Investigation on Using SBS and Active Carbon Filler to Reduce the VOC Emission from Bituminous Materials
Abstract
:1. Introduction
2. Materials and Test Methods
2.1. Raw Materials
| Items | Values |
|---|---|
| PH | 5.0–7.0 |
| Surface area | 1100 m2/g |
| Residual after dried | 90% |
| Dissolved by hydrochloric acid | Less than 0.8% |
| Dissolved by ethanol | Less than 0.2% |
| Property | PJ90 | PJ90 (4% SBS + 4% C) |
|---|---|---|
| Softening point (°C) | 45 | 70 |
| Ductility (15 °C) (cm) | >120 | >120 |
| Penetration (25 °C) (0.1 mm) | 75.6 | 56.7 |
2.2. Principle of TG-MS Test


2.3. Ultraviolet-Visible Spectroscopy Test
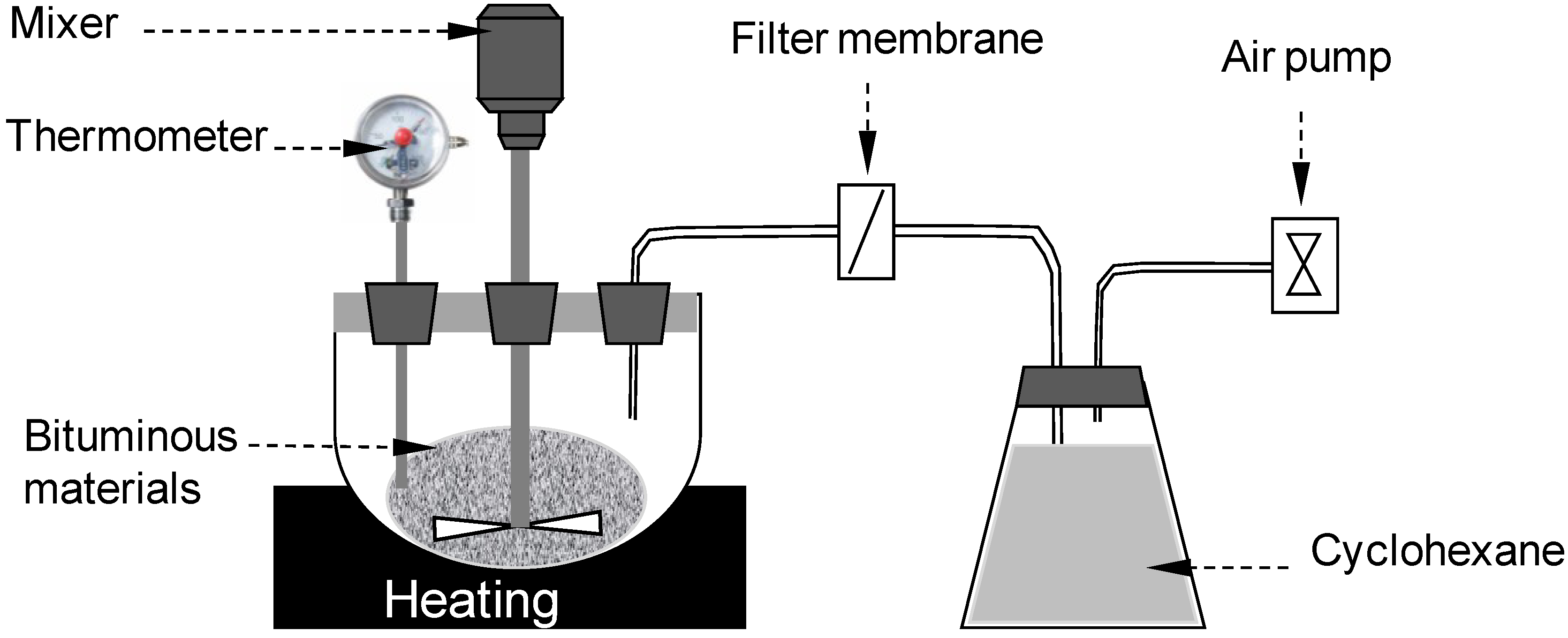
2.4. Rheological Property Analysis
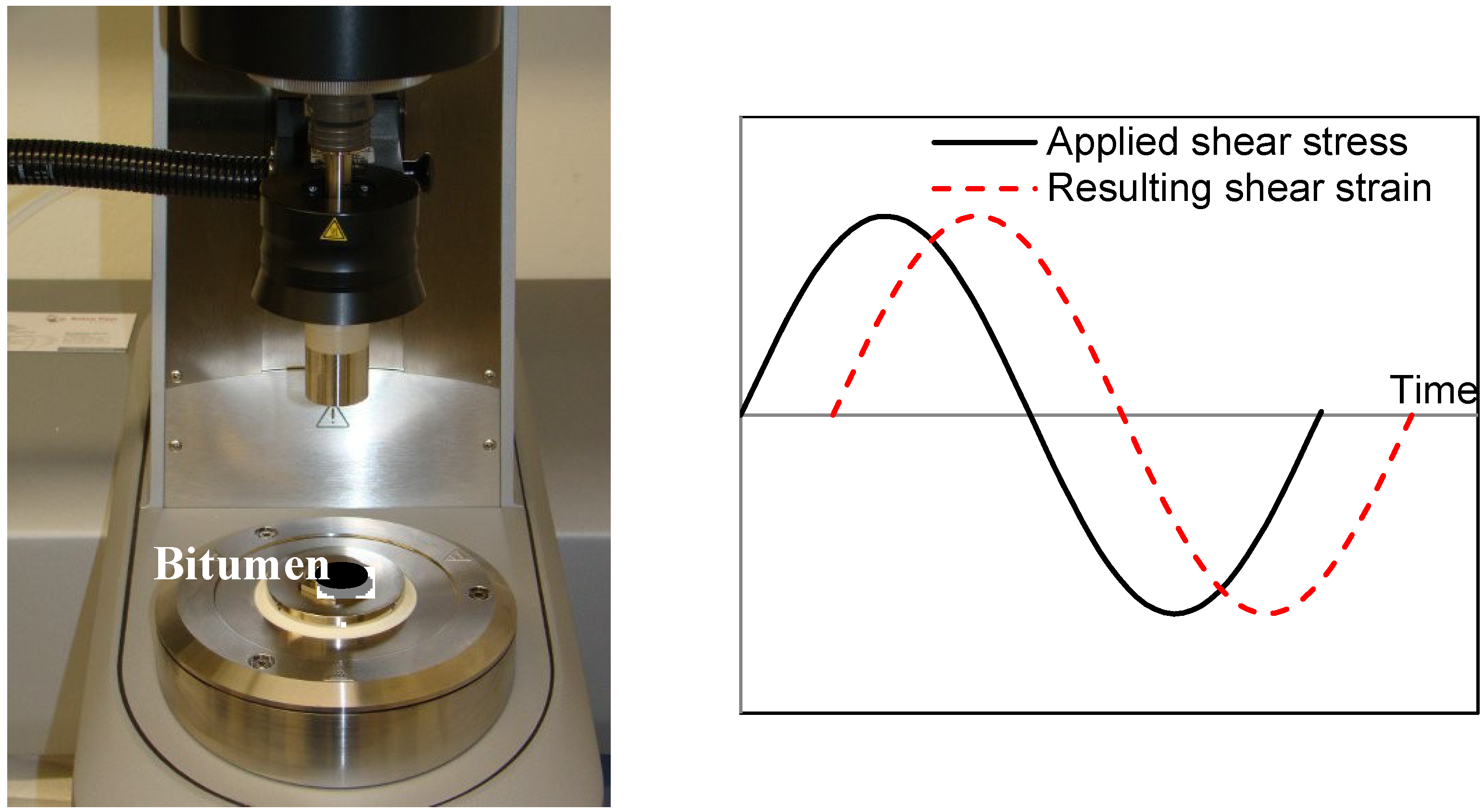
3. Results and Discussions
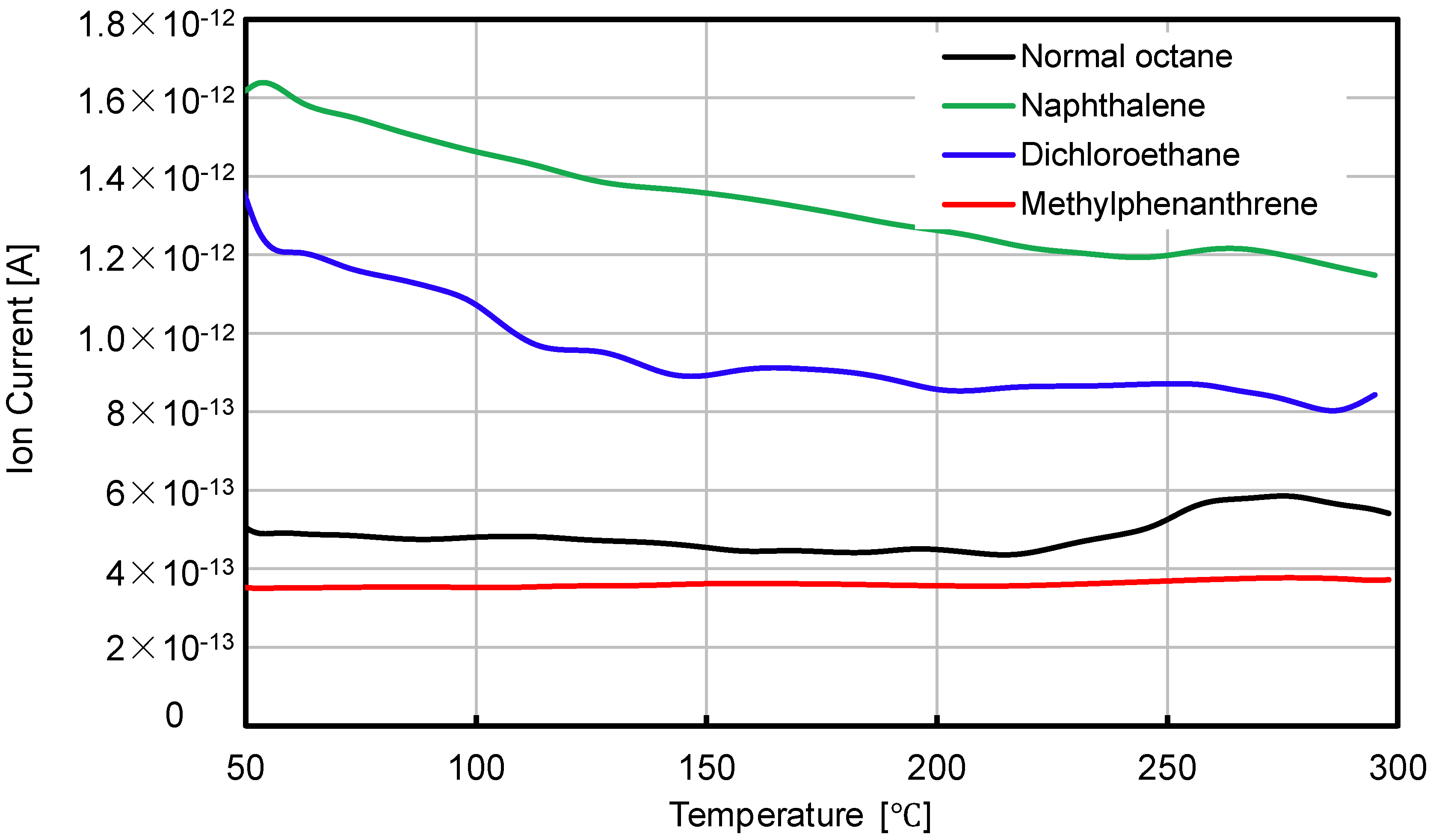
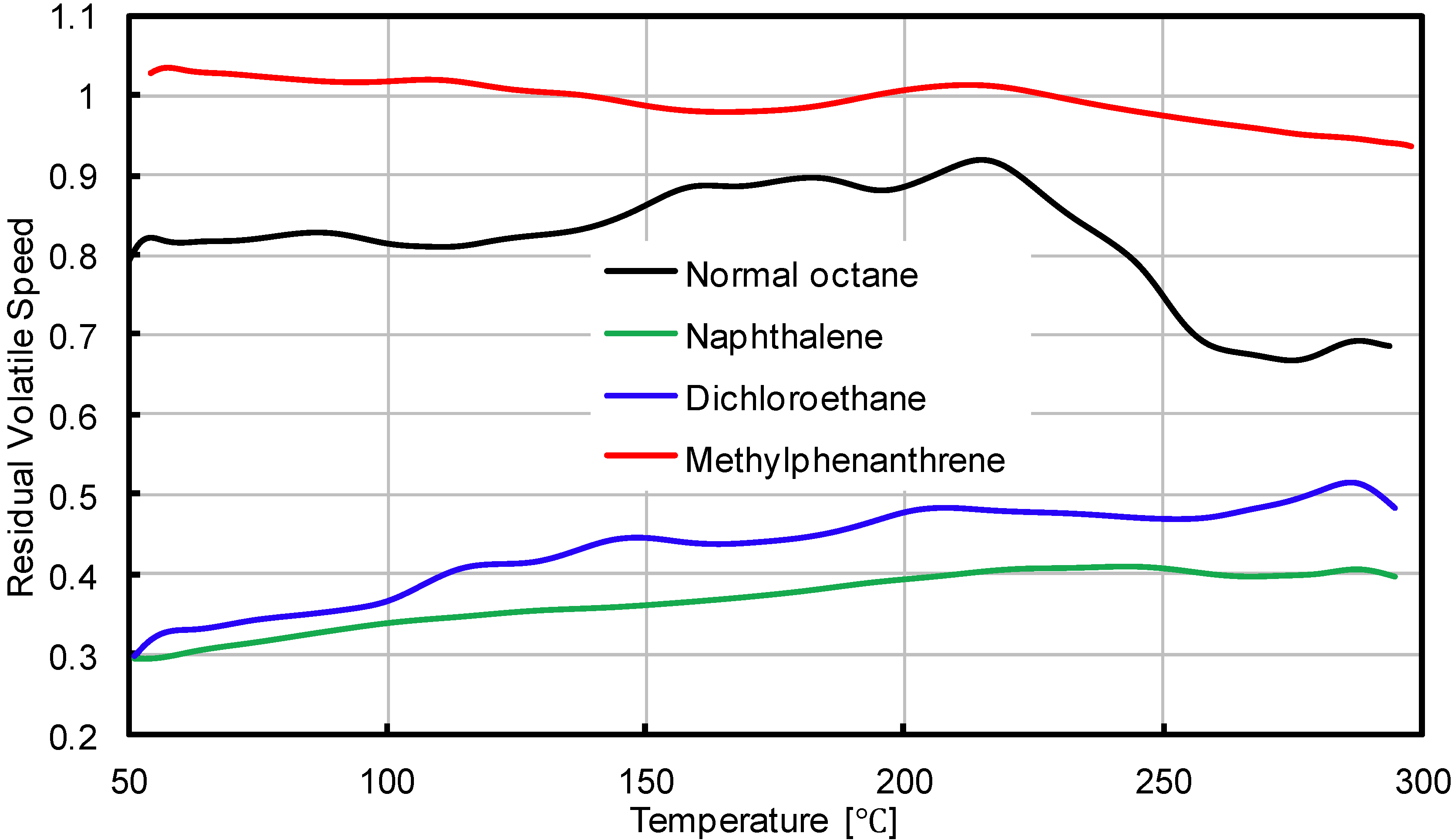
3.1. Residual Volatile Speed

| Volatile Type | Molecular Type | Main Characteristics |
|---|---|---|
| Naphthalene |  | The most volatile components from bituminous materials, may cause cancer |
| Dichloroethane |  | The two main volatile components from bituminous materials, toxicity to humans and aquatic organisms |
| Normal octane |  | |
| Methylphenanthrene |  | Main components of VOC, toxicity to humans and aquatic organisms |
3.2. Ultraviolet-Visible Spectroscopy Test (UV-Vis) Test Analysis
3.2.1. Calibration Curve
| No. | Absorbance (Y) | Concentration of VOC (μg/mL, X) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 0.0686 | 9.4949 |
| 3 | 0.1573 | 28.4848 |
| 4 | 0.3897 | 47.4747 |
| 5 | 0.4813 | 66.4646 |
| 6 | 0.6254 | 75.9596 |
| 7 | 0.7192 | 94.9495 |
| 8 | 0.8242 | 123.4343 |
| 9 | 0.9594 | 142.4242 |
| Mathematical Solver | Y = 0.005X + 0.0078 (Y = aX + b, R2 = 0.998) | |
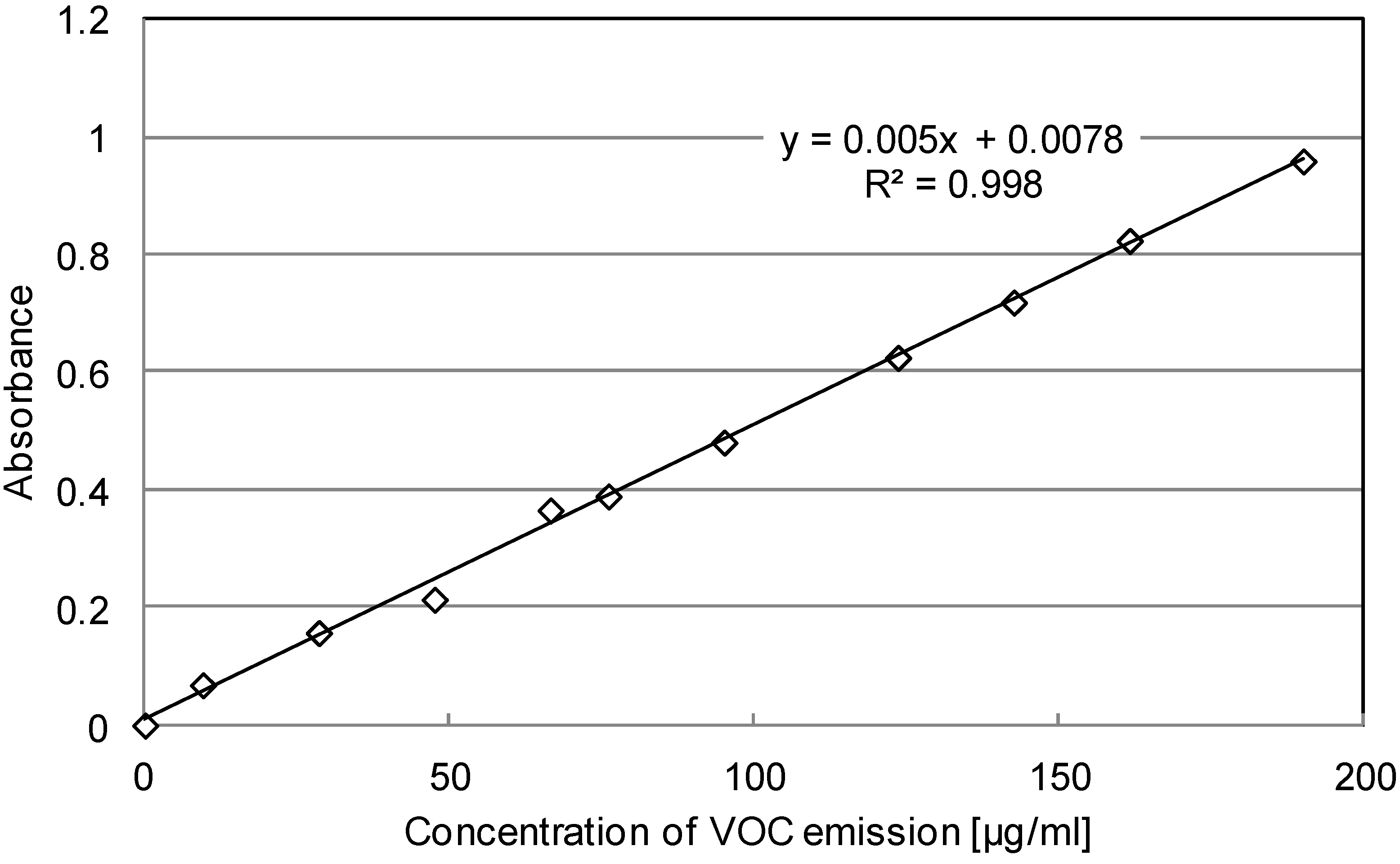
3.2.2. UV-Vis Data

| Parameter | PJ-90 | PJ90 (4% SBS + 4% C) |
|---|---|---|
| M (g) | 935.48 | 1190.94 |
| Y | 2.0569 | 1.2087 |
| V (mL) | 18 | 18 |
| Concentration of VOC in cyclohexane (μg/mL) | 409.82 | 240.18 |
| Total amount of VOC (μg) | 7376.76 | 4323.24 |
| W (μg/g) | 7.886 | 3.63 |
| VOC decrement percentage (%) | 53.96 | |
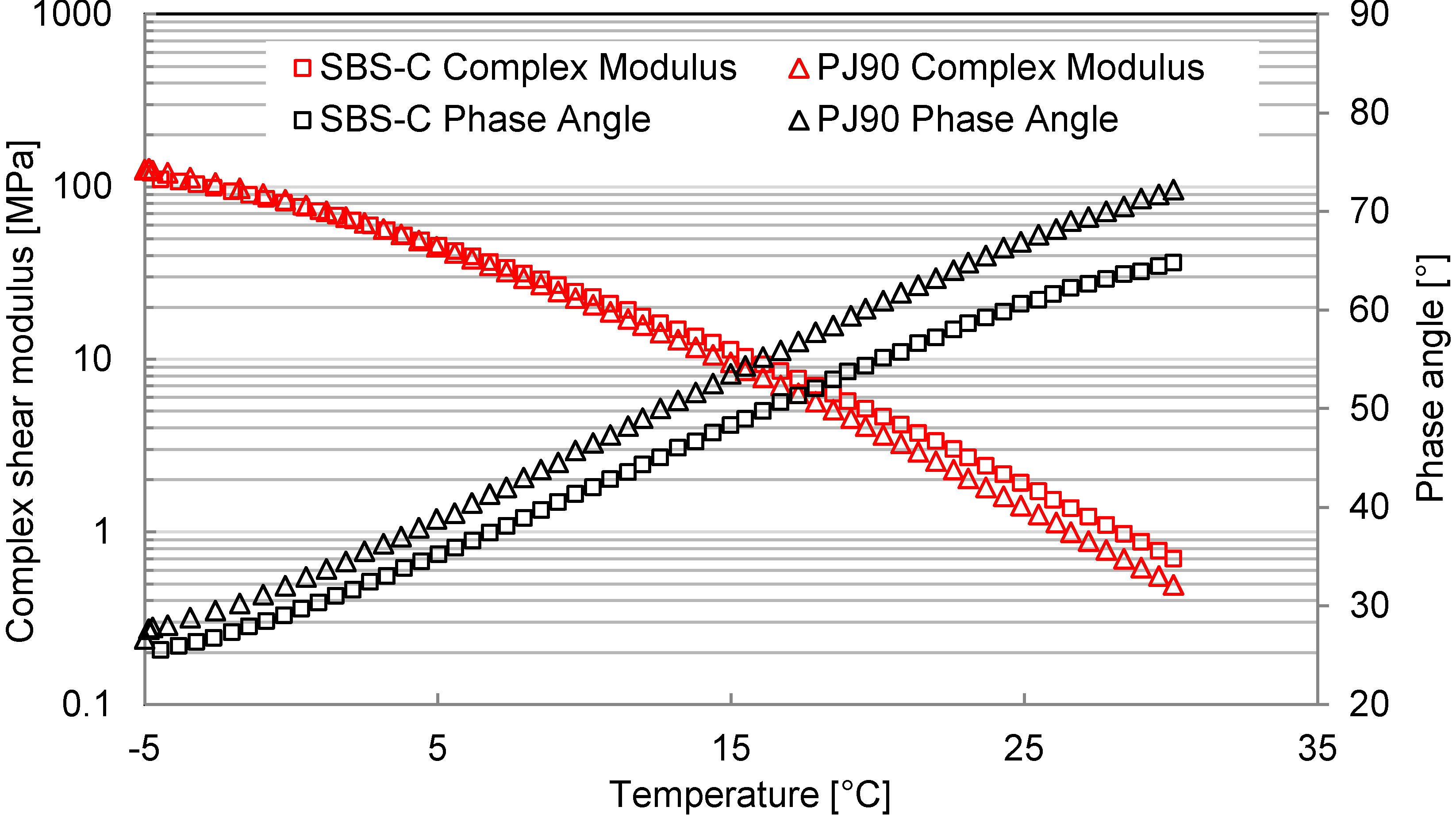
3.3. Rheological Behaviors
3.3.1. Temperature Sweep

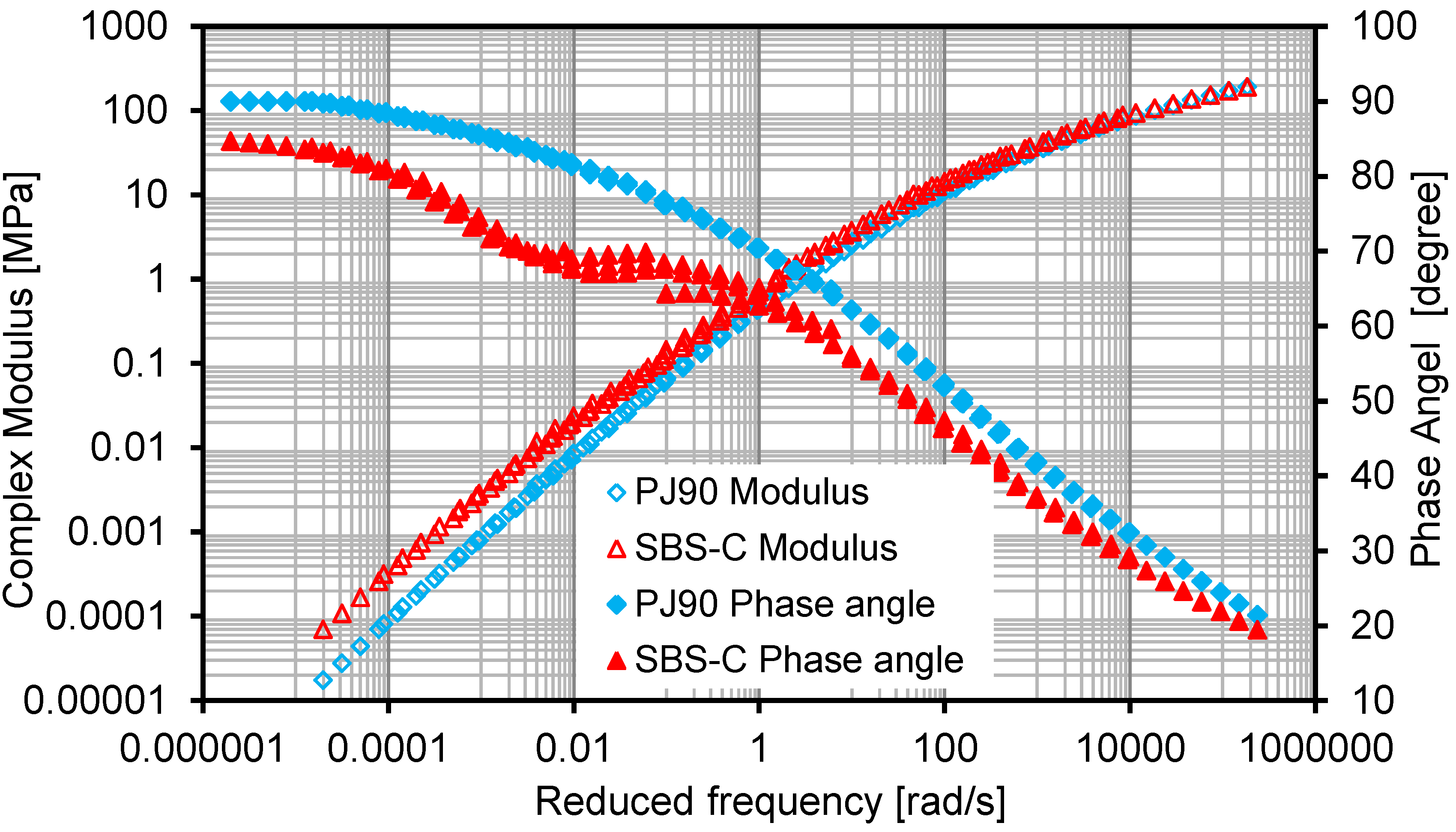
3.3.2. Frequency Sweep
4. Conclusions
- TG-MS, a combination of thermogravimetry and mass spectrometry can be used to identify different VOC components from bituminous materials during a weight lost process.
- The combined introduction of SBS and active carbon filler into bituminous materials can significantly decrease the VOC emission speed, while the decreasing influence is different on different types of gas. Such an inhibitory effect resulting from SBS and active carbon filler is temperature related.
- Under the same temperature condition, the amount of VOC emission from PJ90 bituminous material was much higher than that from bituminous material modified with inhibitors. The combination of using SBS and active carbon filler as inhibitors can prevent more than 50% of VOC molecules from volatilization.
- The combined introduction of SBS and active carbon filler into bituminous materials can not only lower the VOC emission speed and amount, but also improve the deformation resistance behavior at a higher temperature, which was proven by means of a temperature sweep test and a frequency sweep test.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hicks, J.B. Asphalt industry cross-sectional exposure assessment study. Appl. Occup. Environ. Hyg. 1995, 10, 840–848. [Google Scholar] [CrossRef]
- Boffetta, P.; Burstyn, I. Studies of carcinogenicity of bitumen fume in humans. Am. J. Ind. Med. 2003, 43, 1–2. [Google Scholar] [CrossRef]
- Kitto, A.M.; Pirbazari, M.; Badriyha, B.N.; Ravindran, V.; Tyner, R.; Synolakis, C.E. Emissions of volatile and semi-volatile organic compounds and particulate matter from hot asphalts. Environ. Technol. 1997, 18, 121–138. [Google Scholar] [CrossRef]
- Gamble, J.F.; Nicolich, M.J.; Barone, N.J.; Vincent, W.J. Exposure-response of asphalt fumes with changes in pulmonary function and symptoms. Scand. J. Work Environ. Health 1999, 25, 186–206. [Google Scholar] [CrossRef]
- Karakaya, A.; Yücesoy, B.; Turhan, A.; Erdem, O.; Burgaz, S.; Karakaya, A.E. Investigation of some immunological functions in a group of asphalt workers exposed to polycyclic aromatic hydrocarbons. Toxicology 1999, 135, 43–47. [Google Scholar] [CrossRef]
- Xiao, Y.; van de Ven, M.F.C.; Molenaar, A.A.A.; Wu, S.P. Possibility of using epoxy modified bitumen to replace tar-containing binder for pavement antiskid surfaces. Constr. Build. Mater. 2013, 48, 59–66. [Google Scholar] [CrossRef]
- Xiao, Y.; van de Ven, M.F.C.; Molenaar, A.A.A.; Wu, S.P. Environmental concern of using coal tar in road engineering and its possible alternatives. J. Wuhan Univ. Technol. 2010, 32, 1–7. [Google Scholar]
- Gasthauer, E.; Mazé, M.; Marchand, J.P.; Amouroux, J. Characterization of asphalt fume composition by GC/MS and effect of temperature. Fuel 2008, 87, 1428–1434. [Google Scholar] [CrossRef]
- Lange, C.; Stroup-Gardiner, M. Quantification of potentially odorous volatile organic compounds from asphalt binders using head-space gas chromatography. J. Test. Eval. 2005, 33. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Kuang, D.; Zhang, H. Effect of organophilic montmorillonite on thermal-oxidative aging behavior of SBS modified bitumen crack filling material. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 673–676. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, J.; Wu, S. Effect of ageing on rheological properties of storage-stable SBS/sulfur-modified asphalts. J. Hazard. Mater. 2010, 182, 507–517. [Google Scholar] [CrossRef]
- Wu, S.P.; Pang, L.; Mo, L.T.; Chen, Y.C.; Zhu, G.J. Influence of aging on the evolution of structure, morphology and rheology of base and SBS modified bitumen. Constr. Build. Mater. 2009, 23, 1005–1010. [Google Scholar] [CrossRef]
- Chaala, A.; Roy, C.; Ait-Kadi, A. Rheological properties of bitumen modified with pyrolytic carbon black. Fuel 1996, 75, 1575–1583. [Google Scholar] [CrossRef]
- Gentzis, T.; Rahimi, P.; Malhotra, R.; Hirschon, A.S. The effect of carbon additives on the mesophase induction period of Athabasca bitumen. Fuel Process. Technol. 2001, 69, 191–203. [Google Scholar] [CrossRef]
- Boczkaj, G.; Przyjazny, A.; Kamiński, M. Characteristics of volatile organic compounds emission profiles from hot road bitumens. Chemosphere 2014, 107, 23–30. [Google Scholar] [CrossRef]
- Flego, C.; Carati, C.; Gaudio, L.D.; Zannoni, C. Direct Mass Spectrometry of tar sands: A new approach to bitumen identification. Fuel 2013, 111, 357–366. [Google Scholar] [CrossRef]
- Yu, M.; Wu, S.P.; Chen, M.Z.; Zhang, H.H. Evaluation of volatile organic compounds from asphalt using UV-visible spectrometer. Adv. Mater. Res. 2012, 472–475, 432–436. [Google Scholar]
- Jia, C.; Batterman, S. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. Int. J. Environ. Res. Public Health 2010, 7, 2903–2939. [Google Scholar] [CrossRef]
- Xiao, Y.; van de Ven, M.F.C.; Molenaar, A.A.A.; Su, Z.; Zandvoort, F. Characteristics of two-component epoxy modified bitumen. Mater. Struct. 2011, 44, 611–622. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cui, P.; Wu, S.; Li, F.; Xiao, Y.; Zhang, H. Investigation on Using SBS and Active Carbon Filler to Reduce the VOC Emission from Bituminous Materials. Materials 2014, 7, 6130-6143. https://doi.org/10.3390/ma7096130
Cui P, Wu S, Li F, Xiao Y, Zhang H. Investigation on Using SBS and Active Carbon Filler to Reduce the VOC Emission from Bituminous Materials. Materials. 2014; 7(9):6130-6143. https://doi.org/10.3390/ma7096130
Chicago/Turabian StyleCui, Peiqiang, Shaopeng Wu, Fuzhou Li, Yue Xiao, and Honghua Zhang. 2014. "Investigation on Using SBS and Active Carbon Filler to Reduce the VOC Emission from Bituminous Materials" Materials 7, no. 9: 6130-6143. https://doi.org/10.3390/ma7096130






