Preparation, Characterization and Performances of Powdered Polycarboxylate Superplasticizer with Bulk Polymerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Initiator, Macromonomer and Reaction Temperature on the Fluidities of Cement Paste
| Composition | SiO2/% | CaO/% | Al2O3/% | Fe2O3/% | MgO/% | SO3/% | Na2O/% |
| Reference cement | 22.93 | 66.23 | 4.29 | 2.89 | 1.92 | 0.35 | 0.70 |
| Composition | C3S/% | C2S/% | C3A/% | C4AF/% | Loss/% | f-CaO/% | – |
| Reference cement | 58.78 | 21.38 | 6.49 | 8.77 | 1.48 | 0.64 |
| Initiator | Macromonomer | Temperature/°C | Fluidity/mm | ||
|---|---|---|---|---|---|
| 5 min | 1 h | 2 h | |||
| BPO | IPEG | 80 | – | – | – |
| TPEG | 80 | – | – | – | |
| IPEG | 85 | 210 ± 5 | 80 ± 10 | – | |
| TPEG | 85 | – | – | – | |
| IPEG | 90 | – | – | – | |
| TPEG | 90 | – | – | – | |
| AIBN | IPEG | 75 | 315 ± 5 | 180 ± 5 | 165 ± 0 |
| TPEG | 75 | 247.5 ± 2.5 | 160 ± 5 | 100 ± 10 | |
| IPEG | 80 | 295 ± 0 | 130 ± 10 | 120 ± 5 | |
| TPEG | 80 | 260 ± 0 | 255 ± 5 | 250 ± 0 | |
| IPEG | 85 | 187.5 ± 2.5 | 85 ± 10 | – | |
| TPEG | 85 | 197.5 ± 2.5 | 190 ± 5 | 147.5 ± 2.5 | |
2.2. Effects of Addition of the Third Monomer on the Fluidities of Cement Paste
| Third Monomer | Fluidity/mm | ||
|---|---|---|---|
| 5 min | 1 h | 2 h | |
| MA | 137.5 ± 2.5 | – | – |
| FA | 280 ± 5 | 245 ± 5 | 210 ± 10 |
| None | 260 ± 0 | 255 ± 5 | 250 ± 0 |
2.3. 1H NMR of PCE

2.4. Molecular Weight of PCE
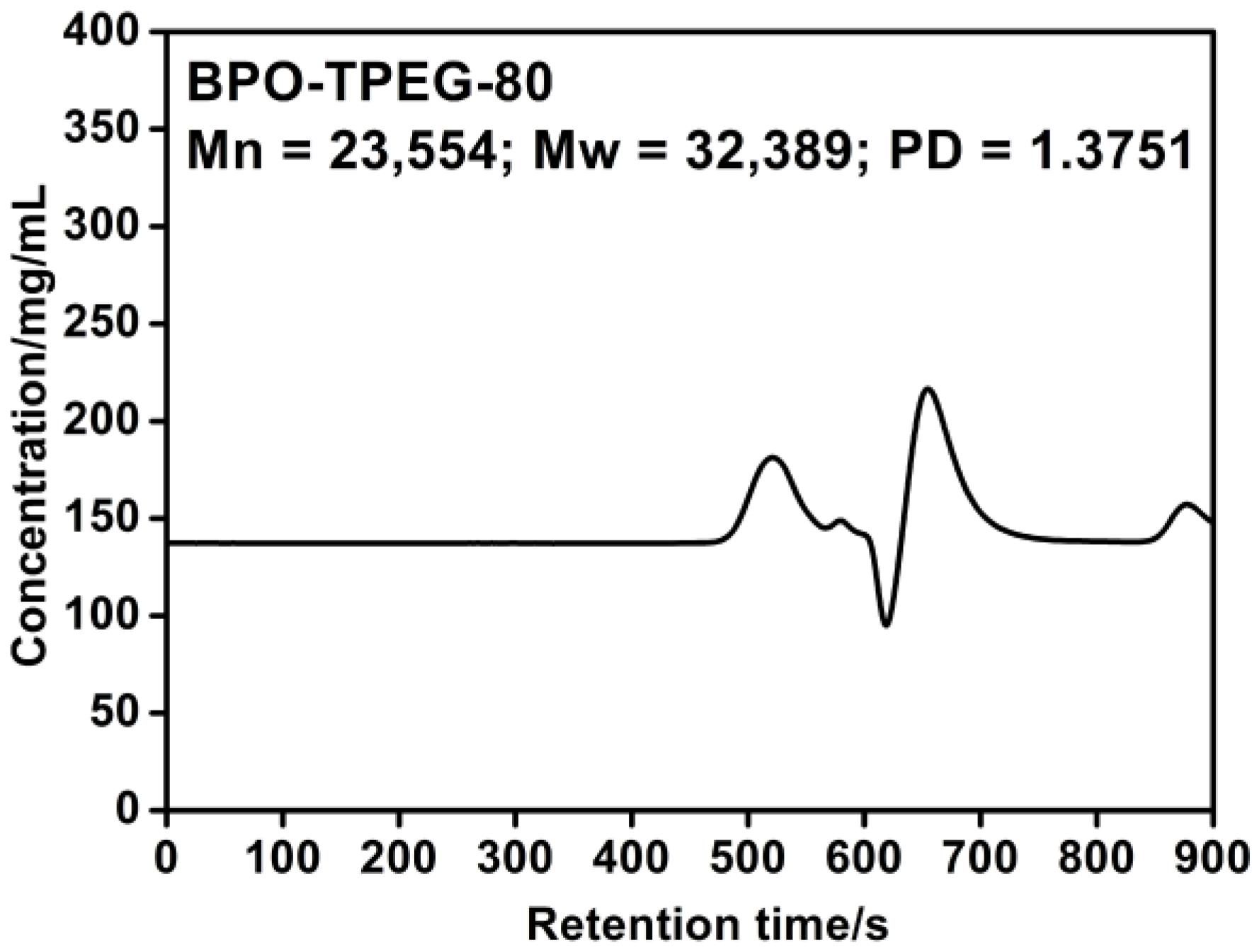
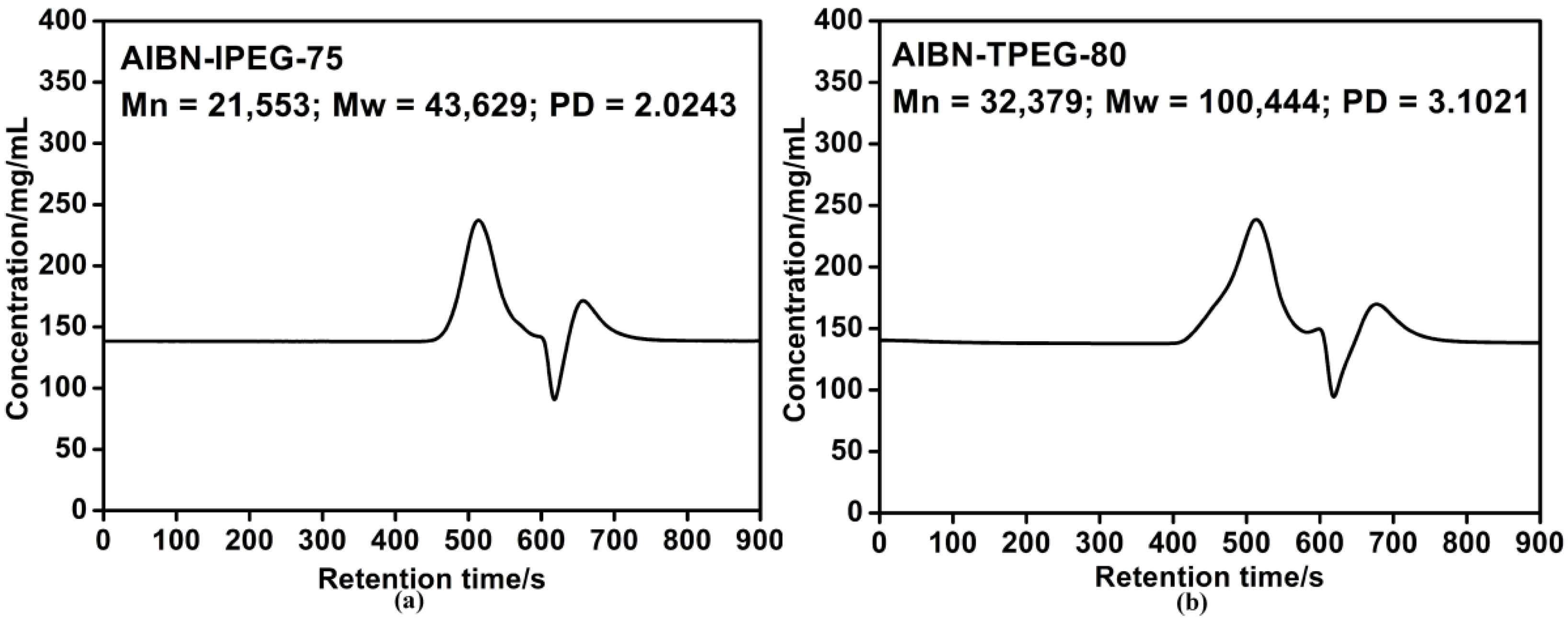
2.5. Setting Times of Cement Pastes
| Sample | Dosage/% | Water/g | Setting Time | |
|---|---|---|---|---|
| Initial Setting/min | Final Setting/min | |||
| Blank | 0 | 138 | 197 ± 3 | 250 ± 5 |
| AIBN-IPEG-75 | 0.2 | 115.1 | 197 ± 5 | 240 ± 2 |
| AIBN-IPEG-80 | 0.2 | 117.4 | 178 ± 1 | 235 ± 3 |
| AIBN-TPEG-75 | 0.2 | 116.2 | 160 ± 2 | 210 ± 5 |
| AIBN-TPEG-80 | 0.2 | 124.2 | 184 ± 2 | 259 ± 3 |
| AIBN-TPEG-85 | 0.2 | 122.9 | 201 ± 3 | 233 ± 3 |
| AIBN-TPEG-FA-80 | 0.2 | 118.1 | 189 ± 1 | 225 ± 4 |
2.6. Hydration Heat
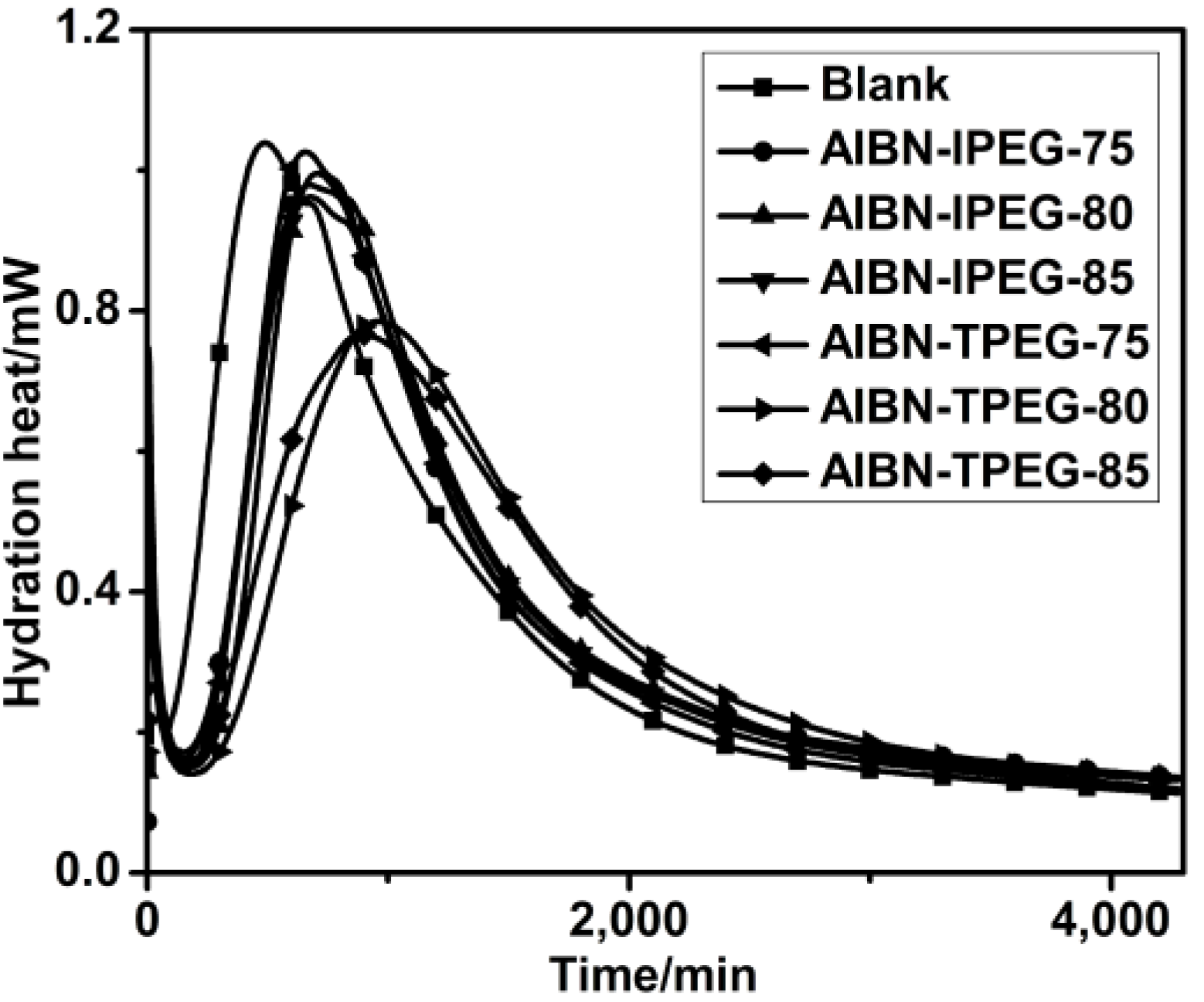
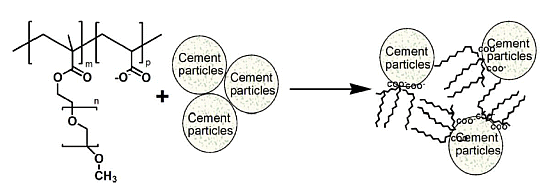
2.7. Preparation and Dispersion Mechanisms of PCE
3. Experimental Section
3.1. Materials
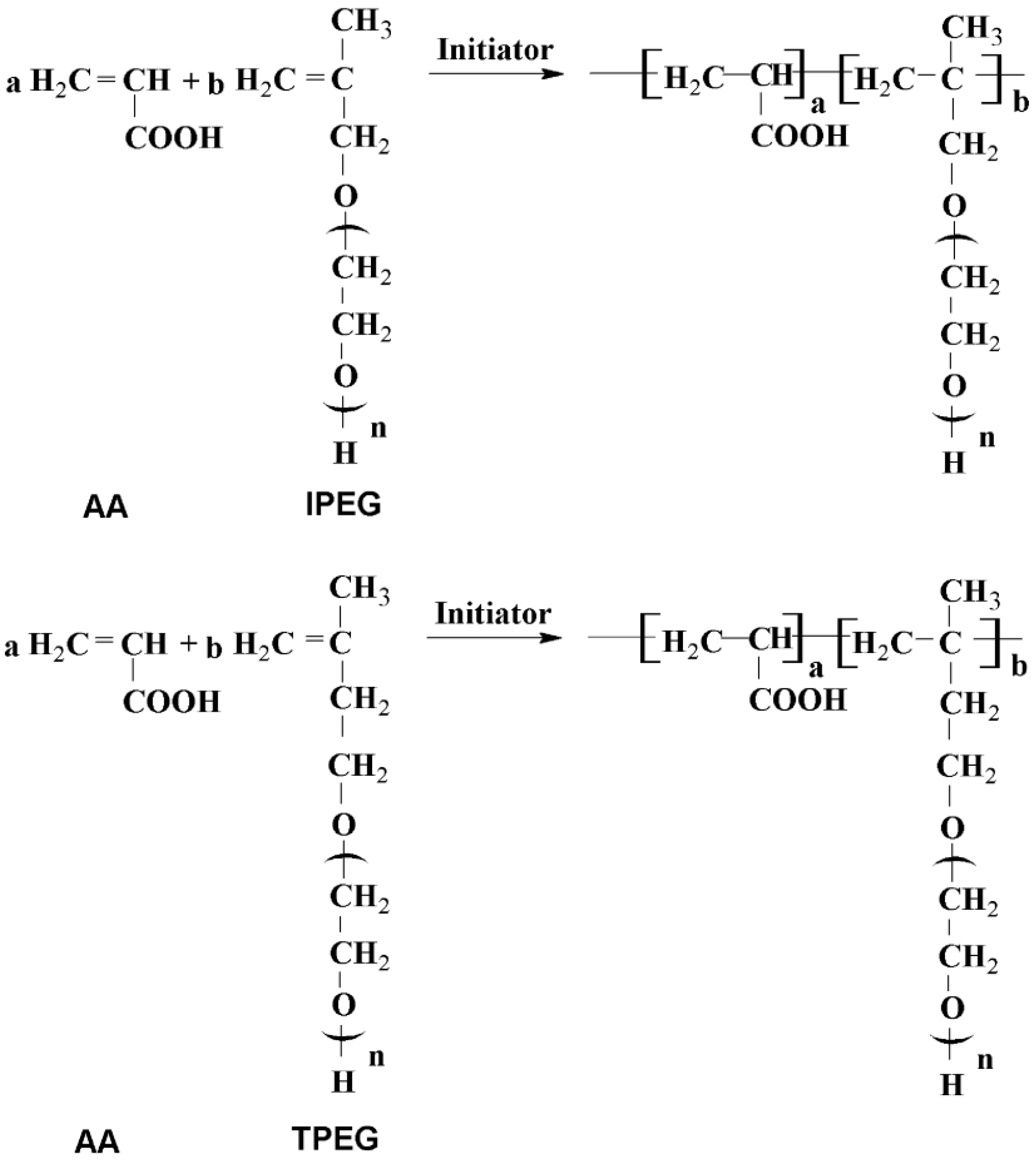
3.2. Synthesis of PCE
3.3. Preparation of Powdered PCE
3.4. Tests and Measurements
3.4.1. 1H NMR Analysis
3.4.2. Molecular Weight and Its Distribution
3.4.3. Fluidity of Cement Paste
3.4.4. Setting Times of Cement Paste
3.4.5. Hydration Heat
4. Conclusions
Acknowledgments
Author Contrbutions
Conflicts of Interest
References
- Meddah, M.S.; Suzuki, M.; Sato, R. Influence of a combination of expansive and shrinkage-reducing admixture on autogenous deformation and self-stress of silica fume high-performance concrete. Constr. Build. Mater. 2011, 25, 239–250. [Google Scholar] [CrossRef]
- Piekarczyk, B.Ł. The type of air-entraining and viscosity modifying admixtures and porosity and frost durability of high performance self-compacting concrete. Constr. Build. Mater. 2013, 40, 659–671. [Google Scholar] [CrossRef]
- Laskar, A.I.; Talukdar, S. Rheological behavior of high performance concrete with mineral admixtures and their blending. Constr. Build. Mater. 2008, 22, 2345–2354. [Google Scholar] [CrossRef]
- Pingkun, C.; Yawnan, P. Influence of mixing techniques on properties of high performance concrete. Cem. Concr. Res. 2001, 31, 87–95. [Google Scholar] [CrossRef]
- Kinoshita, M.; Nawa, T.; Lida, M.; Lchiboji, H. Effect of chemical structure on fluidizing mechanism of concrete superplasticizer containing polyethylene oxide graft chains. In Proceedings of the 6th International Conferences on Superplasticizers and other chemical admixtures in concrete (CANMET/ACI), Nice, France, 10–13 October 2000.
- Lu, S.H.; Liu, G.; Ma, Y.F.; Li, F. Synthesis and application of a new vinyl copolymer superplasticizer. J. Appl. Polym. Sci. 2010, 117, 273–280. [Google Scholar]
- Sakai, E.; Kawakami, A.; Daimon, M. Dispersion mechanisms of comb-type superplasticizers containing grafted poly (ethylene oxide) chains. Macromol. Symp. 2001, 175, 367–376. [Google Scholar] [CrossRef]
- Comparet, C.; Nonat, A.; Pourchet, S.; Mosquet, M.; Maitrasse, P. The molecular parameters and the effect of comb-type superplasticizers on self-compacting concrete: A comparison of comb-type superplasticizer adsorption onto a basic calcium carbonate medium in the presence of sodium sulphate. In Proceedings of the 7th CANMET/ACI International Conference on Superplasticizers and other Chemical Admixtures in Concrete, Berlin, Germany, 20–24 October 2003.
- Plank, J.; Dai, Z.M.; Keller, H.; Hossle, H.; Seidl, W. Fundamental mechanisms for polycarboxylate intercalation into C3A hydrate phases and the role of sulfate present in cement. Cem. Concr. Res. 2010, 40, 45–57. [Google Scholar] [CrossRef]
- Hirata, T. Cement Dispersant. JP Patent 84,2022; (S59–018338), 26 November 1981. [Google Scholar]
- Zhao, H.J.; Yan, Y.; Hu, Z.H.; Gu, T. Study on solid polycarboxylic water-reducer. Cem. Eng. 2009, 1, 5–7. [Google Scholar]
- Yang, G.W.; Mei, M.H.; Liu, J.Y.; Guo, C.; Ren, X.M.; Hou, X.L.; Wu, Z.G. Solid polycarboxylate superplasticizer prepared by the method of vacuum evaporation thin layer. In Proceedings of the Annual Symposium of 3rd National Specialty Committee for Application Technology of Concrete Admixtures, Beijing, China, 20–21 June 2007.
- Plank, J.; Sachsenhauser, B. Impact of molecular structure on zeta potential and adsorbed conformation of α-allyl-ω-methoxy polyethylene glycol-maleic anhydride superplasticizers. J. Adv. Concr. Technol. 2006, 4, 233–239. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B.; de Reese, J. Experimental determination of the thermodynamic parameters affecting the adsorption behaviour and dispersion effectiveness of PCE superplasticizers. Cem. Concr. Res. 2010, 40, 699–709. [Google Scholar] [CrossRef]
- Iida, M.; Kinoshita, M.; Saitou, K.; Tamaki, S. Multi-Functional Admixtures for Hydraulic Cement Compositions. U.S. Patent 7265191B2, 4 September 2007. [Google Scholar]
- Mezger, J.; Kraus, A.; Grassl, H.; Becher, K. Vinyl Ester Maleic Acid Derivative Copolymers. U.S. Patent 8227559B2, 24 July 2012. [Google Scholar]
- Okada, T.; Ikeda, A.; Suga, A.; Koshisaka, S. Cement Dispersant. CN Patent 101516801B, 18 July 2012. [Google Scholar]
- Gui, M.M.; Fang, Y.H.; Lin, Q.C.; Yu, F.Y.; Lin, T.X.; Ma, X.X. The preparation research of powder polycarboxylate superplasticizer. Appl. Mech. Mater. 2011, 71–78, 677–683. [Google Scholar]
- Jiang, B.; Zhou, S.; Ji, H.; Liao, B.; Pang, H. Dispersion and rheological properties of ceramic suspensions using linear polyacrylate copolymers with carboxylic groups as superplasticizer. Colloids Surf. A Physicochem. Eng. Asp. 2011, 396, 310–316. [Google Scholar]
- Najafi, F.; Sarbolouki, M.N. Preparation of biodegradable poly [(dimethyldichlorosilane)-alt- (fumaric acid/sebacic acid)]-co-PEG block copolymer. Polymer 2002, 43, 6363–6368. [Google Scholar] [CrossRef]
- Flatt, R.J. Towards a prediction of superplasticized concrete rheology. Mater. Struct. 2004, 27, 289–300. [Google Scholar] [CrossRef]
- Lim, G.G.; Hong, S.S.; Kim, D.S.; Lee, B.J.; Rho, J.S. Slump loss control of cement paste by adding polycarboxylic type slump-releasing dispersant. Cem. Concr. Compos. 2009, 29, 223–229. [Google Scholar]
- Uchikawa, H.; Sawaki, D.; Hanegara, S. Influence of kind and added timing of organic admixture on the composition, structure and property of fresh cement paste. Cem. Concr. Compos. 1995, 25, 353–364. [Google Scholar] [CrossRef]
- Yin, S.; Aishah, F.; Salmiah, A. Adsorptive behavior of surfactants on surface of Portland cement. Cem. Concr. Compos. 2001, 31, 1009–1015. [Google Scholar] [CrossRef]
- Flatt, R.J. Dispersion forces in cement suspensions. Cem. Concr. Res. 2004, 34, 399–408. [Google Scholar] [CrossRef]
- Flatt, R.J.; Bowen, P. Electrostatic repulsion between particles in cement suspensions: Domain of validity of linearized Poisson-Boltzmann equation for nonideal electrolytes. Cem. Concr. Res. 2003, 33, 781–791. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Laurow, R.K. Influence of melamine superplasticizer on some characteristics of concrete. In Proceedings of the 10th International Congress on the Chemistry of Cement, Goteborg, Sweden, 2–6 June 1997.
- Sakai, E.; Kang, J.K.; Daimon, M. Action mechanisms of comb-type superplasticizers containing grafted polyethylene oxide chains. In Proceedings of the 6th CANMET/ACI International Conference on Superplasticizers and other Chemical Admixtures in Concrete, Nice, France, 1 January 2000.
- Yoshioka, K.; Sakai, E.; Daimon, M. Role of steric hindrance in the performance of superplasticizer for concrete. J. Am. Ceram. Soc. 1997, 80, 2667–2671. [Google Scholar] [CrossRef]
- Kim, B.G.; Jiang, S.; Jolicoeur, C.; Aïtcin, P.C. The adsorption behavior of PNS superplasticizer and its relation to fluidity of cement paste. Cem. Concr. Compos. 2000, 30, 887–893. [Google Scholar] [CrossRef]
- Roussel, N.; Ovarlez, G.; Garrault, S.; Brumaud, C. The origins of thixotropy of fresh cement pastes. Cem. Concr. Res. 2012, 42, 148–157. [Google Scholar] [CrossRef]
- Perrot, A.; Lecompte, T.; Khelifi, H.; Brumaud, C.; Hot, J.; Roussel, N. Yield stress and bleeding of fresh cement pastes. Cem. Concr. Res. 2012, 42, 937–944. [Google Scholar] [CrossRef]
- Huang, Z.P.; Ma, X.G.; Jiang, G.J.; Cao, Q.W. Measurement of BAG molecular weight and its distribution using laser light scattering method. J. Solid Rocket. Technol. 2004, 27, 233–237. [Google Scholar]
- Methods for Testing Uniformity of Concrete Admixture; GB/T8077–2000. 2000. Available online: http://cx.spsp.gov.cn/ (accessed on 18 December 2000).
- Standard for Test Method of Performance on Ordinary Fresh Concrete; GB/T50080–2002; 2002. Available online: http://cx.spsp.gov.cn/ (accessed on 10 January 2003).
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Figi, R.; Gauckler, L. Interaction of polycarboxylate-based superplasticizers with cements containing different C3A amounts. Cem. Concr. Compos. 2009, 31, 153–162. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, X.; Wang, Z.; Zheng, Y.; Cui, S.; Lan, M.; Li, H.; Zhu, J.; Liang, X. Preparation, Characterization and Performances of Powdered Polycarboxylate Superplasticizer with Bulk Polymerization. Materials 2014, 7, 6169-6183. https://doi.org/10.3390/ma7096169
Liu X, Wang Z, Zheng Y, Cui S, Lan M, Li H, Zhu J, Liang X. Preparation, Characterization and Performances of Powdered Polycarboxylate Superplasticizer with Bulk Polymerization. Materials. 2014; 7(9):6169-6183. https://doi.org/10.3390/ma7096169
Chicago/Turabian StyleLiu, Xiao, Ziming Wang, Yunsheng Zheng, Suping Cui, Mingzhang Lan, Huiqun Li, Jie Zhu, and Xu Liang. 2014. "Preparation, Characterization and Performances of Powdered Polycarboxylate Superplasticizer with Bulk Polymerization" Materials 7, no. 9: 6169-6183. https://doi.org/10.3390/ma7096169