Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring
Abstract
:1. Introduction
1.1. General Background
1.2. Polyacrylonitrile Electrospun Fibers as a Strain Sensor in Structural Health Monitoring Applications
2. Results and Discussion
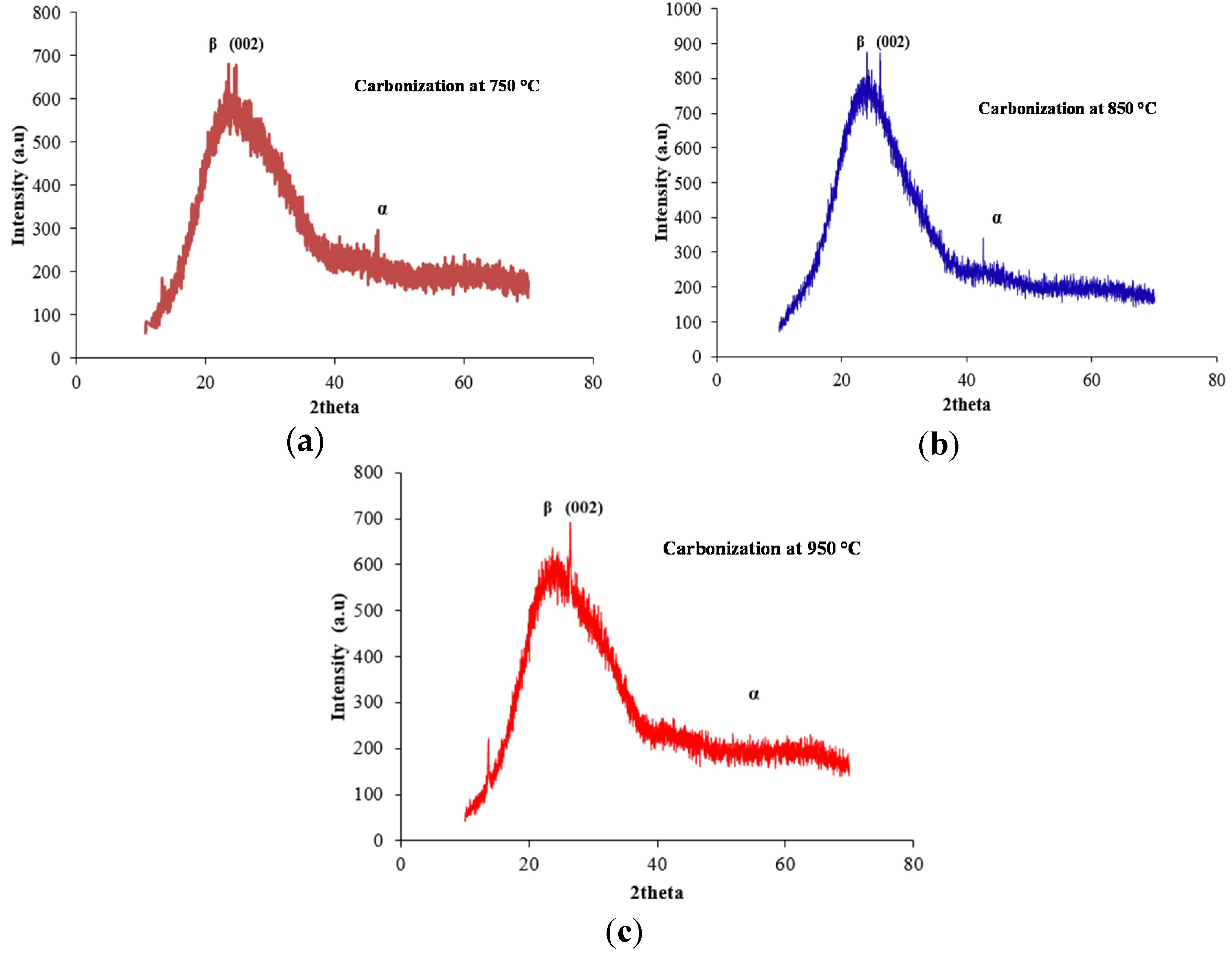
2.1. X-ray Diffraction (XRD) Analysis
| Temperature (°C) | d(002) nm | Lc nm | Lc/d(002) |
|---|---|---|---|
| 750 | 0.4053 | 2.66 | 1.87 |
| 850 | 0.4128 | 2.76 | 1.58 |
| 950 | 0.4226 | 2.76 | 1.58 |

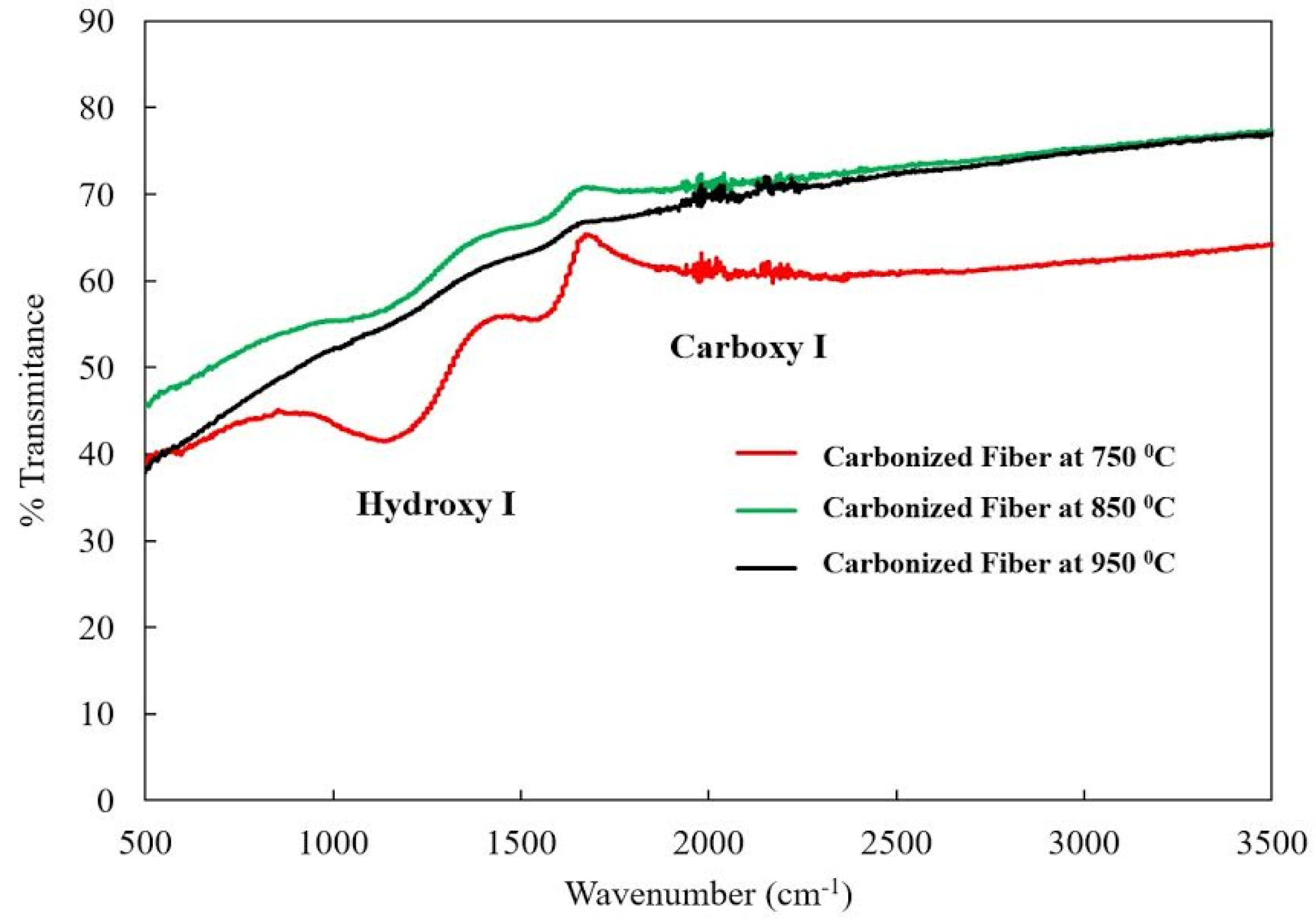
2.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
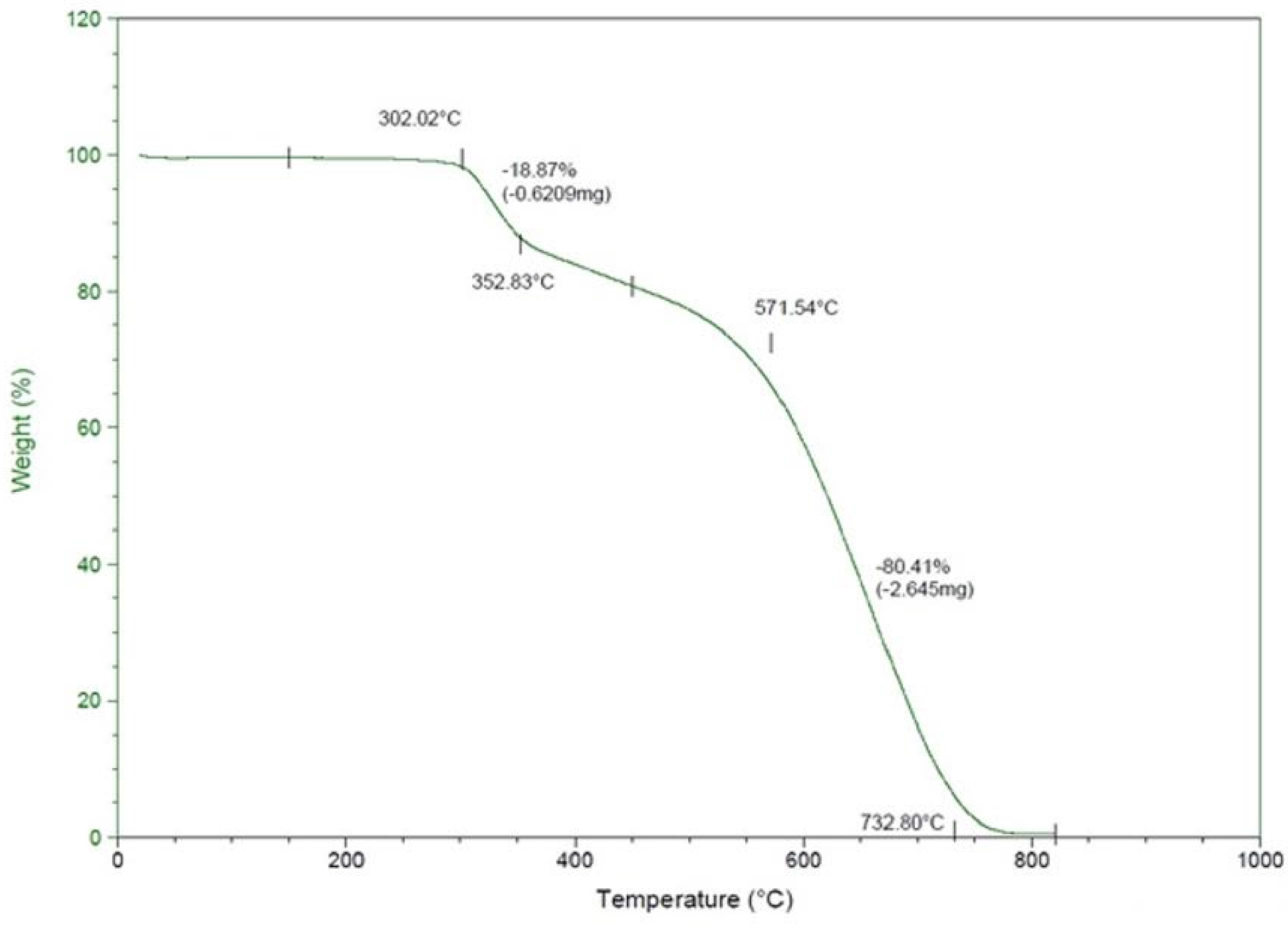
2.3. Thermogravimetry Analysis (TGA) Analysis

2.4. Differential Scanning Calorimeter (DSC) Analysis
2.5. Ionic Conductivity Test
| Temperature (°C) | Conductivity (σ) S/cm (×103) |
|---|---|
| 24.85 | 2.13 |
| 25.17 | 1.85 |
| 35.10 | 2.78 |
| 39.13 | 3.31 |
| 40.10 | 4.31 |
| 42.01 | 4.50 |
| 43.88 | 4.48 |
| 57.60 | 4.49 |
| 77.40 | 4.51 |
2.6. Surface Hydrophobicity of Carbonized Fibers
| No. | Water Contact Angle (°) | |||||
|---|---|---|---|---|---|---|
| 750 °C | 850 °C | 950 °C | ||||
| Right | Left | Right | Left | Right | Left | |
| 1 | 149.46 | 152.36 | 156.22 | 156.32 | 149.21 | 154.78 |
| 2 | 151.32 | 154.85 | 155.23 | 157.22 | 152.55 | 153.22 |
| 3 | 152.63 | 149.98 | 155.69 | 153.45 | 156.33 | 154.32 |
| 4 | 153.55 | 151.11 | 159.63 | 155.25 | 157.54 | 156.33 |
| 5 | 159.90 | 148.79 | 159.58 | 156.08 | 158.64 | 152.52 |
| Mean | 152.40 | 156.47 | 154.54 | |||
| Standard Deviation (SD) | ±3.23 | ±1.92 | ±2.80 | |||
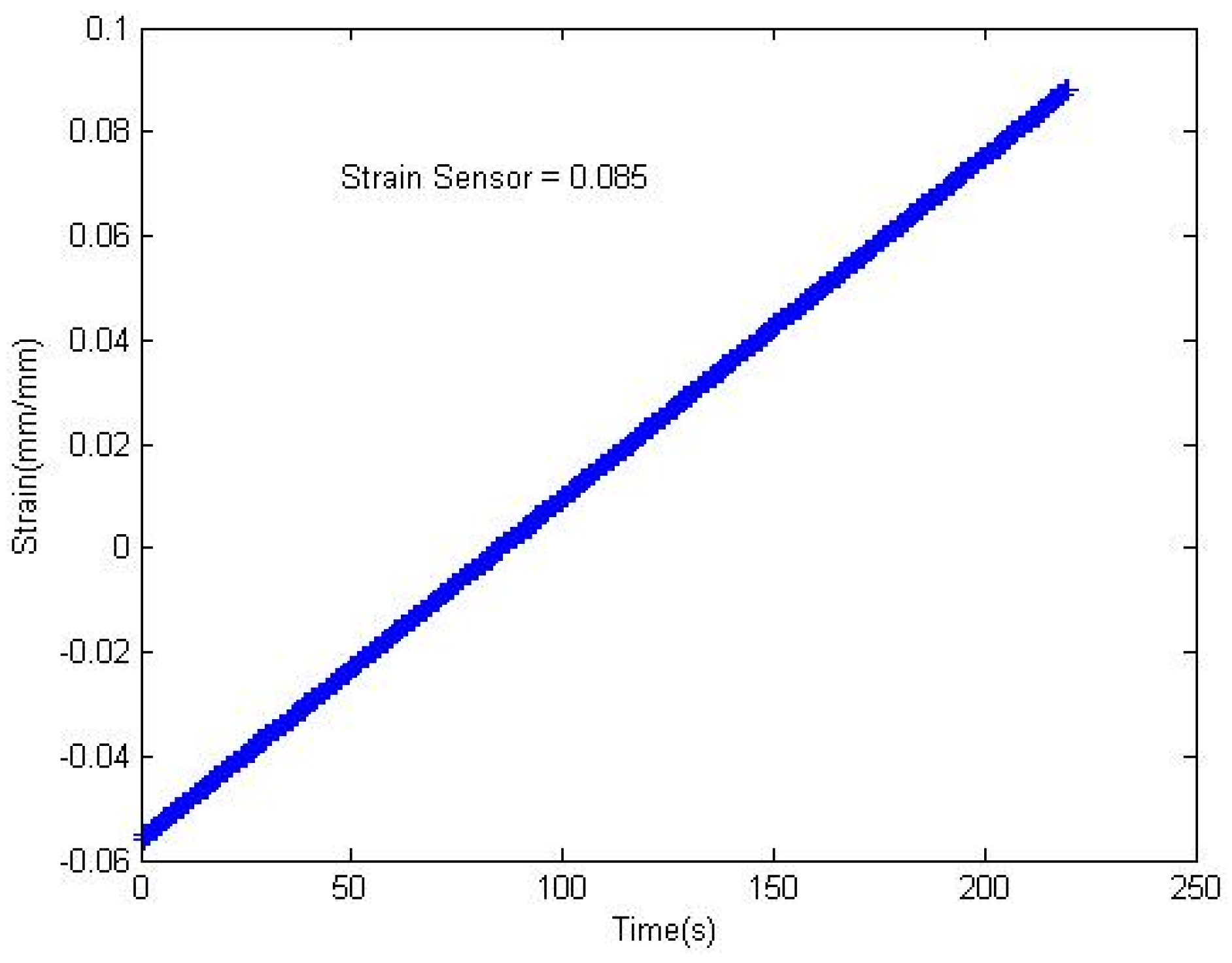
2.7. Strain Sensing Performance

3. Experimental
3.1. Materials
3.2. Method
Carbonization of Polyacrylonitrile (PAN) Nanofibers
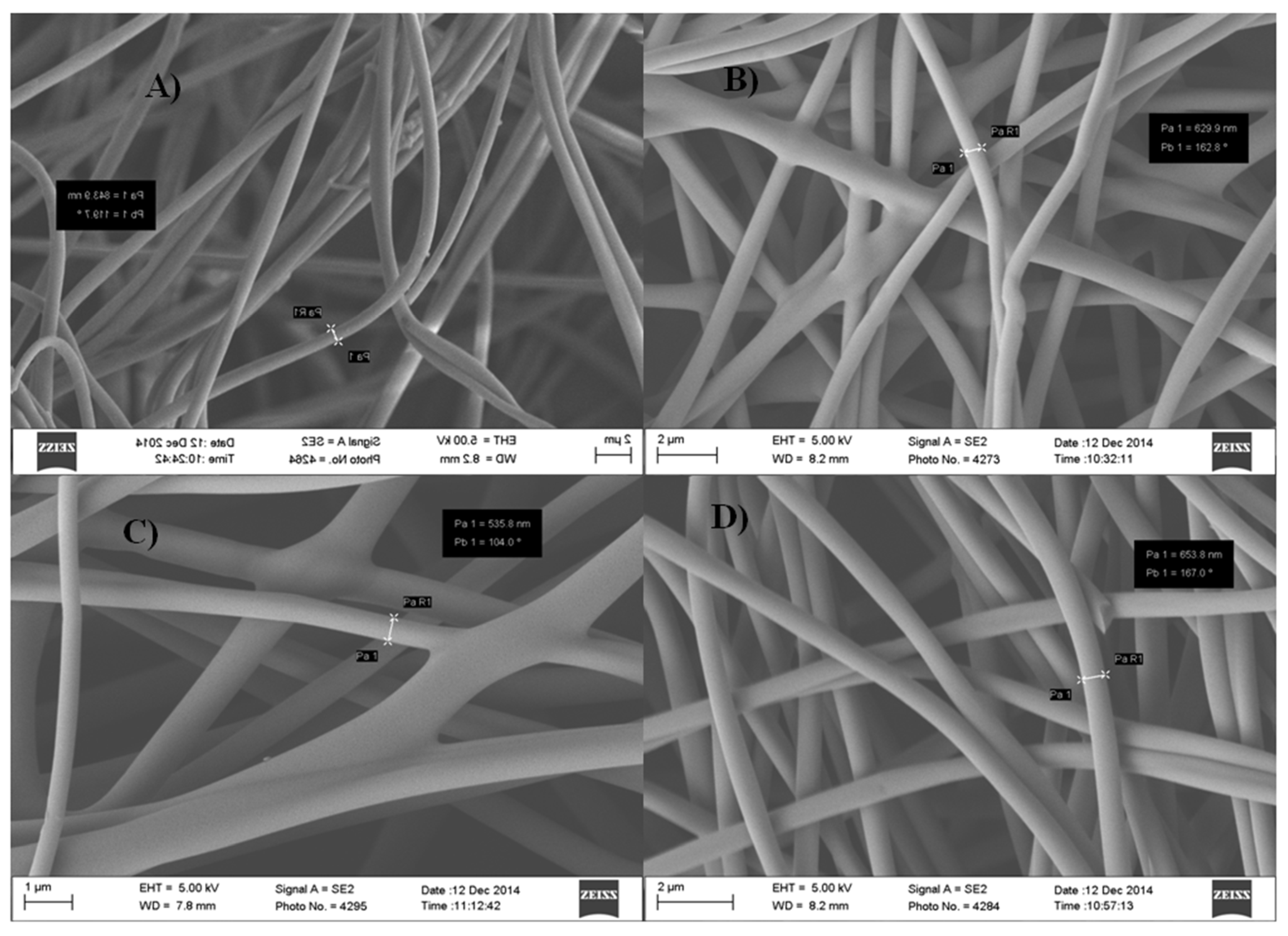
3.3. Fabrication of Nanocomposite for Sensors
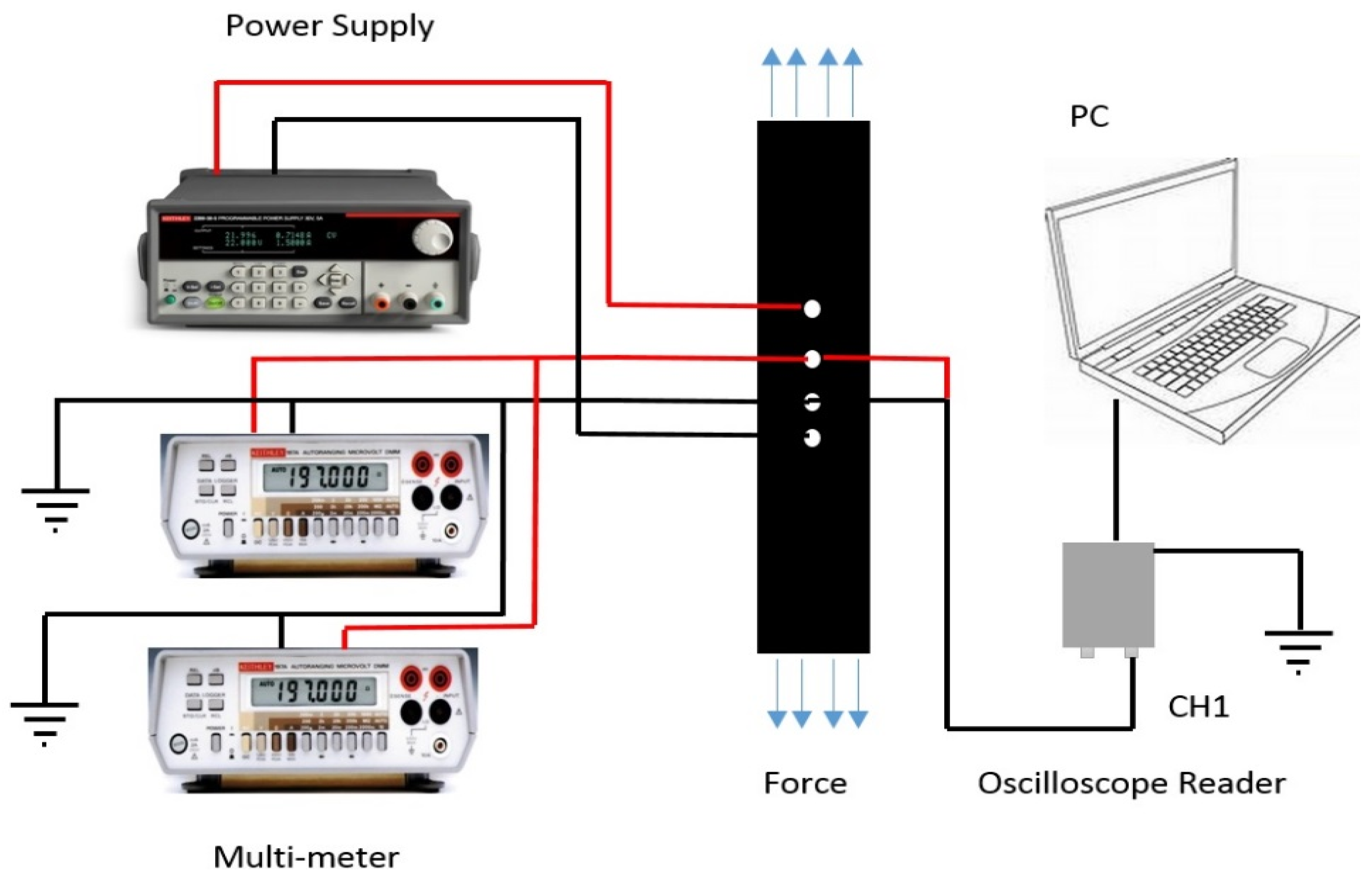
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farrar, C.R.; Worden, K. An introduction to structural health monitoring. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2007, 365, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Cawley, P. Long Range Inspection of Structures Using Low Frequency Ultrasound. In Structural Damage Assessment Using Advanced Signal Processing Procedures; Sheffield Academic Press: Sheffield, UK, 1997; pp. 1–17. [Google Scholar]
- Bartelds, G. Aircraft Structural Health Monitoring, Prospects for Smart Solutions from a European Viewpoint. In Structural Health Monitoring, Current Status and Perspectives; CRC Press, Inc.: Boca Raton, FL, USA, 1997; pp. 293–300. [Google Scholar]
- Staszewski, W.; Biemans, C.; Boller, C.; Tomlinson, G. Impact Damage Detection in Composite Structures—Recent Advances. In Structural Health Monitoring; CRC Press: Palo Alto, CA, USA, 2000; pp. 754–763. [Google Scholar]
- Spencer, B.F., Jr.; Ruiz-Sandoval, M.; Kurata, N. Smart Sensing Technology for Structural Health Monitoring. In Proceedings of the 13th World Conference on Earthquake Engineering, Vancouver, BC, Canada, 1–6 August 2004.
- Beese, A.M.; Papkov, D.; Li, S.; Dzenis, Y.; Espinosa, H.D. In situ transmission electron microscope tensile testing reveals structure–Property relationships in carbon nanofibers. Carbon 2013, 60, 246–253. [Google Scholar] [CrossRef]
- Nataraj, S.; Yang, K.; Aminabhavi, T. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Chand, S. Review carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Yun, K.M.; Hogan, C.J.; Matsubayashi, Y.; Kawabe, M.; Iskandar, F.; Okuyama, K. Nanoparticle filtration by electrospun polymer fibers. Chem. Eng. Sci. 2007, 62, 4751–4759. [Google Scholar] [CrossRef]
- Yalcinkaya, B.; Yener, F.; Jirsak, O.; Cengiz-Callioglu, F. On the nature of electric current in the electrospinning process. J. Nanomater. 2013, 6. [Google Scholar] [CrossRef]
- Ko, T.-H.; Day, T.-C.; Perng, J.-A.; Lin, M.-F. The characterization of pan-based carbon fibers developed by two-stage continuous carbonization. Carbon 1993, 31, 765–771. [Google Scholar]
- Cho, C.W.; Cho, D.; Ko, Y.-G.; Kwon, O.H. Stabilization, carbonization, and characterization of pan precursor webs processed by electrospinning technique. Carbon Lett. 2007, 8, 313–320. [Google Scholar]
- Mohammed, A.A.; Moussa, W.A.; Lou, E. High sensitivity mems strain sensor: Design and simulation. Sensors 2008, 8, 2642–2661. [Google Scholar] [CrossRef]
- Nagy, M.L. A user-friendly, high-sensitivity strain gauge. Sensors 2001, 18, 20–27. [Google Scholar]
- Hrovat, M.; Belavic, D.; Samardzija, Z.; Holc, J. An Investigation of Thick-Film Resistor, Fired at Different Temperatures, for Strain Sensors. In Proceeding of the 24th International Spring Seminar conference on Electronics Technology: Concurrent Engineering in Electronic Packaging, Calimanesti-Caciulata, Romania, 5–9 May 2001; IEEE: Washington DC, USA; pp. 32–36.
- Wen, S.; Chung, D. Carbon fiber-reinforced cement as a strain-sensing coating. Cem. Concr. Res. 2001, 31, 665–667. [Google Scholar] [CrossRef]
- Wang, X.; Fu, X.; Chung, D. Strain sensing using carbon fiber. J. Mater. Res. 1999, 14, 790–802. [Google Scholar] [CrossRef]
- Mäder, T.; Nestler, D.; Wielage, B. Strain Sensing Using Single Carbon Fibres. In Proceeding of the 18th International Conference on Composite Materials, Jeju Island, Korea, 21–26 August 2011.
- Iwashita, N.; Park, C.R.; Fujimoto, H.; Shiraishi, M.; Inagaki, M. Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon 2004, 42, 701–714. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, C.; Liu, X. Mechanical, microstructure and surface characterizations of carbon fibers prepared from cellulose after liquefying and curing. Materials 2013, 7, 75–84. [Google Scholar] [CrossRef]
- Farsani, R.E.; Raissi, S.; Shokuhfar, A.; Sedghi, A. FT-IR study of stabilized pan fibers for fabrication of carbon fibers. World Acad. Sci. Eng. Technol. 2009, 50, 430–433. [Google Scholar]
- Saufi, S.M.; Ismail, A.F. Development and characterization of polyacrylonitrile (pan) based carbon hollow fiber membrane. Songklanakarin J. Sci. Technol. 2002, 24, 843–854. [Google Scholar]
- Su, C.-I.; Huang, Y.-X.; Wong, J.-W.; Lu, C.-H.; Wang, C.-M. Pan-based carbon nanofiber absorbents prepared using electrospinning. Fibers Polym. 2012, 13, 436–442. [Google Scholar] [CrossRef]
- Goracheva, V.; Mikhailova, T.; Fedorkina, S.; Konnova, N.; Azarova, M.; Konkin, A. Thermographic and thermogravimetric analysis of the thermal behaviour of polyacrylonitrile fibres. Fibre Chem. 1974, 5, 496–498. [Google Scholar] [CrossRef]
- Xiao, S.; Lv, H.; Tong, Y.; Xu, L.; Chen, B. Thermal behavior and kinetics during the stabilization of polyacrylonitrile precursor in inert gas. J. Appl. Polym. Sci. 2011, 122, 480–488. [Google Scholar] [CrossRef]
- Boulet, P.; Brissinger, D.; Collin, A.; Acem, Z.; Parent, G. On the influence of the sample absorptivity when studying the thermal degradation of materials. Materials 2015, 8, 5398–5413. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Avilés, M.; Ginés, J.; del Rio, J.; Pascual, J.; Pérez-Rodríguez, J.; Sánchez-Soto, P. Thermal analysis of acrylonitrile polymerization and cyclization in the presence of n, n-dimethylformamide. J. Therm. Anal. Calorim. 2002, 67, 177–188. [Google Scholar] [CrossRef]
- Moafi, H.F.; Fallah Shojaie, A.; Ali Zanjanchi, M. Photoactive polyacrylonitrile fibers coated by nano-sized titanium dioxide: Synthesis, characterization, thermal investigation. J. Chil. Chem. Soc. 2011, 56, 610–615. [Google Scholar] [CrossRef]
- Barrall, E.; Johnson, J.; Slack, P., Jr.; Jenkins, L. Thermal Characterization Techniques; Marcel Dekker, Inc.: New York, NY, USA, 1970; p. 28. [Google Scholar]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Pub Co: Danvers, MA, USA, 2005. [Google Scholar]
- Wendorff, J.H.; Agarwal, S.; Greiner, A. Electrospinning: Materials, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lee, J.K.; An, K.W.; Ju, J.B.; Cho, B.W.; Cho, W.I.; Park, D.; Yun, K.S. Electrochemical properties of pan-based carbon fibers as anodes for rechargeable lithium ion batteries. Carbon 2001, 39, 1299–1305. [Google Scholar] [CrossRef]
- Ra, E.J.; An, K.H.; Kim, K.K.; Jeong, S.Y.; Lee, Y.H. Anisotropic electrical conductivity of MWCNT/PAN nanofiber paper. Chem. Phys. Lett. 2005, 413, 188–193. [Google Scholar] [CrossRef]
- Khan, W.S.; Asmatulu, R.; Eltabey, M.M. Electrical and thermal characterization of electrospun pvp nanocomposite fibers. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Groszek, A.; Partyka, S. Measurements of hydrophobic and hydrophilic surface sites by flow microcalorimetry. Langmuir 1993, 9, 2721–2725. [Google Scholar] [CrossRef]
- Nuraje, N.; Khan, W.S.; Lei, Y.; Ceylan, M.; Asmatulu, R. Superhydrophobic electrospun nanofibers. J. Mater. Chem. A 2013, 1, 1929–1946. [Google Scholar] [CrossRef]
- Dong, J.; Yao, Z.; Yang, T.; Jiang, L.; Shen, C. Control of superhydrophilic and superhydrophobic graphene interface. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Vadlamani, V.K.; Chalivendra, V.B.; Shukla, A.; Yang, S. Evaluation of Resistance Measurement Techniques in Carbon Black and Carbon Nano-Tubes Reinforced Epoxy. In Mems and Nanotechnology, Volume 4; Springer: Berlin, Germany, 2011; pp. 17–23. [Google Scholar]
- Nsami, J.N.; Mbadcam, J.K. The adsorption efficiency of chemically prepared activated carbon from cola nut shells by zncl 2 on methylene blue. J. Chem. 2013. [Google Scholar] [CrossRef]
- Abechi, S.E.; Gimba, C.E.; Uzairu, A.; Dallatu, Y.A. Preparation and characterization of activated carbon from palm kernel shell by chemical activation. Res. J. Chem. Sci. 2013, 3, 54–61. [Google Scholar]
- Rodrigues, L.A.; de Sousa Ribeiro, L.A.; Thim, G.P.; Ferreira, R.R.; Alvarez-Mendez, M.O.; dos Reis Coutinho, A. Activated carbon derived from macadamia nut shells: An effective adsorbent for phenol removal. J. Porous Mater. 2013, 20, 619–627. [Google Scholar] [CrossRef]
- Memon, S.A.; Liao, W.; Yang, S.; Cui, H.; Shah, S.F.A. Development of composite pcms by incorporation of paraffin into various building materials. Materials 2015, 8, 499–518. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarifi, I.M.; Alharbi, A.; Khan, W.S.; Swindle, A.; Asmatulu, R. Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring. Materials 2015, 8, 7017-7031. https://doi.org/10.3390/ma8105356
Alarifi IM, Alharbi A, Khan WS, Swindle A, Asmatulu R. Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring. Materials. 2015; 8(10):7017-7031. https://doi.org/10.3390/ma8105356
Chicago/Turabian StyleAlarifi, Ibrahim M., Abdulaziz Alharbi, Waseem S. Khan, Andrew Swindle, and Ramazan Asmatulu. 2015. "Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring" Materials 8, no. 10: 7017-7031. https://doi.org/10.3390/ma8105356
APA StyleAlarifi, I. M., Alharbi, A., Khan, W. S., Swindle, A., & Asmatulu, R. (2015). Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring. Materials, 8(10), 7017-7031. https://doi.org/10.3390/ma8105356






