Synthesis of Co3O4 Cotton-Like Nanostructures for Cholesterol Biosensor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological and Structural Characteristics
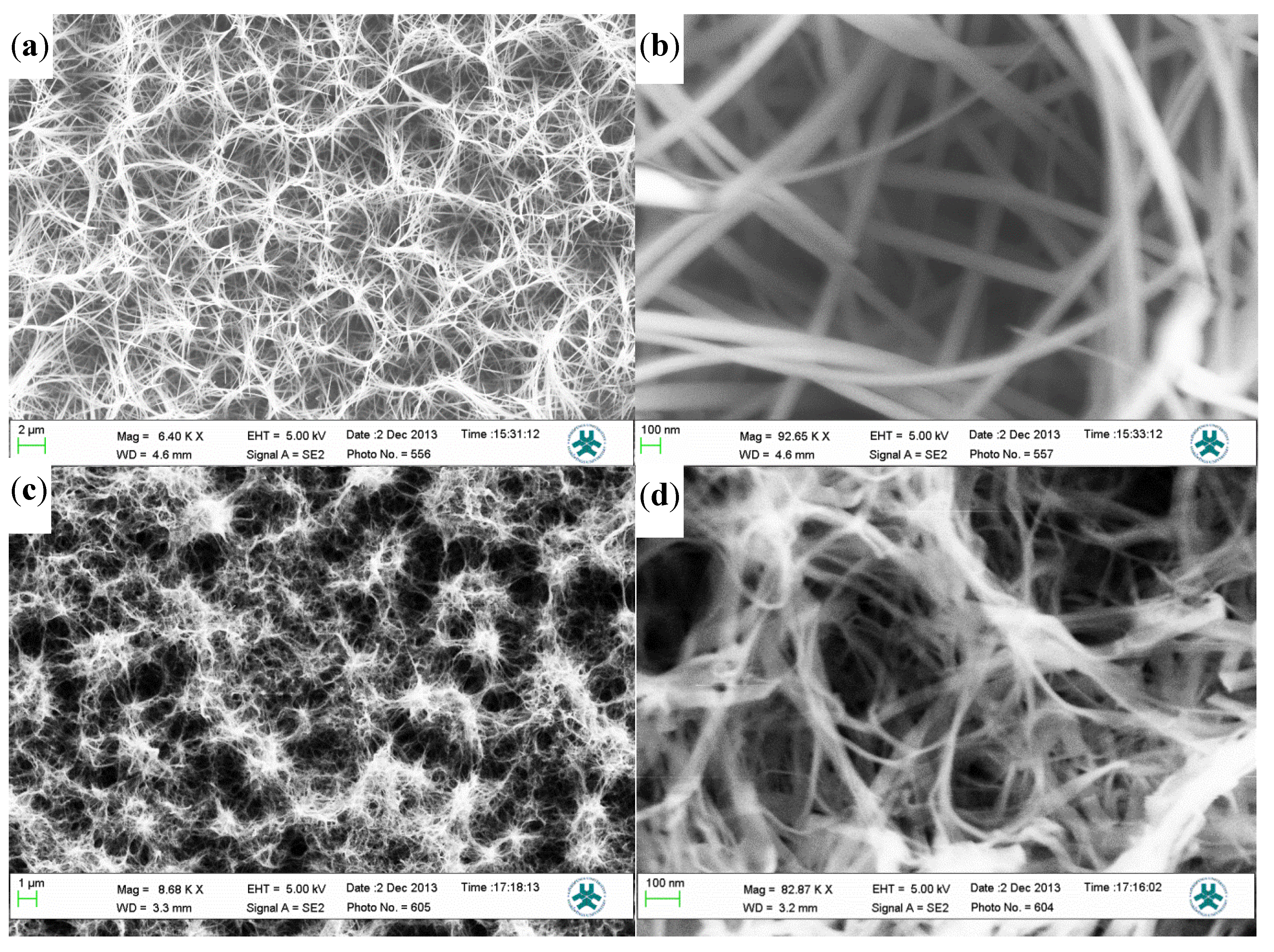

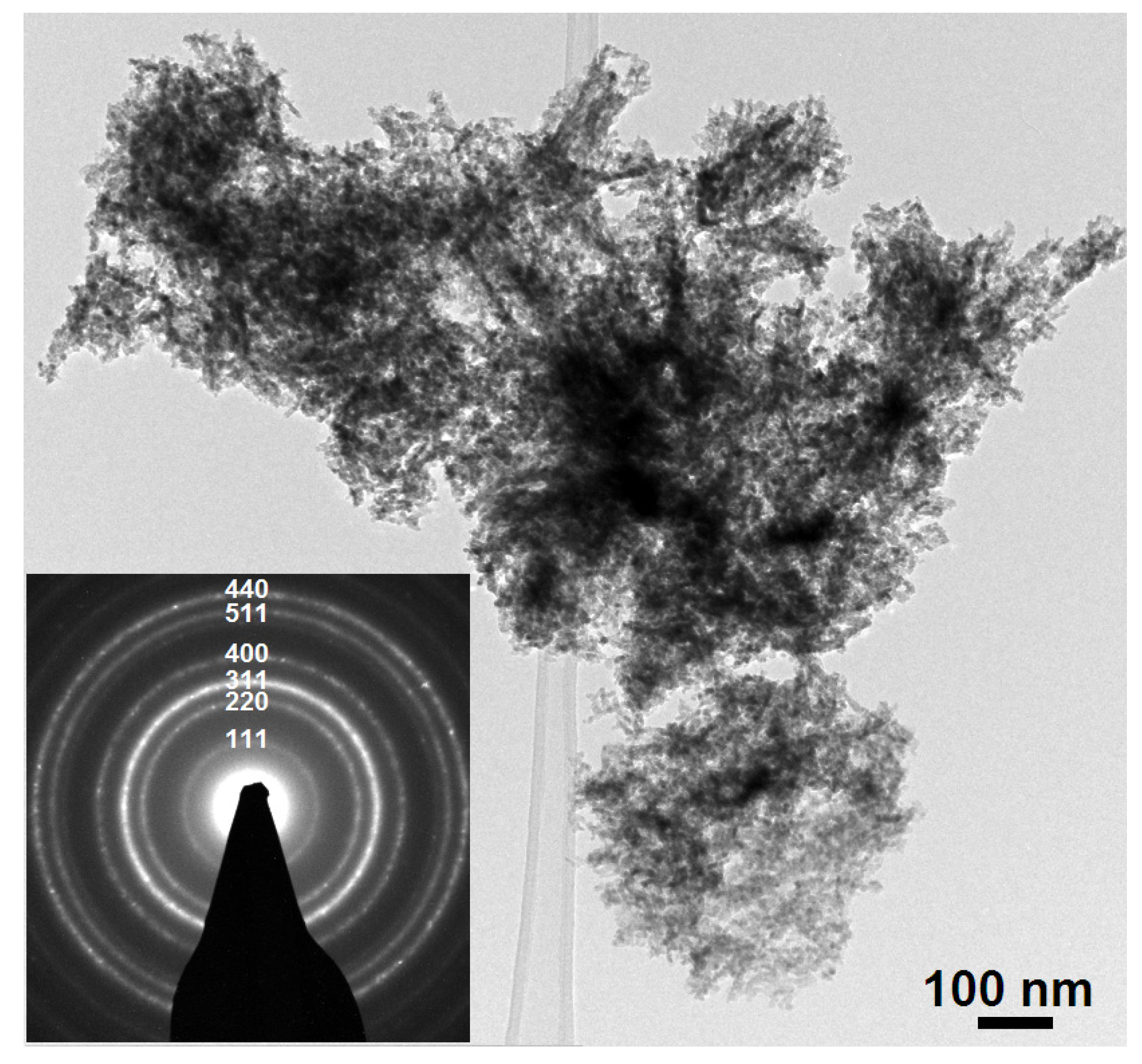


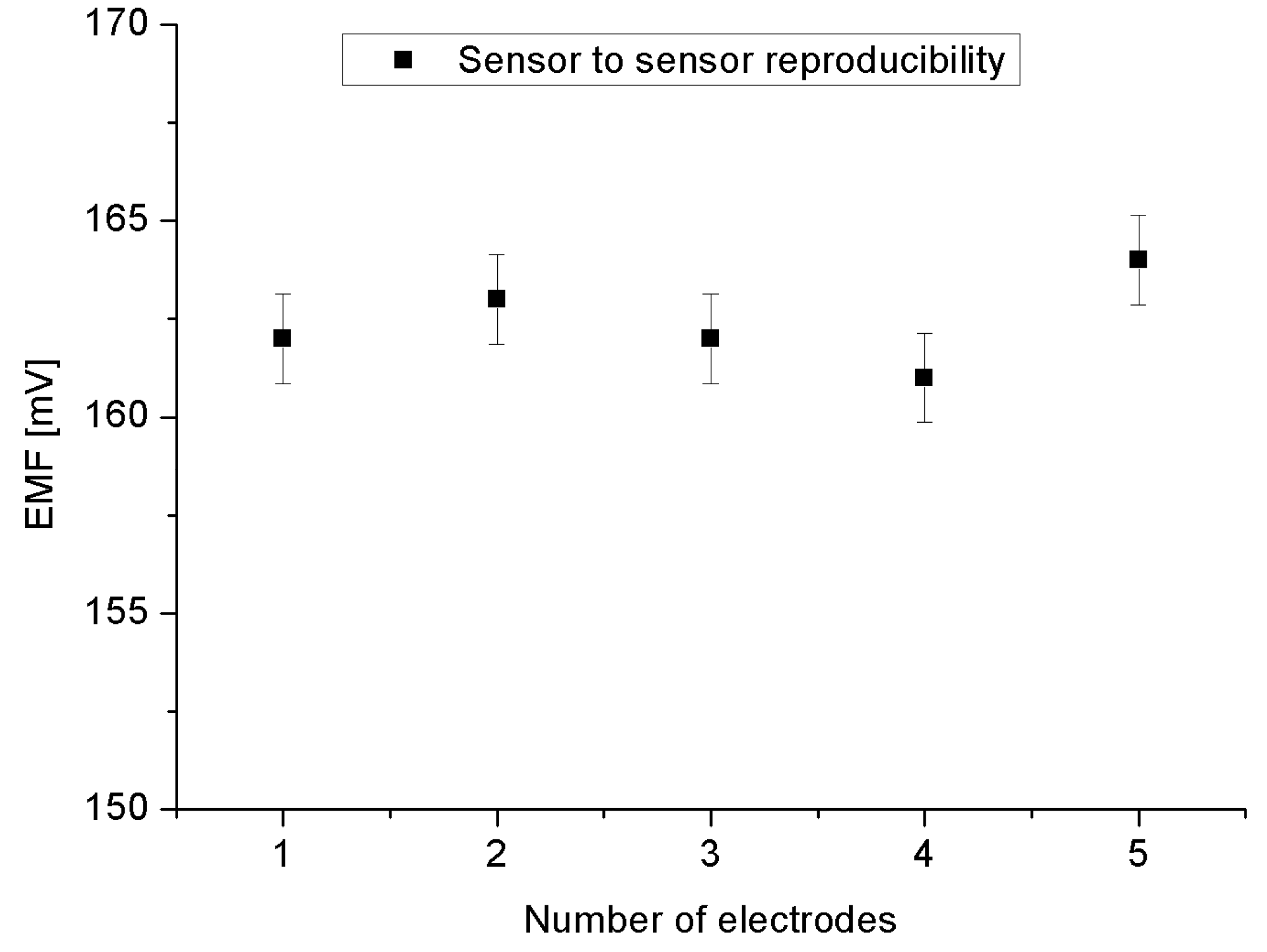
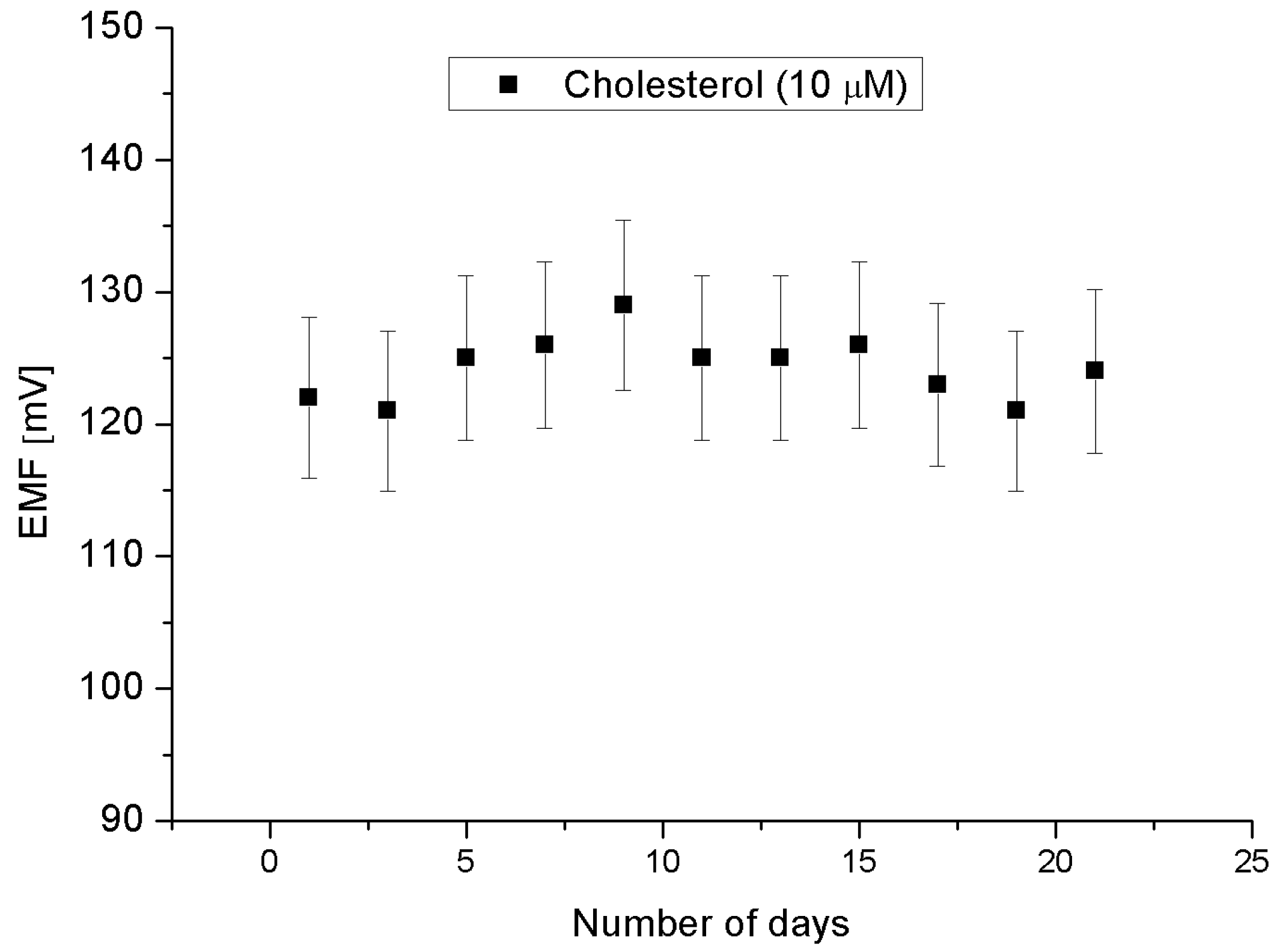
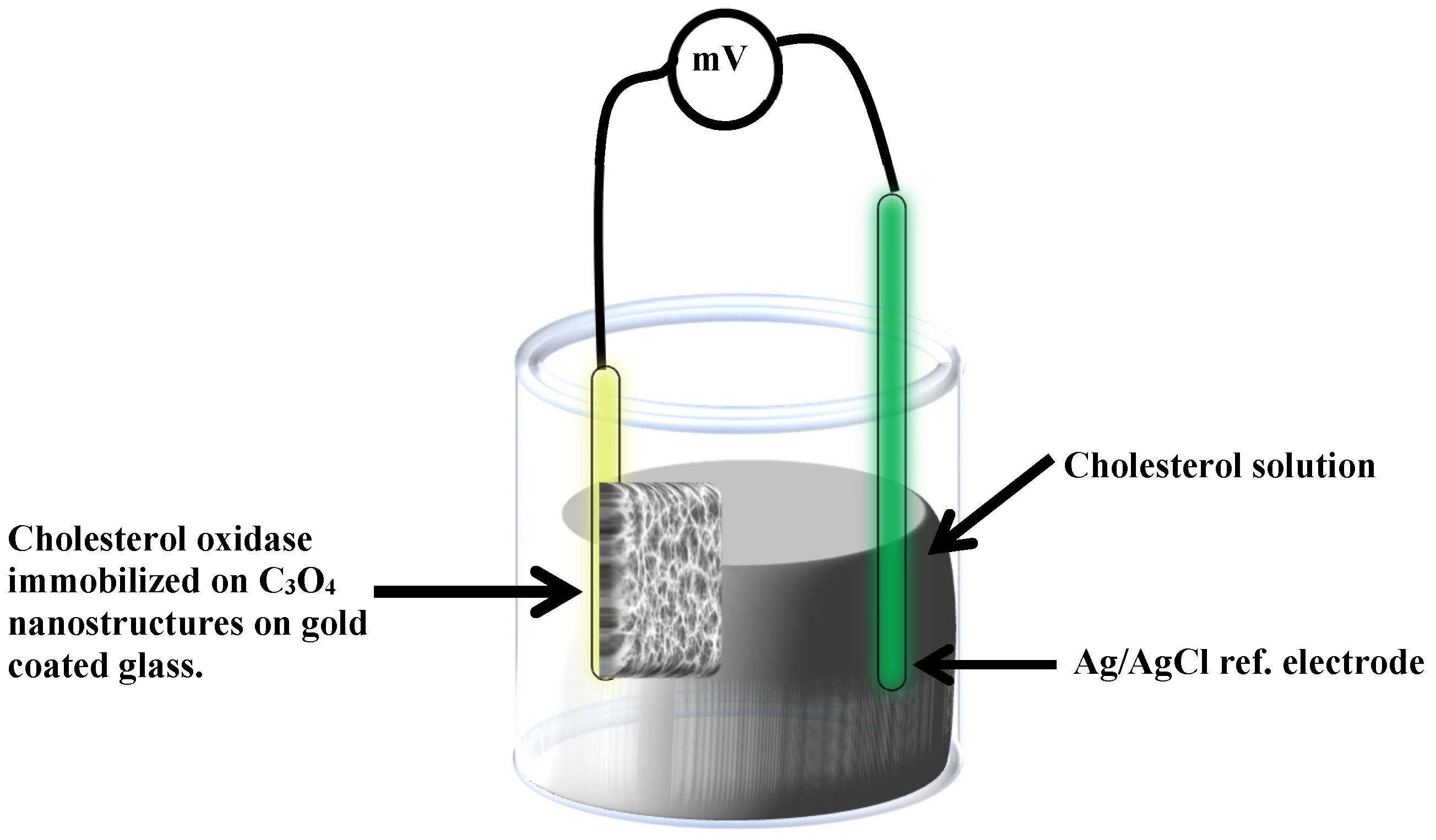
2.2. Cholesterol Biosensor Performance



3. Experimental Section
3.1. Chemicals
3.2. Synthesis of Co3O4 Nanostructures
3.3. Instrumentations
3.4. Immobilization of Co3O4 Nanostructures with Cholesterol Oxidase and Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patnaik, P. Handbook of Inorganic Chemicals; The McGraw-Hill Companies: New York, NY, USA, 2003; p. 252. [Google Scholar]
- Koumoto, K.; Yanagida, H. Electrical conduction in pure and Li-substituted Co3O4. Am. Ceram. Soc. 1981, 64, C-156–C-157. [Google Scholar] [CrossRef]
- Jansson, J.; Palmqvist, A.E.C.; Fridell, E.; Skoglundh, M.; Österlund, L.; Thormählen, P.; Langer, V. On the catalytic activity of Co3O4 in low-temperature CO oxidation. J. Catal. 2002, 211, 387–397. [Google Scholar] [CrossRef]
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Patil, P.S.; Kadam, L.D.; Lokhande, C.D. Preparation and characterization of spray pyrolysed cobalt oxide thin films. Thin Solid Films 1996, 272, 29–32. [Google Scholar] [CrossRef]
- Roth, W.L. The magnetic structure of Co3O4. J. Phys. Chem. Solids 1964, 25, l–10. [Google Scholar] [CrossRef]
- Chen, J.; Selloni, A. Electronic states and magnetic structure at the Co3O4 (110) surface: A first-principles study. Phys. Rev. B 2012, 85, 085306:1–085306:29. [Google Scholar]
- Li, W.-Y.; Xu, L.-N.; Chen, J. Co3O4 Nanomaterials in lithium-ion batteries and gas sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, J.; Lou, Z.; Zhang, T. Cross-linked p-type Co3O4 octahedral nanoparticles in 1D n-type TiO2 nanofibers for high-performance sensing devices. J. Mater. Chem. A 2014, 2, 10022–10028. [Google Scholar] [CrossRef]
- Narayan, R.V.; Kanniah, V.; Dhathathreyan, A. Tuning size and catalytic activity of nano-clusters of cobalt oxide. J. Chem. Sci. 2006, 118, 179–184. [Google Scholar] [CrossRef]
- Kittaka, S.; Morimoto, T. Isoelectric point of metal oxides and binary metal oxides having spinel structure. J. Colloid Interface 1980, 75, 398–403. [Google Scholar] [CrossRef]
- Henrich, V.E. The nature of transition-metal-oxide surfaces. Prog. Surf. Sci. 1983, 14, 175–200. [Google Scholar] [CrossRef]
- Liao, L.; Lu, H.B.; Li, J.C.; He, H.; Wang, D.F.; Fu, D.J.; Liu, C.; Zhang, W.F. Size dependence of gas sensitivity of ZnO nanorods. J. Phys. Chem. C 2007, 111, 1900–1903. [Google Scholar] [CrossRef]
- Pearce, T.C.; Schiffman, S.S.; Nagle, H.T.; Gardner, J.W. Introduction to chemosensors. In Handbook of Machine Olfaction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; Chapter 4; pp. 79–103. [Google Scholar]
- Dan, Y.; Evoy, S.; Johnson, A.T. Chemical gas sensors based on nanowires. In Nanowire Research Progress; Nova Science Publisher: Hauppauge, NY, USA, 2008; Chapter 3. [Google Scholar]
- Ibupoto, Z.H.; Elhag, S.; Nur, O.; Willander, M. Fabrication of sensitive potentiometric cholesterol biosensor based on Co3O4 interconnected nanowires. Electroanalysis 2014, 26, 1928–1934. [Google Scholar] [CrossRef]
- Morris, T.G. A comparison of methods for the estimation of serum cholesterol and values in random samples of populations in the 55–64 age group. J. Clin. Pathol. 1959, 12, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, E.; Nicelli, M.; Capri, E.; Trevisan, M.; Del Re, A.A.M. Cholesterol, β-sitosterol, ergosterol, and coprostanol in agricultural soils. J. Environ. Qual. 2003, 32, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Sperry, W.M.; Brand, F.C. The colorimetric determination of cholesterol. J. Biol. Chem. 1943, 150, 315–324. [Google Scholar]
- Missel, P.J.; Mazer, N.A.; Benedek, G.B.; Young, C.Y.; Carey, M.C. Thermodynamic analysis of the growth of sodium dodecyl sulfate micelles. J. Phys. Chem. 1980, 84, 1044–1057. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta 2000, 1508, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Baghalha, M.; Ahmadi, M.K. The effects of sodium dodecyl sulfate and carbon nanotubes on copper corrosion rates. In Proceedings of the 220th ECS Meeting, Boston, MA, USA, 9–14 October 2011; The Electrochemical Society: Pennington, NJ, USA, 2011; p. 1670. [Google Scholar]
- Inamdar, A.I.; Mujawar, S.H.; Ganesan, V.; Patil, P.S. Surfactant-mediated growth of nanostructured zinc oxide thin films via electrodeposition and their photoelectrochemical performance. Nanotechnology 2008, 19, 325706:1–325706:7. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Holze, R.; Jadhav, H.S.; Lokhande, C.D.; Park, C.-J. Surfactant-assisted morphological tuning of hierarchical CuO thin films for electrochemical supercapacitors. Dalton Trans. 2013, 42, 6459–6467. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Raitano, J.M.; Zhang, L.; Chan, S.-W. Controlled synthesis of Co3O4 nanopolyhedrons and nanosheets at low temperature. Chem. Commun. 2009, 48, 7569–7571. [Google Scholar] [CrossRef]
- Klepper, K.B.; Nilsen, O.; Fjellvåg, H. Growth of thin films of Co3O4 by atomic layer deposition. Thin Solid Films 2007, 515, 7772–7781. [Google Scholar] [CrossRef]
- Miedzinska, K.M.E.; Hollebone, B.R. An assignment of the optical absorption spectrum of mixed valence Co3O4 spinel films. J. Phys. Chem. Solids 1987, 48, 649–656. [Google Scholar] [CrossRef]
- McNaught, A.D. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-G.; Wu, Q.-S.; Liu, W.-Z.; Ding, Y.-P. Simultaneous determination of dinitrophenol isomers with electrochemical method enhanced by surfactant and their mechanisms research. Electrochim. Acta 2006, 52, 589–594. [Google Scholar] [CrossRef]
- Elhag, S.; Ibupotoa, Z.H.; Liu, X.; Nur, O.; Willander, M. Dopamine wide range detection sensor based on modified Co3O4 nanowires electrode. Sens. Actuators B 2014, 203, 543–549. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Lan, W.-J.; Chen, C.-H. Redox preparation of mixed-valence cobalt manganese oxide nanostructured materials: Highly efficient noble metal-free electrocatalysts for sensing hydrogen peroxide. Nanoscale 2014, 6, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.P.; Linder, E. Recommendations for nomenclature of Ion-selective electrodes. Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Zhuang, Z.; Sheng, W.; Yan, Y. Synthesis of monodispere Au Co3O4 core shell nanocrystals and their enhanced activity for oxygen evolution reaction. Adv. Mater. 2014, 26, 3950–3955. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhag, S.; Ibupoto, Z.H.; Nour, O.; Willander, M. Synthesis of Co3O4 Cotton-Like Nanostructures for Cholesterol Biosensor. Materials 2015, 8, 149-161. https://doi.org/10.3390/ma8010149
Elhag S, Ibupoto ZH, Nour O, Willander M. Synthesis of Co3O4 Cotton-Like Nanostructures for Cholesterol Biosensor. Materials. 2015; 8(1):149-161. https://doi.org/10.3390/ma8010149
Chicago/Turabian StyleElhag, Sami, Zafar Hussain Ibupoto, Omer Nour, and Magnus Willander. 2015. "Synthesis of Co3O4 Cotton-Like Nanostructures for Cholesterol Biosensor" Materials 8, no. 1: 149-161. https://doi.org/10.3390/ma8010149
APA StyleElhag, S., Ibupoto, Z. H., Nour, O., & Willander, M. (2015). Synthesis of Co3O4 Cotton-Like Nanostructures for Cholesterol Biosensor. Materials, 8(1), 149-161. https://doi.org/10.3390/ma8010149





