1. Introduction
Hydrogen storage is one of the key enabling technologies for realization of hydrogen energy economy [
1]. Hydrogen storage materials, taking metal hydrides as a typical example, are commercially prepared by solvent-based synthesis methods or by direct gas–solid hydrogenation reactions. In contrast to the traditional gas–solid hydrogenation process, which is achieved at temperatures far above room temperature, an attractive method—so-called reactive ball milling (RBM) [
2,
3]—was developed in the 1990s to conduct the exothermic reactions between the gas- and metallic solid phases at almost room temperature. This relatively new process has been considered as a powerful tool for fabrication of different nanocrystalline metallic nitrides and hydrides [
4]. In their room-temperature process, the starting metallic powders are subjected to dramatic shear and impact forces generated by the milling media (balls). The powders are, therefore, disintegrated into smaller particles with large surface area, and very clean or fresh oxygen-free active surfaces of the powders are created. The reactive milling atmosphere (nitrogen or hydrogen gases) was gettered and absorbed completely by the first atomically clean surfaces of the metallic ball-milled powders to react in a same manner as a typical gas–solid reaction [
5]. Since then, the RBM process has become a common technique successfully used for preparing nanocrystalline metal hydrides, including magnesium hydride (MgH
2) and their composite powders [
1,
6].
High capacity hydrogen storage materials such as MgH
2 have been receiving much attention as promising solid-state hydrogen storage systems due to their high hydrogen storage capacity (7.60 wt. %), reversibility, cost effectiveness, availability and cyclability [
7,
8,
9]. The international interest in the development of hydrogen based technologies, particularly the area of fuel cell electric vehicles, has greatly increased in recent years [
9].
Unfortunately, and in contrast to the obvious advantages seen in MgH
2 binary hydrogen storage systems, the high thermal stability and the difficulty to decompose this hydride system into metal and hydrogen gas, plus the poor hydrogenation and consequence dehydrogenation kinetics, lead to restricting utilization of such a light-weight system in real automobile applications [
7,
9,
10].
Even though and in spite of the serious drawbacks found in MgH
2, the worldwide interest in such an attractive binary metal hydride has been increased, especially after improving its hydrogen absorption and desorption kinetics by applying a longer ball milling time that led to destabilizing the β-MgH
2 phase and increasing the volume fractions of the metastable γ-MgH
2 phase [
11]. Long mechanical ball milling time always is one key approach for releasing the crystalline stored energy, leading to refining the MgH
2 grains along their grain boundaries resulting in a fine-grained structure. Such fine grains with their short-distance grain boundaries always facilitate a short diffusion path, allowing fast diffusion of the hydrogen atoms into the Mg lattice [
12].
Moreover, ball milling the MgH
2 with pure metallic catalysts (e.g., Ti, Fe, Ni, Nb, V) [
13], intermetallic compounds (e.g., Zr
100−xNi
x, and Ti-based alloys) [
14,
15,
16], metal carbides such as TiC [
17], metal oxides such as Nb
2O
5 [
18], metal chlorides such as MgCl
2 [
19], rare earth chlorides such as LaCl
3 [
20], and nanocomposite Ni/Nb
2O
5 powders [
21] led to remarkable improvement in the hydrogen absorption/desorption kinetics and lowering the thermal stability of MgH
2. It has been shown by Ismail [
20] that the improved hydrogen storage properties of MgH
2 doped with LaCl
3 were due to the catalytic effects of the La-Mg alloy and MgCl
2. Such ultrafine micro-scaled/nano-scaled powders serving as catalysts have shown the possibility of improving the hydrogenation/dehydrogenation properties of MgH
2 to open up a new horizon for its real application.
In the present study, we have investigated the effect of FeCr contamination introduced to the MgH2 powders upon ball milling in the long term on improving the hydrogenation/dehydrogenation properties of the metal hydride phase. Moreover, the effect of doping the as-synthesized MgH2 nanocrystalline powders with TiC nanopowders on the hydrogen storage capacity and cyclability of MgH2 was studied in terms of morphology and kinetics.
3. Results
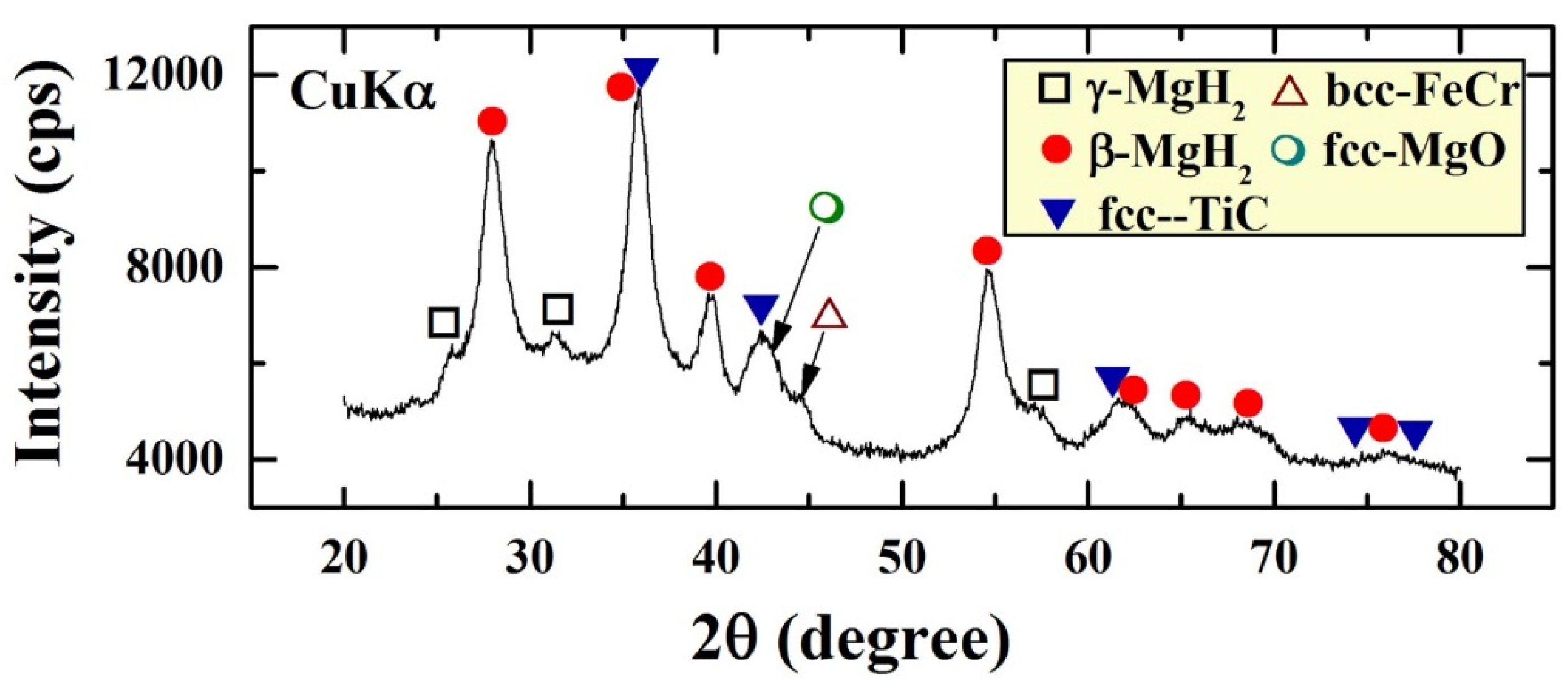
The XRD pattern of the end-product of MgH
2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h of ball milling is shown in
Figure 1. The powders composed of β-MgH
2 (PDF file #: 03-065-3365) and γ-MgH
2 (PDF file #: 00-035-1184) phases mixed with fcc-TiC phase (PDF file #: 00-031-1400). This end-product was significantly contaminated (~2.3 wt. %) with bcc-FeCr alloy (PDF file #: 00-054-0331) introduced to the powders upon using FeCr stainless steel as milling tool. A significant amount of bcc-FeCr was obtained as shown in
Figure 1. Moreover, handling the powders outside of the glove box led to a surface oxidation of the powders and the formation of magnesium oxide layers, as indicated by the Bragg-peaks belonging to fcc-MgO phase (PDF file #: 00-004-0829) shown in
Figure 1. Obviously, the as-prepared nanocomposite powders revealed broad Bragg peaks, suggesting the formation of nanocrystalline grains.
Figure 1.
XRD patterns of MgH2 nanocrystalline powders obtained after 200 h of RBM time and then ball-milled with TiC powders for 50 h.
Figure 1.
XRD patterns of MgH2 nanocrystalline powders obtained after 200 h of RBM time and then ball-milled with TiC powders for 50 h.
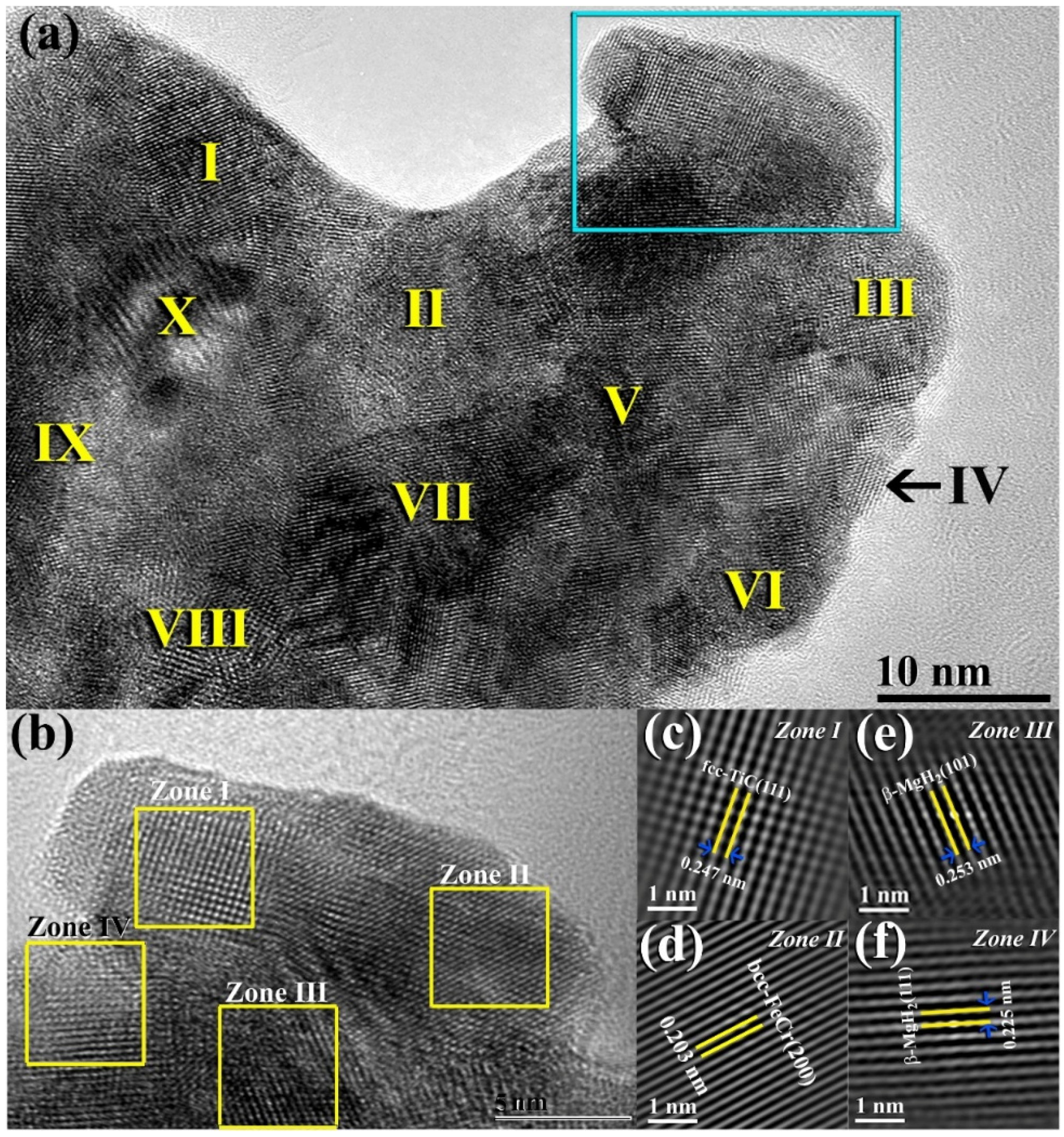
The bright field image (BFI) of nanocomposite MgH
2/5.2TiC/4.6FeCr powders obtained after 50 h of ball milling is displayed in
Figure 2a. The powders revealed Moiré-fringes of different phases. This is suggested by the dissimilarity in the interplanar spacing (2d), as shown in
Figure 2a. The HRTEM image of the indexed square region shown at the edge of the powders in
Figure 2a is presented in
Figure 2b. The fast Fourier transform (FFT) patterns corresponding to the examined square regions presented in
Figure 2b are displayed in
Figure 2c,f. The atomic array with a long-range ordered structure that was presented in Zone I corresponding to nanocrystalline TiC grain. This was confirmed by the interplanar spacing of 0.247 nm (
Figure 2c) that well matches with fcc-TiC (111). Zone II
Figure 2b refers to the precipitation of bcc-FeCr contamination, as confirmed by the interplanar spacing of 0.203 nm for (200), as presented in
Figure 2d. Zones III and IV display two individual regions in the MgH
2 matrix corresponding to β-MgH
2 (101) and (111), which are well matching with the interplanar spacing of 0.253 nm (
Figure 2c) and 0.225 nm (
Figure 2f), respectively.
Figure 2.
(
a) BFI micrograph of as-milled MgH
2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h of ball milling time. The Roman Numerals presented in (
a) refer to the points used for EDS local analysis (
Table 1). The atomic-resolution TEM image of the squared zone indexed shown in (
a) is presented in (
b). The FFT lattice images for zones I, II, III, and IV, shown in
Figure 2b are displayed in (
c–
f), respectively.
Figure 2.
(
a) BFI micrograph of as-milled MgH
2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h of ball milling time. The Roman Numerals presented in (
a) refer to the points used for EDS local analysis (
Table 1). The atomic-resolution TEM image of the squared zone indexed shown in (
a) is presented in (
b). The FFT lattice images for zones I, II, III, and IV, shown in
Figure 2b are displayed in (
c–
f), respectively.
The distribution of TiC/FeCr into the MgH
2 matrix was examined by intensive EDS local analysis performed at selected points (Roman Numerals symbols shown in
Figure 2a) and listed in
Table 1. The results show that the concentration of TiC/FeCr is remarkably varied from one region to another beyond the nano-level, as shown in
Table 1. It is worth mentioning that significant FeCr contamination was evident within those TiC-rich areas (II, IV, VII, X), as shown in
Table 1. This is attributed to the existence of high FeCr contamination content in the as-prepared nanocrystalline TiC powders. However, a considerable amount of FeCr contamination content existed in the as-prepared MgH
2 nanocrystalline powders, as can be seen in the rich Mg-area presented in
Table 1 (I, V, VI, and VIII).
Table 1.
Local EDS elemental analysis of the points presented in
Figure 2a for MgH
2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h of ball milling. The oxygen content introduced to the sample during TEM sample preparations is not included.
Table 1.
Local EDS elemental analysis of the points presented in Figure 2a for MgH2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h of ball milling. The oxygen content introduced to the sample during TEM sample preparations is not included.
| Point | Elements (wt. %) |
|---|
| Mg | Ti | C | Fe | Cr | Total |
|---|
| I | 97.03 | 1.60 | 0.38 | 3.42 | 0.57 | 100 |
| II | 15.65 | 40.30 | 10.12 | 29.21 | 4.72 | 100 |
| III | 95.79 | 0.73 | 0.18 | 2.83 | 0.47 | 100 |
| IV | 48.70 | 28.63 | 7.16 | 12.87 | 2.64 | 100 |
| V | 76.03 | 9.82 | 3.96 | 8.26 | 1.93 | 100 |
| VI | 97.29 | 0.88 | 0.22 | 1.38 | 0.23 | 100 |
| VII | 39.70 | 30.57 | 7.16 | 18.93 | 3.64 | 100 |
| VIII | 98.59 | 0.24 | 0.08 | 0.93 | 0.16 | 100 |
| IX | 97.20 | 1.13 | 0.26 | 1.18 | 0.23 | 100 |
| X | 61.2 | 18.65 | 4.83 | 12.79 | 2.53 | 100 |
In order to get more information about the TiC/FeCr distribution embedded into the host MgH
2 matrix, STEM-EDS X-ray elemental mapping was performed.
Figure 3 presents the images of STEM-(bright field) BF (a), STEM-(dark field) DF (b) and the corresponding EDS chemical mapping for Mg (c), O (d), Ti (e), C (f) Fe (g), and Cr (h) of an agglomerated powder obtained after 50 h of the ball milling. The powder had nearly a spherical-like morphology with a size of about 520 nm in diameter (
Figure 3a). Obviously, the powder after this stage of milling had a rough surface topology related to attachment with TiC nanocrystalline particles (
Figure 3b). As a result of SEM sample preparations and handling the powders outside of the glove box, the MgH
2 powder (
Figure 3c) was oxidized, as indicated by a thin-layer of MgO coat with a thickness of about 68 nm, as shown in
Figure 3c. Nanocrystalline TiC (
Figure 3a,e,f) was homogeneously distributed onto the surface of MgH
2 powders. The individual TiC particle size was in the range of 10–20 nm in diameter, as shown in
Figure 3e). However, some agglomerated TiC particles with apparent sizes ranging between 80 nm and 220 nm were bonded onto the MgH
2 surfaces, as shown in
Figure 3e. The FeCr contamination introduced to the powders upon using steel balls was homogeneously distributed in the MgH
2 matrix, as elucidated in
Figure 3g,h. We should emphasize that the concentration of FeCr contamination was higher in the regions containing TiC-particles when compared with the MgH
2-matrix region, as shown in
Figure 3e–h.
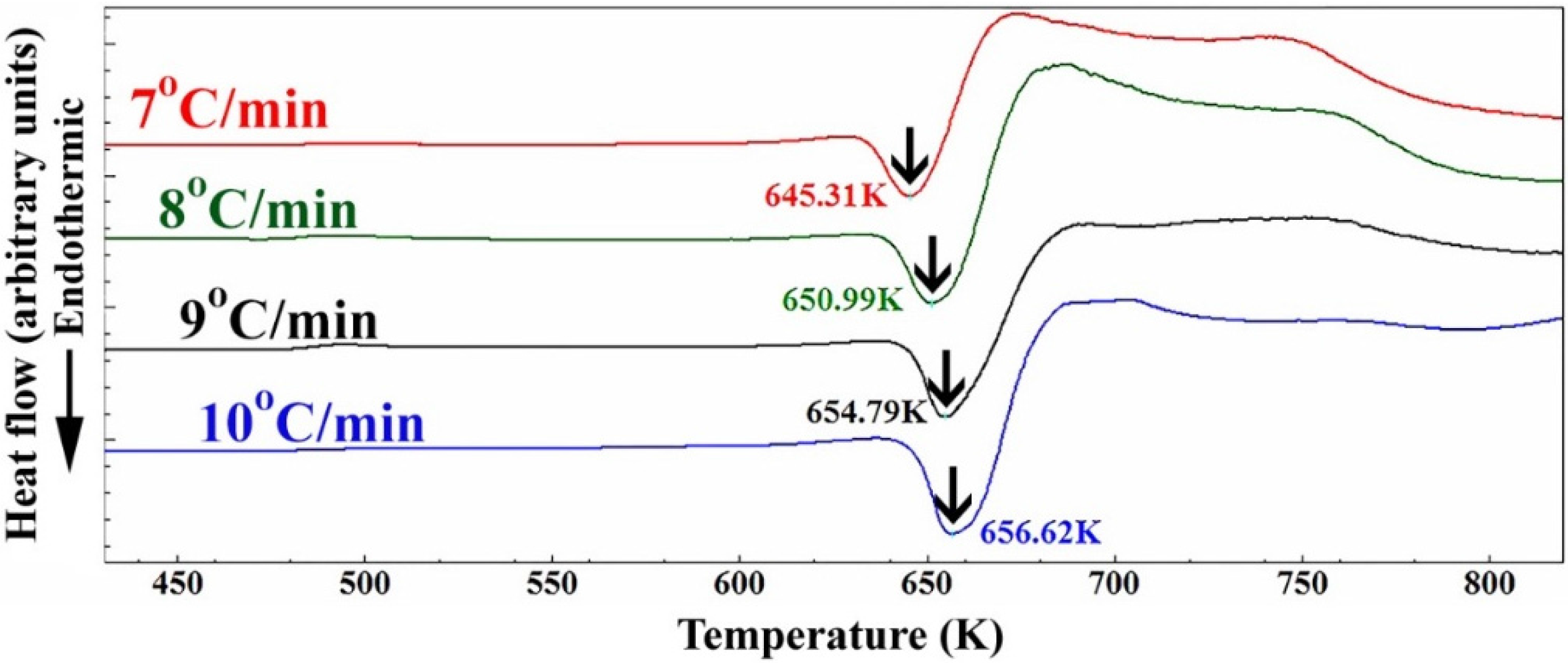
The thermal stability of nanocomposite MgH
2/5.2TiC/4.6FeCr powders obtained after 50 h of the ball milling was investigated by DSC analysis conducted with heating rates (
k) of 7, 8, 9, and 10 °C/min and presented in
Figure 4. All the scans revealed single endothermic events related to the decomposition of MgH
2 phase. While the peak height increased proportionally with increasing heating rates, the peak temperatures (
Tp) were significantly shifted to the higher temperature side upon increasing the heating rates from 7 °C/min to 10 °C/min, as shown in
Figure 4. The peak decomposition temperature performed at a heating rate of 10 °C/min was 658 K (385 °C). When comparing this value with that (441 °C) obtained for nanocrystalline MgH
2 powders [
12], one can say that doping MgH
2 with 5.2 wt. % TiC/4.6 wt. % FeCr powders led to destabilizing the metal hydride phase and decreasing the decomposition temperature by 56 °C.
Figure 3.
(a) STEM-BF; (b) STEM-DF micrographs and the corresponding X-ray elemental mapping of (c) Mg; (d) O; (e) Ti; (f) C; (g) Fe; and (h) Cr for aggregated MgH2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h of ball milling.
Figure 3.
(a) STEM-BF; (b) STEM-DF micrographs and the corresponding X-ray elemental mapping of (c) Mg; (d) O; (e) Ti; (f) C; (g) Fe; and (h) Cr for aggregated MgH2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h of ball milling.
Figure 4.
DSC curves achieved at different heating rates (7, 8, 9, and 10 °C/min) of nanocomposite MgH2/5.2TiC/4.6FeCr powders obtained after 50 h of milling.
Figure 4.
DSC curves achieved at different heating rates (7, 8, 9, and 10 °C/min) of nanocomposite MgH2/5.2TiC/4.6FeCr powders obtained after 50 h of milling.
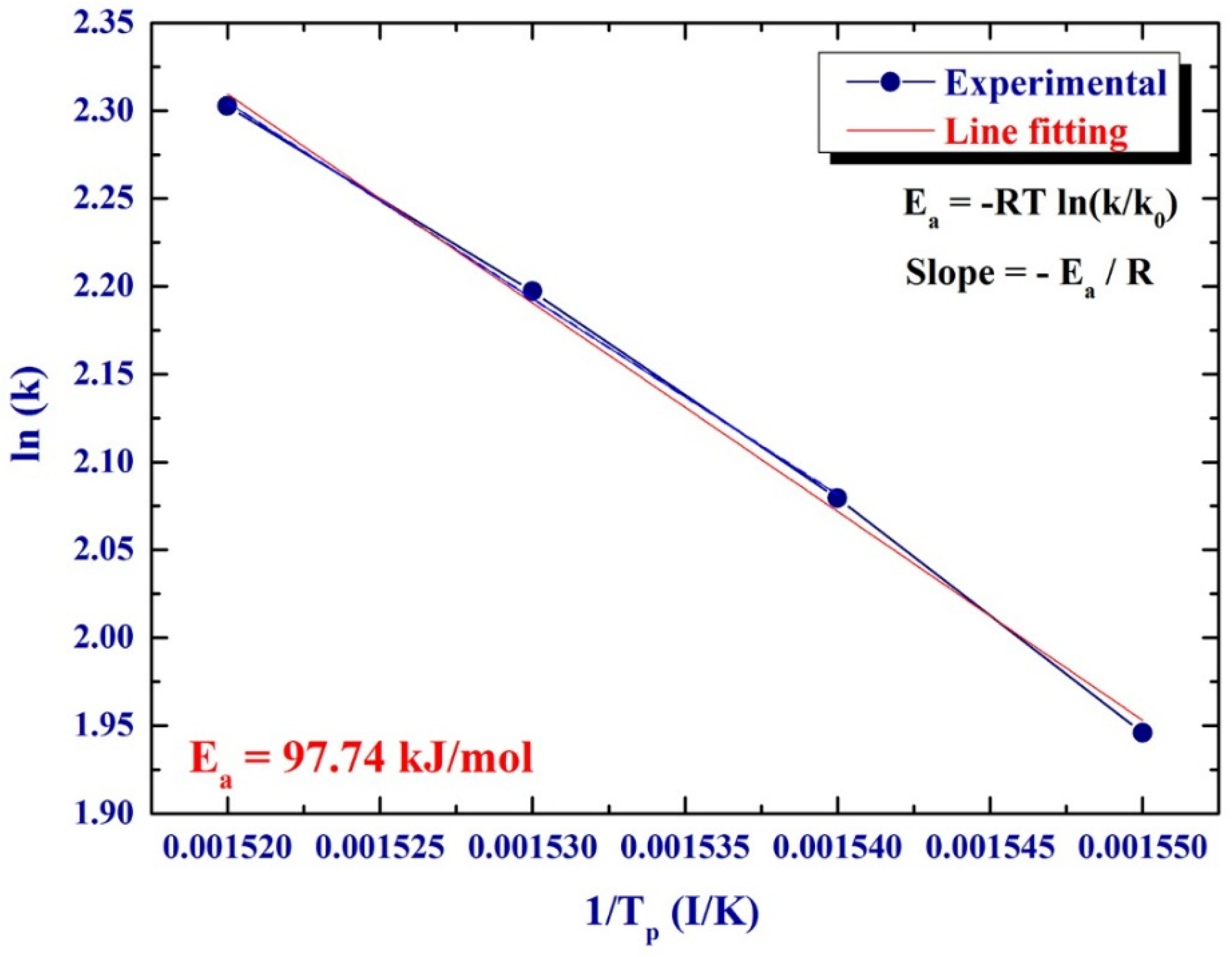
The improved dehydrogenation kinetics in a helium gas atmosphere was investigated by calculating the activation energy (
Ea) of the decomposition reaction. In the present work, the activation energy for dehydrogenation of MgH
2 doped with TiC/FeCr was calculated according to the Arrhenius Equation:
where
k is a temperature-dependent reaction rate constant,
R is the gas constant, and
T is the absolute temperature. The value
Ea of the reaction was determined by measuring the decomposition the
Tp corresponded to the different heating rates (
k) and then plotting ln(
k)
versus 1/
Tp, as shown in
Figure 5. A best fit for the results was calculated by the least-square method. It follows from
Figure 5 that all data points lie closely on the same straight line. The
Ea of 97.74 kJ/mol was obtained from the slope of line (−
E/
R). This value, which is far below than that one (146.53 kJ/mol) calculated for pure MgH
2 powders [
12], indicating a significant improvement of the dehydrogenation kinetics of the MgH
2 upon doping with 5.2TiC/4.6FeCr.
Figure 5.
Arrhenius plot displayed the natural logarithmic values of the heating rates (
k)
versus the inverse of the peak temperature (1/
Tp) denoted in the DSC curves of
Figure 4.
Figure 5.
Arrhenius plot displayed the natural logarithmic values of the heating rates (
k)
versus the inverse of the peak temperature (1/
Tp) denoted in the DSC curves of
Figure 4.
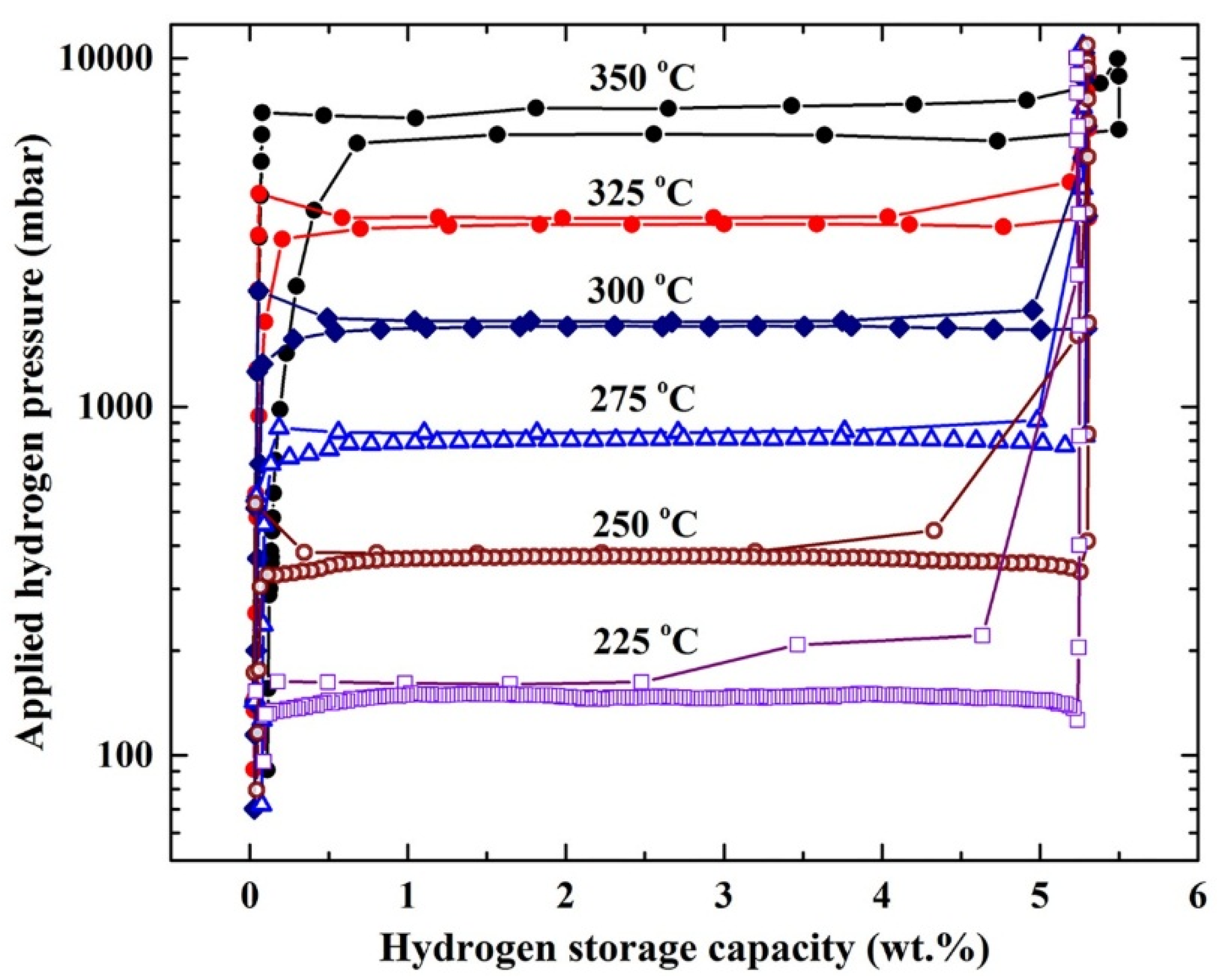
The pressure-composition temperature (PCT) relations of ball-milled MgH
2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h were volumetrically investigated by Sievert’s approach at different temperatures of 225, 250, 275, 300, 325, and 350 °C, as elucidated in
Figure 6. A single reversible hydrogenation/dehydrogenation cycle was developed for each applied temperature. The presence of clear hydrogenation plateaus can be seen in the range between 0.25 and 5.25 wt. % H
2 at temperatures ranging between 275 and 350 °C, as shown in
Figure 6. However, the hydrogen uptake plateau was visible only in the range of 0.25–2.5 and 0.25–3.25 wt. % H
2, at temperatures of 225 °C and 250 °C, respectively. On the other hand, smooth plateaus of hydrogen release were characterized in the whole hydrogen concentrations range (0.25–5.25 wt. % H
2) for all applied temperatures, as presented in
Figure 6. The hydrogen equilibrium pressure measurements were used in the present study to investigate the heat of hydrogen absorption, using van’t Hoff equation:
where
is the hydrogen pressure under equilibrium at a given specific temperature,
T;
P0 is a reference pressure of 1 bar;
R is the gas constant (0.0083145 J/K.mol); Δ
H is the molar enthalpy of metal hydride formation (MgH
2); and Δ
S is the entropy of absorption. Thus, Δ
H can be directly calculated from plotting the natural log of each
point
versus the corresponding 1/
T, as shown in
Figure 7a. In the present work, the calculated ∆
H and ∆
S for MgH
2 doped with 5.2TiC/4.6FeCr was −72.74 kJ/mol and 112.79 J/mol H
2/K, respectively.
The strength of Mg–H bonds, which can be expressed by the enthalpy of decomposition can be calculated by van’t Hoff approach, using the equilibrium dehydrogenation pressure in the PCT measurements. A van’t Hoff plot illustrating the relationship between ln(
P) and 1/
T for the decomposition of MgH
2 powders doped with 5.2TiC/4.6FeCr is shown in
Figure 7b. Both of ∆
H and ∆
S were directly calculated from the slope of the curve presented in
Figure 7b and found to be 76.76 kJ/mol and 119.15 J/mol H
2/K, respectively. Comparing these values with those reported by Reilly (77.4 kJ/mol, 138.3 J/mol H
2/K) [
22], and Klose (81.86 kJ/mol, 146.1 J/mol H
2/K) [
23], one can say that long-term ball milling led to the formation of homogeneous nanocomposite MgH
2/5.2TiC/4.6FeCr powders, destabilizing the chemically stable phase of MgH
2, implied by the obvious increase in the ∆
H of decomposition. Until recently, it was believed that ∆
S has a constant value of about 130 J/mol H
2/K [
24]. It has been suggested by Zhao-Karger
et al. [
24] that ∆
S of the dehydrogenation process can be varied based on the MgH
2 particle size. Based on the
ab initio Hartree-Fock and density functional theory calculations shown by Wagemans
et al. [
25], magnesium hydride becomes less stable with decreases in the cluster size to less than 20 atoms. Accordingly, and based on that study, the ∆
H of hydrogen desorption decreases significantly when the grain size is smaller than 1.3 nm [
25].
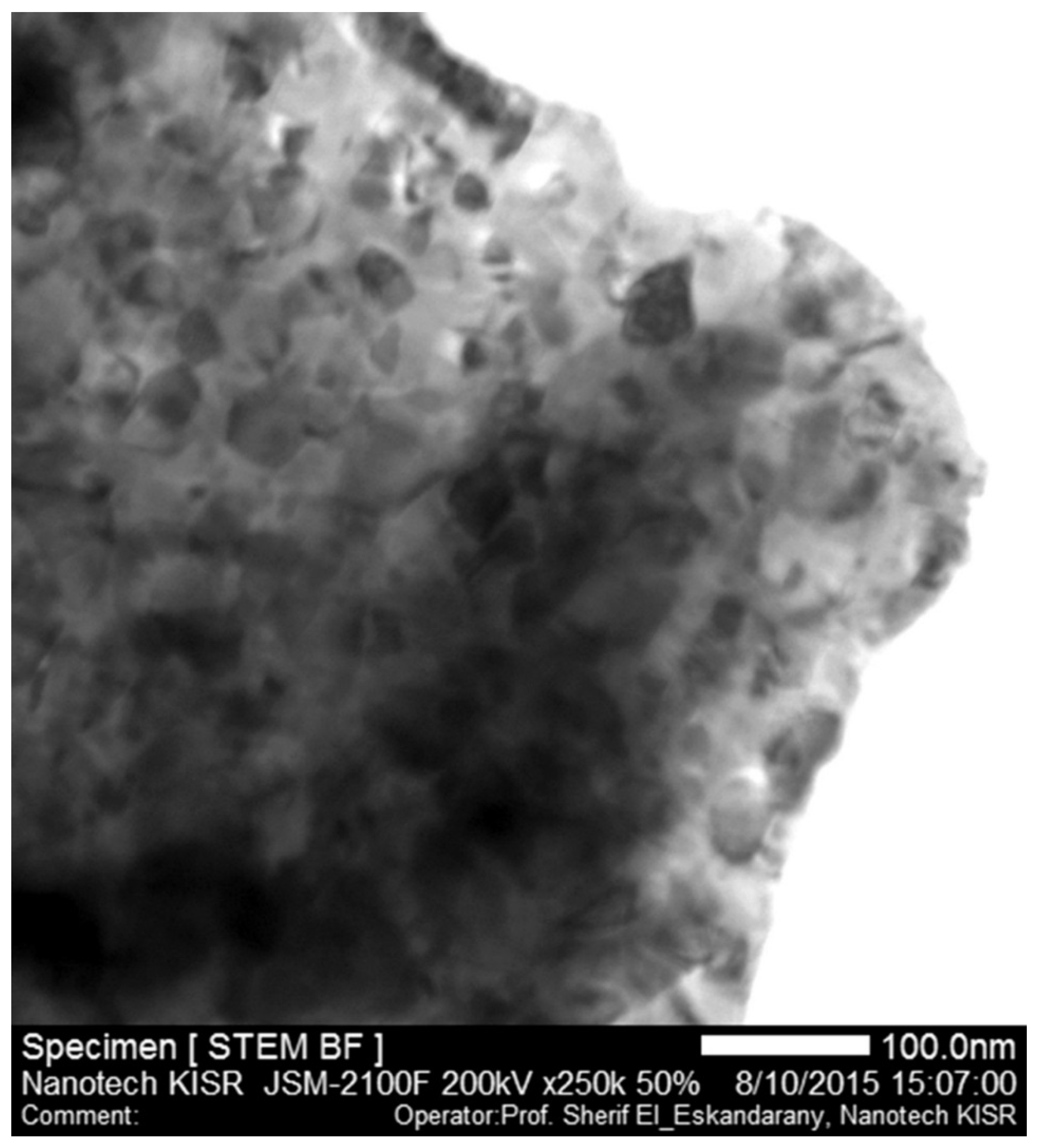
Figure 8 displays the STEM/BF image of the ball-milled nanocomposite sample after the PCT hydrogenation/dehydrogenation measurements under hydrogen gas pressure and temperatures ranging between 0 and 10 bar, and 225 and 350 °C, respectively. Obviously, the sample maintained its nanocrystalline structure ranging between 18 and 67 nm for MgH
2 matrix (light gray-scale particles) and 8 and 27 nm for TiC (dark particles), as shown in
Figure 8. We should emphasize that the as-prepared ultrafine powders in the present study with their nanostructured grains facilitated better hydrogen desorption and shortened the diffusion distance required to accomplish a complete dehydrogenation process. In addition, TiC refractory nanoparticles acted as grain growth inhibitors maintaining the MgH
2 particles, especially when the samples were subjected to the high temperature side (300–350 °C) during the PCT analysis.
Figure 6.
Pressure-composition-temperature (PCT) curves of ball-milled MgH2/5.2 TiC/4.6 FeCr nanocomposite powders obtained after 50 h at different temperatures of 225, 250, 275, 300, 325, and 350 °C.
Figure 6.
Pressure-composition-temperature (PCT) curves of ball-milled MgH2/5.2 TiC/4.6 FeCr nanocomposite powders obtained after 50 h at different temperatures of 225, 250, 275, 300, 325, and 350 °C.
Figure 7.
van’t Hoff plot of the plateaus shown in
Figure 6 for the (
a) hydrogenation, and (
b) dehydrogenation of ball-milled MgH
2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h.
Figure 7.
van’t Hoff plot of the plateaus shown in
Figure 6 for the (
a) hydrogenation, and (
b) dehydrogenation of ball-milled MgH
2/5.2TiC/4.6FeCr nanocomposite powders obtained after 50 h.
Figure 8.
STEM/BF image of ball-milled MgH2/5.2TiC/4.6FeCr nanocomposite sample after achieving the PCT hydrogenation/dehydrogenation measurements under hydrogen gas pressure and temperatures ranging between 0 and 10 bar, and 225 and 350 °C, respectively.
Figure 8.
STEM/BF image of ball-milled MgH2/5.2TiC/4.6FeCr nanocomposite sample after achieving the PCT hydrogenation/dehydrogenation measurements under hydrogen gas pressure and temperatures ranging between 0 and 10 bar, and 225 and 350 °C, respectively.
Figure 9 displays the temperature effect on the hydrogen absorption (a) and consequence desorption (b,c) kinetics of nanocomposite MgH
2/5.2TiC/4.6FeCr powders obtained after 50 h of the ball milling. In general, the synthesized nanocomposite powders showed excellent potential for absorbing hydrogen gas in a short time at temperatures ranging from 250 to 275 °C under pressure ranging from 100 mbar to 8 bar, as shown in
Figure 9a. After 1 min, the powders examined at 250 and 275 °C were able to uptake 3.66 and 4.55 wt. % H
2, respectively as elucidated in
Figure 9a. After 11.2 min of the absorption, the sample examined at 275 °C reached its saturated value with hydrogen storage reaching 5.51 wt. %. In contrast, 19.2 min was required for the sample examined at 250 °C to absorb 5.41 wt. % H
2, as shown in
Figure 9a.
Figure 9.
Effect of temperature and time on the (
a) hydrogenation; and (
b) dehydrogenation kinetics of nanocomposite MgH
2/5.2TiC/4.6FeCr powders obtained after ball milling for 50 h. The dehydrogenation kinetics measured at 250 °C (open symbols) and 275 °C (closed symbols) after 5 min of desorption are presented in
Figure 9c.
Figure 9.
Effect of temperature and time on the (
a) hydrogenation; and (
b) dehydrogenation kinetics of nanocomposite MgH
2/5.2TiC/4.6FeCr powders obtained after ball milling for 50 h. The dehydrogenation kinetics measured at 250 °C (open symbols) and 275 °C (closed symbols) after 5 min of desorption are presented in
Figure 9c.
The corresponding desorption kinetics of the nanocomposite powders investigated at 250 °C and 275 °C are shown in
Figure 9b,c. The powders examined at 275 °C showed excellent desorption kinetics, indexed by the relatively short time (~10 min) required to release about 5.51 wt. % of hydrogen, as shown in
Figure 9b. The sample examined at this temperature desorbed 1.62 wt. % of hydrogen within a short desorption time of 2.5 min, as shown in
Figure 9c. At this applied temperature, the sample released about 3.43 wt. % of its hydrogen storage capacity after 5 min of desorption, as elucidated in
Figure 7c. In contrast to such fast desorption kinetics achieved at 275 °C, the sample examined at 250 °C showed a slow dehydrogenation behavior, indexed by the long time required to release its full hydrogen content (~5.5 wt. %), 79 min, as shown in
Figure 9b. After 2.5 and 5 min of desorption conducted at 250 °C (
Figure 9c), the sample was unable to release more than 0.32, and 0.80 H
2 wt. %, respectively, as presented in
Figure 9c. Aside from the particle size effect on the ∆
H and ∆
S of hydrogen desorption for MgH
2, the dehydrogenation temperature decreased from 400 °C in bulk MgH
2 to be 250–275 °C, when the crystallite size of MgH
2 was less than 10 nm in diameter (
Figure 2a).
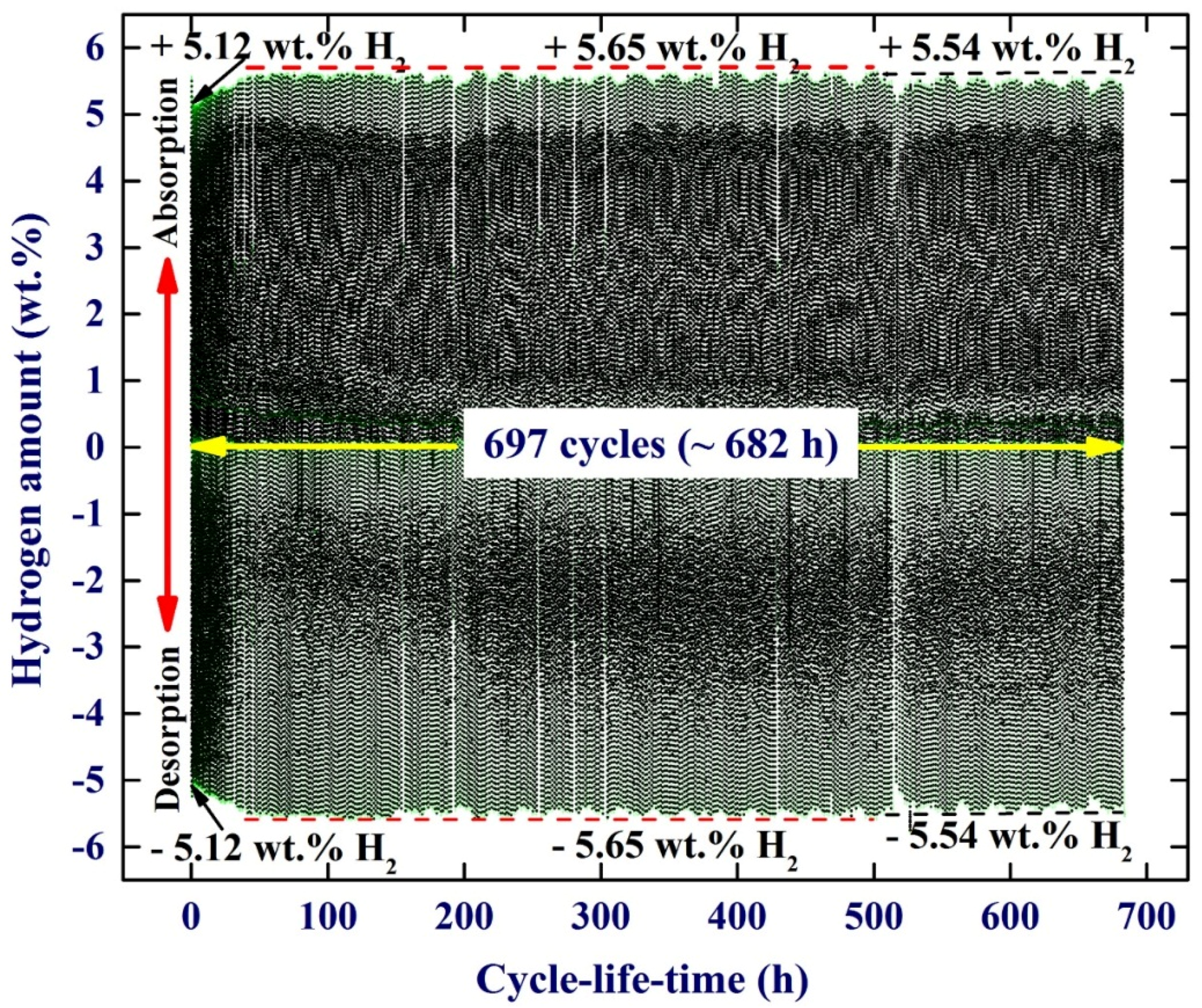
Apart from the fast kinetics of hydrogenation/dehydrogenations characterizations shown by MgH
2/5.2TiC/4.6FeCr ternary system, the cyclic-reversibility of the fabricated nanocomposite powders examined at 275 °C under repeated hydrogenation/dehydrogenation pressure of 0/8 bar was investigated.
Figure 10 shows the cycle-life-time performed at 275 °C for the nanocomposite powders obtained after 50 h of ball milling. Obviously, this new nanocomposite system exhibits excellent cyclic-reversible properties, indexed by its high cyclic stability without failure, even after about 682 h (679 cycles), as shown in
Figure 10. Comparing the number of cycles achieved at 275 °C by this nanocomposite system with those performed in MgH
2/Mn
3.6Ti
2.4, 1000 cycles/275 °C [
16], MgH
2/5Ni5Nb
2O
5, 180 cycles/250 °C [
26], MgH
2/5Fe 47 cycles/300 °C [
27], and MgH
2/10Co 350 °C [
25] systems, one can consider the MgH
2/TiC/FeCr system as one of the most stable and capable MgH
2-based nanocomposite systems used for hydrogen storage applications.
Figure 10.
(a) Hydrogenation and consequent dehydrogenation curves of 697 complete cycles performed within 682 h for nanocomposite MgH2/5.2TiC/4.6FeCr powders obtained after 50 h of ball milling. The hydrogen absorption and desorption processes were achieved at a constant temperature of 275 °C with an applied pressure of 100 mbar/8 bar.
Figure 10.
(a) Hydrogenation and consequent dehydrogenation curves of 697 complete cycles performed within 682 h for nanocomposite MgH2/5.2TiC/4.6FeCr powders obtained after 50 h of ball milling. The hydrogen absorption and desorption processes were achieved at a constant temperature of 275 °C with an applied pressure of 100 mbar/8 bar.