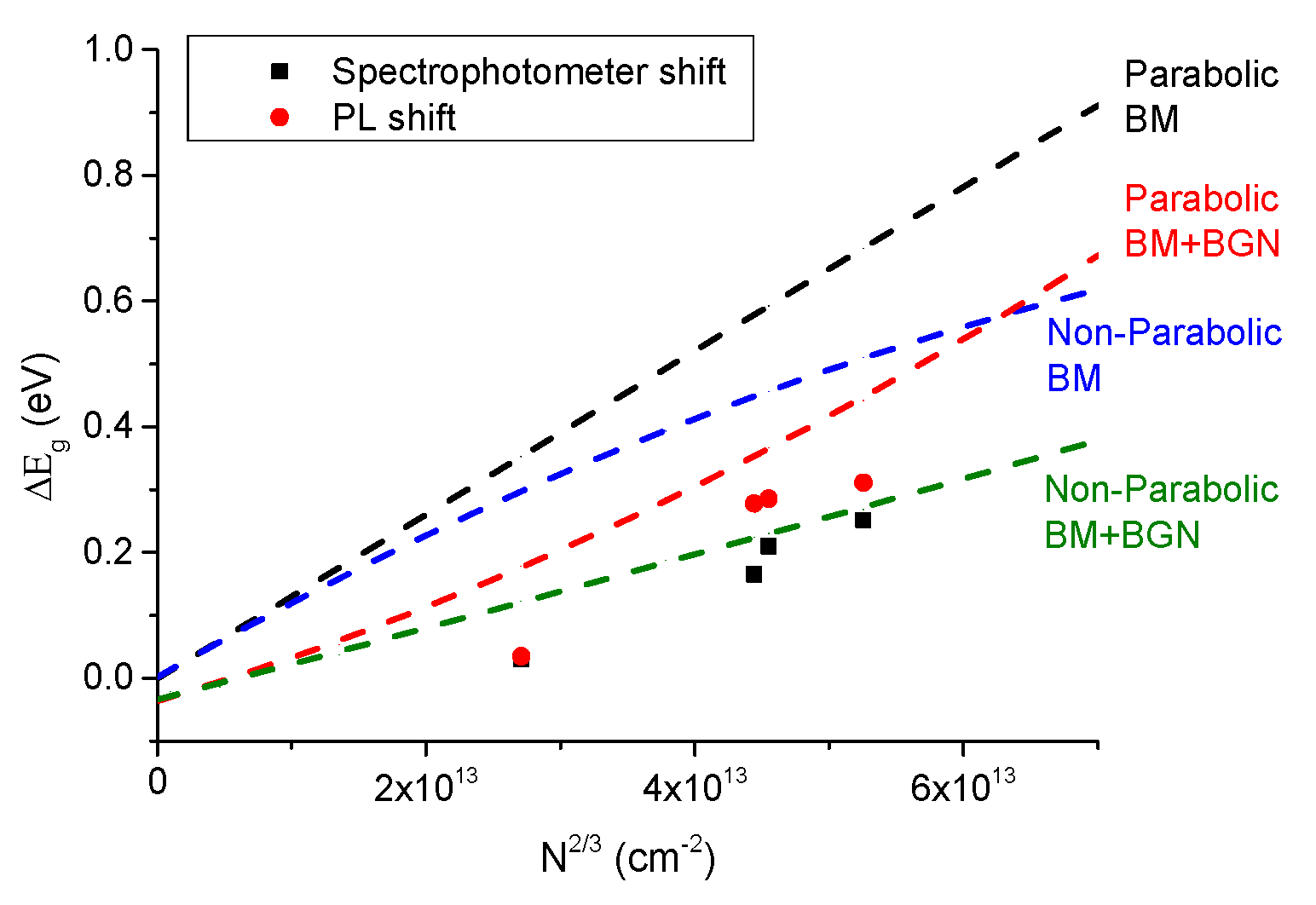
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klingshirn, C. ZnO: From basics towards applications. Phys. Status Solidi B 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Lewis, B.G.; Paine, D.C. Applications and processing of transparent conducting oxides. MRS Bull. 2000, 25, 22–27. [Google Scholar] [CrossRef]
- Walsh, A.; Da Silva, J.L.F.; Wei, S. Multi-component transparent conducting oxides: Progress in materials modelling. J. Phys. Condens. Matter 2011, 23. [Google Scholar] [CrossRef] [PubMed]
- Ellmer, K. Resistivity of polycrystalline zinc oxide films: Current status and physical limit. J. Phys. D Appl. Phys. 2001, 34, 3097–3108. [Google Scholar] [CrossRef]
- Minami, T. Transparent conducting oxide semiconductors for transparent electrodes. Semicond. Sci. Technol. 2005, 20, S35–S44. [Google Scholar] [CrossRef]
- Faÿ, S.; Steinhauser, J.; Nicolay, S.; Ballif, C. Polycrystalline ZnO:B grown by LPCVD as TCO for thin film silicon solar cells. Thin Solid Film. 2010, 518, 2961–2966. [Google Scholar] [CrossRef]
- Faÿ, S.; Steinhauser, J.; Oliveira, N.; Vallat-Sauvain, E.; Ballif, C. Opto-electronic properties of rough LP-CVD ZnO:B for use as TCO in thin-film silicon solar cells. Thin Solid Film. 2007, 515, 8558–8561. [Google Scholar] [CrossRef]
- Ilican, S.; Caglar, Y.; Caglar, M.; Demirci, B. Polycrystalline indium-doped ZnO thin films: Preparation and characterization. J. Optoelectron. Adv. Mater. 2008, 10, 2592–2598. [Google Scholar]
- Huang, C.; Wang, M.; Deng, Z.; Cao, Y.; Liu, Q.; Huang, Z.; Huang, Q. Low content indium-doped zinc oxide films with tunable work function fabricated through magnetron sputtering. Semicond. Sci. Technol. 2010, 25. [Google Scholar] [CrossRef]
- Wang, C.C.; Man, B.Y.; Liu, M.; Chen, C.S.; Jiang, S.Z.; Yang, S.Y.; Xu, S.C.; Gao, X.G.; Hu, B. The intrinsic room-temperature ferromagnetism in ZnO:Co thin films deposited by PLD. Adv. Condens. Matter Phys. 2012, 2012. [Google Scholar] [CrossRef]
- Lin, M.; Chang, Y.; Chen, M.; Chu, C. Characteristics of Zr-doped ZnO thin films grown by atomic layer deposition. J. Electrochem. Soc. 2011, 158, D395–D399. [Google Scholar] [CrossRef]
- Gokulakrishnan, V.; Parthiban, S.; Jeganathan, K.; Ramamurthi, K. Investigation on the effect of Zr doping in ZnO thin films by spray pyrolysis. Appl. Surf. Sci. 2011, 257, 9068–9072. [Google Scholar] [CrossRef]
- Chalker, P.R.; Marshall, P.A.; King, P.J.; Dawson, K.; Romani, S.; Williams, P.A.; Rosseinsky, M.J. Atomic layer deposition of germanium-doped zinc oxide films with tunable ultraviolet emission. J. Mater. Chem. 2012, 22, 12824–12829. [Google Scholar] [CrossRef]
- Ahn, C.H.; Kim, J.H.; Cho, H.K. Tunable electrical and optical properties in composition controlled Hf:ZnO thin films grown by atomic layer deposition. J. Electrochem. Soc. 2012, 159, H384–H387. [Google Scholar] [CrossRef]
- Tsay, C.; Lee, W. Effect of dopants on the structural, optical and electrical properties of sol-gel derived ZnO semiconductor thin films. Curr. Appl. Phys. 2013, 13, 60–65. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.; Kwon, J.; Choi, H.; Kim, S.; Kim, K. Structural and electrical properties of atomic layer deposited Al-doped ZnO films. Adv. Funct. Mater. 2011, 21, 448–455. [Google Scholar] [CrossRef]
- Dasgupta, N.P.; Neubert, S.; Lee, W.; Trejo, O.; Lee, J.; Prinz, F.B. Atomic layer deposition of Al-doped ZnO films: Effect of grain orientation on conductivity. Chem. Mater. 2010, 22, 4769–4775. [Google Scholar] [CrossRef]
- Chalker, P.R.; Marshall, P.A.; Romani, S.; Rosseinsky, M.J.; Rushworth, S.; Williams, P.A.; Ridealgh, J. Atomic layer deposition of gallium-doped zinc oxide transparent conducting oxide films. MRS Proc. 2012, 1315, 39–44. [Google Scholar] [CrossRef]
- Chalker, P.R.; Marshall, P.A.; Romani, S.; Roberts, J.W.; Irvine, S.J.C.; Lamb, D.A.; Williams, P.A. Atomic layer deposition of Ga-doped ZnO transparent conducting oxide substrates for CdTe-based photovoltaics. J. Vac. Sci. Technol. 2013, 31. [Google Scholar] [CrossRef]
- Saito, K.; Hiratsuka, Y.; Omata, A.; Makino, H.; Kishimoto, S.; Yamamoto, T.; Hirayama, H. Atomic layer deposition and characterization of Ga-doped ZnO thin films. Superlattices Microstruct. 2007, 42, 172–175. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. TCO/metal/TCO structures for energy and flexible electronics. Thin Solid Film. 2011, 520, 1–17. [Google Scholar] [CrossRef]
- Duan, X.M.; Stampfl, C.; Bilek, M.M.; McKenzie, D.R.; Wei, S. Design of shallow acceptors in ZnO through early transition metals codoped with N acceptors. Phys. Rev. B 2011, 83. [Google Scholar] [CrossRef]
- Wang, F.; Lv, M.; Pang, Z.; Yang, T.; Dai, Y.; Han, S. Theoretical study of structural, optical and electrical properties of zirconium-doped zinc oxide. Appl. Surf. Sci. 2008, 254, 6983–6986. [Google Scholar] [CrossRef]
- Gabás, M.; Landa-Cánovas, A.; Luis Costa-Krämer, J.; Agulló-Rueda, F.; González-Elipe, A.R.; Díaz-Carrasco, P.; Ramón Ramos-Barrado, J. Differences in n-type doping efficiency between Al- and Ga-ZnO films. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Highly efficient, solar active, and reusable photocatalyst: Zr-loaded Ag-ZnO for reactive red 120 dye degradation with synergistic effect and dye-sensitized mechanism. Langmuir 2013, 29, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Bahedi, K.; Addou, M.; Jouad, M.E.; Bayoud, S.; Sofiani, Z. Effects of deposition temperature on the surface roughness and the nonlinear optical susceptibility of sprayed deposited ZnO:Zr thin films. Appl. Surf. Sci. 2009, 255, 9054–9057. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Vijaya, J.J.; Kennedy, L.J. Effects of morphology and Zr doping on structural, optical, and photocatalytic properties of ZnO nanostructures. Ind. Eng. Chem. Res. 2012, 51, 16333–16345. [Google Scholar] [CrossRef]
- Paul, G.K.; Bandyopadhyay, S.; Sen, S.K.; Sen, S. Structural, optical and electrical studies on sol-gel deposited Zr doped ZnO films. Mater. Chem. Phys. 2003, 79, 71–75. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Yang, G.; Zhang, J.; Shi, Z.; Zhang, Z.; Xue, D. Synthesis and magnetic properties of Zr doped ZnO nanoparticles. Nanoscale Res. Lett. 2011, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tsay, C.; Fan, K. Optimization of Zr-doped ZnO thin films prepared by sol-gel method. Mater. Trans. 2008, 49, 1900–1904. [Google Scholar] [CrossRef]
- Zhang, H.; Lei, C.; Liu, H.; Yuan, C. Low-temperature deposition of transparent conducting ZnO:Zr films on PET substrates by DC magnetron sputtering. Appl. Surf. Sci. 2009, 255, 6054–6056. [Google Scholar] [CrossRef]
- Yadav, S.K.; Vyas, S.; Chandra, R.; Chaudhary, G.P.; Nath, S.K. Study of electrical and optical properties of Zr-doped ZnO thin films prepared by dc reactive magnetron sputtering. Adv. Mater. Res. 2009, 67, 161–166. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Feng, L. Influence of annealing temperature on the properties of ZnO:Zr films deposited by direct current magnetron sputtering. Vacuum 2010, 84, 833–836. [Google Scholar] [CrossRef]
- Kim, H.; Horwitz, J.S.; Kim, W.H.; Qadri, S.B.; Kafafi, Z.H. Anode material based on Zr-doped ZnO thin films for organic light-emitting diodes. Appl. Phys. Lett. 2003, 83, 3809–3811. [Google Scholar] [CrossRef]
- Yu, S.F.; Yuen, C.; Lau, S.P.; Wang, Y.G.; Lee, H.W.; Tay, B.K. Ultraviolet amplified spontaneous emission from zinc oxide ridge waveguides on silicon substrate. Appl. Phys. Lett. 2003, 83, 4288–4290. [Google Scholar] [CrossRef]
- Pala, R.G.S.; Metiu, H. Modification of the oxidative power of ZnO (1010) surface by substituting some surface Zn atoms with other metals. J. Phys. Chem. C 2007, 111, 8617–8622. [Google Scholar] [CrossRef]
- Lv, M.; Xiu, X.; Pang, Z.; Dai, Y.; Ye, L.; Cheng, C.; Han, S. Structural, electrical and optical properties of zirconium-doped zinc oxide films prepared by radio frequency magnetron sputtering. Thin Solid Film. 2008, 516, 2017–2021. [Google Scholar] [CrossRef]
- Van de Walle, C.G.; Neugebauer, J. Universal alignment of hydrogen levels in semiconductors, insulators and solutions. Lett. Nat. 2003, 423, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Chaaya, A.A.; Viter, R.; Bechelany, M.; Alute, Z.; Erts, D.; Zalesskaya, A.; Miele, P. Evolution of microstructure and related optical properties of ZnO grown by atomic layer deposition. Beilstein J. Nanotechnol. 2013, 4, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72. [Google Scholar] [CrossRef]
- Priester, L. Grain Boundaries: From Theory to Engineering; Springer Science & Business Media: Medford, MA, USA, 2012; pp. 135–137. [Google Scholar]
- Kim, K.; Kim, G.; Woo, J.; Kim, C. Characteristics of nickel-doped zinc oxide thin films prepared by sol-gel method. Surf. Coat. Technol. 2008, 202, 5650–5653. [Google Scholar] [CrossRef]
- Pankove, J.I. Optical Processes in Semiconductors; Courier Corporation: North Chelmsford, MA, USA, 1971. [Google Scholar]
- Rossnagel, S.M.; Kuan, T.S. Alteration of Cu conductivity in the size effect regime. J. Vac. Sci. Technol. B 2004, 22, 240–247. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Li, R.; Zhang, Y.; Xu, G.; Cheng, H. Fabrication of highly transparent and conductive indium-tin oxide thin films with a high figure of merit via solution processing. Langmuir 2013, 29, 13836–13842. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Lee, W.; Bae, K.; Lee, S.B.; Rubloff, G.W. Structural, electrical, and optical properties of atomic layer deposition Al-doped ZnO films. J. Appl. Phys. 2010, 108. [Google Scholar] [CrossRef]
- Illiberi, A.; Scherpenborg, R.; Roozeboom, F.; Poodt, P. Atmospheric spatial atomic layer deposition of In-doped ZnO. ECS J. Solid State Sci. Technol. 2014, 3, P111–P114. [Google Scholar] [CrossRef]
- Ye, Z.-Y.; Lu, H.-L.; Geng, Y.; Geng, Y.; Gu, Y.-Z.; Xie, Z.-Y.; Zhang, Y.; Sun, Q.-Q.; Ding, S.-J.; Zhang, D.V. Structural, electrical, and optical properties of Ti-doped ZnO films fabricated by atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Burstein, E. Anomalous optical absorption limit in InSb. Phys. Review 1954, 93, 632–633. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Pisarkiewicz, T.; Zakrzewska, K.; Leja, E. Scattering of charge carriers in transparent and conducting thin oxide films with a non-parabolic conduction band. Thin Solid Film. 1989, 174, 217–223. [Google Scholar] [CrossRef]
- Jain, A.; Sagar, P.; Mehra, R.M. Band gap widening and narrowing in moderately and heavily doped n-ZnO films. Solid State Electron. 2006, 50, 1420–1424. [Google Scholar] [CrossRef]
- Kim, W.M.; Kim, J.S.; Jeong, J.; Park, J.; Baik, Y.; Seong, T. Analysis of optical band-gap shift in impurity doped ZnO thin films by using nonparabolic conduction band parameters. Thin Solid Film. 2013, 531, 430–435. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herodotou, S.; Treharne, R.E.; Durose, K.; Tatlock, G.J.; Potter, R.J. The Effects of Zr Doping on the Optical, Electrical and Microstructural Properties of Thin ZnO Films Deposited by Atomic Layer Deposition. Materials 2015, 8, 7230-7240. https://doi.org/10.3390/ma8105369
Herodotou S, Treharne RE, Durose K, Tatlock GJ, Potter RJ. The Effects of Zr Doping on the Optical, Electrical and Microstructural Properties of Thin ZnO Films Deposited by Atomic Layer Deposition. Materials. 2015; 8(10):7230-7240. https://doi.org/10.3390/ma8105369
Chicago/Turabian StyleHerodotou, Stephania, Robert E. Treharne, Ken Durose, Gordon J. Tatlock, and Richard J. Potter. 2015. "The Effects of Zr Doping on the Optical, Electrical and Microstructural Properties of Thin ZnO Films Deposited by Atomic Layer Deposition" Materials 8, no. 10: 7230-7240. https://doi.org/10.3390/ma8105369




