Bioceramics for Hip Joints: The Physical Chemistry Viewpoint
Abstract
:1. Introduction
2. Background on Physical Chemistry of Bioceramics
2.1. A serious Inquiry on Bioinertness of Ceramic Oxides
2.2. Oxygen Sub-Lattice in Bioceramic Oxide Surfaces
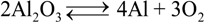
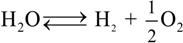
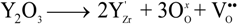
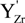 represents the Y3+ ions on regular cationic sites (i.e., replacing Zr4+ ions) associated with a relative charge of −1;
represents the Y3+ ions on regular cationic sites (i.e., replacing Zr4+ ions) associated with a relative charge of −1; 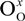 stands for oxygen ions on regular anionic sites and
stands for oxygen ions on regular anionic sites and 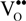 represents the double positively charged (with respect to the charge of regular oxygen ions) oxygen vacancies. The above notation is referred to as the Kröger-Vink notation for lattice elements and point defects in crystal structures [66]. This formalism is useful to concisely describe the atomistic reactions related to the replacement of Zr4+ by Y3+. Note that two ions of Y-dopant are necessary to electrically balance a single oxygen vacancy. Accordingly, the concentration of vacancies in the anionic sublattice can, in principle, be calculated from the concentration of Y-ions at cationic sites and might easily reach the range of few percent, as follows:
represents the double positively charged (with respect to the charge of regular oxygen ions) oxygen vacancies. The above notation is referred to as the Kröger-Vink notation for lattice elements and point defects in crystal structures [66]. This formalism is useful to concisely describe the atomistic reactions related to the replacement of Zr4+ by Y3+. Note that two ions of Y-dopant are necessary to electrically balance a single oxygen vacancy. Accordingly, the concentration of vacancies in the anionic sublattice can, in principle, be calculated from the concentration of Y-ions at cationic sites and might easily reach the range of few percent, as follows:
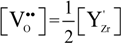
2.3. Monitoring Surface Off-Stoichiometry on Hip Bearing Surfaces
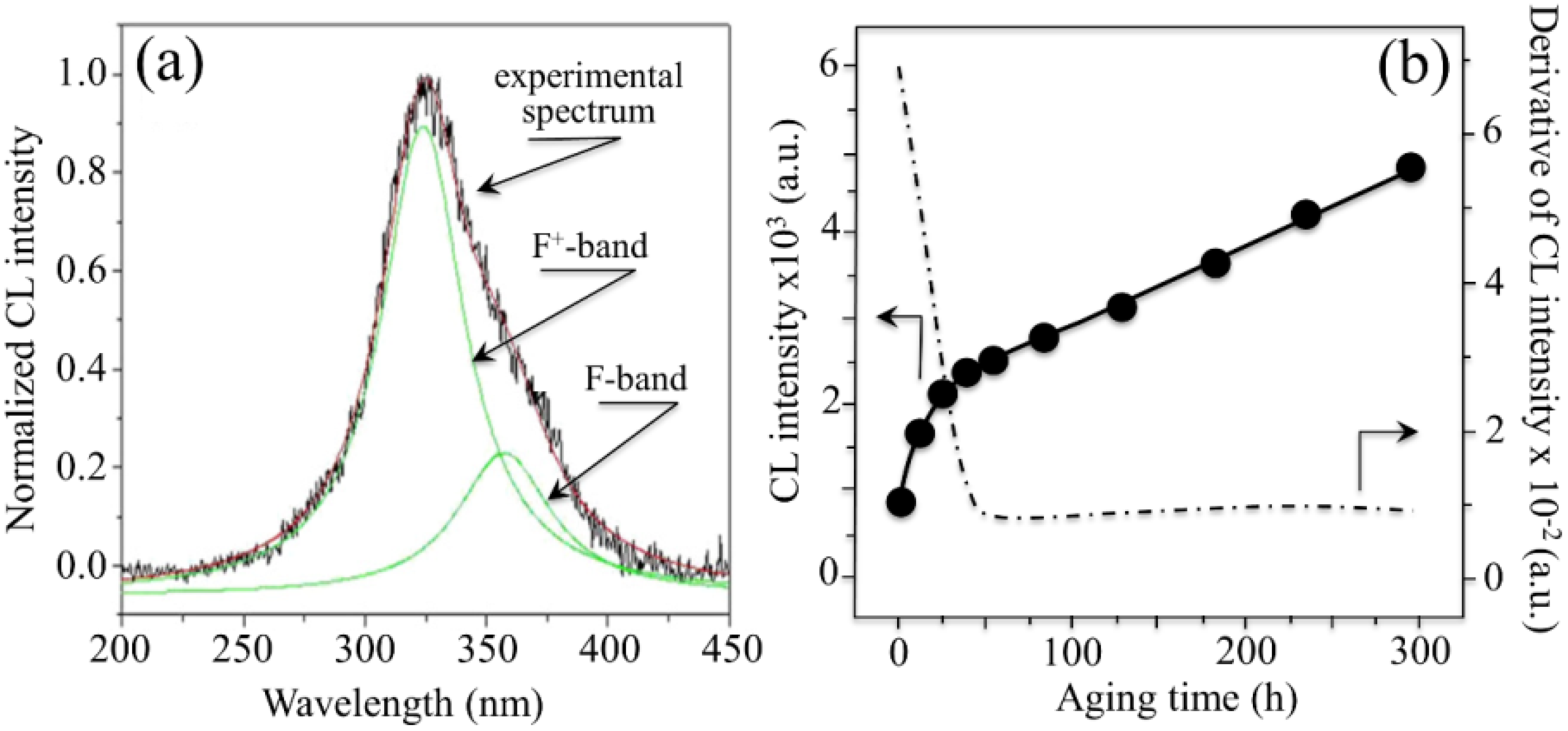

3. Experimental Evidence and Future Strategies
3.1. The Limits of Monolithic Bioceramics
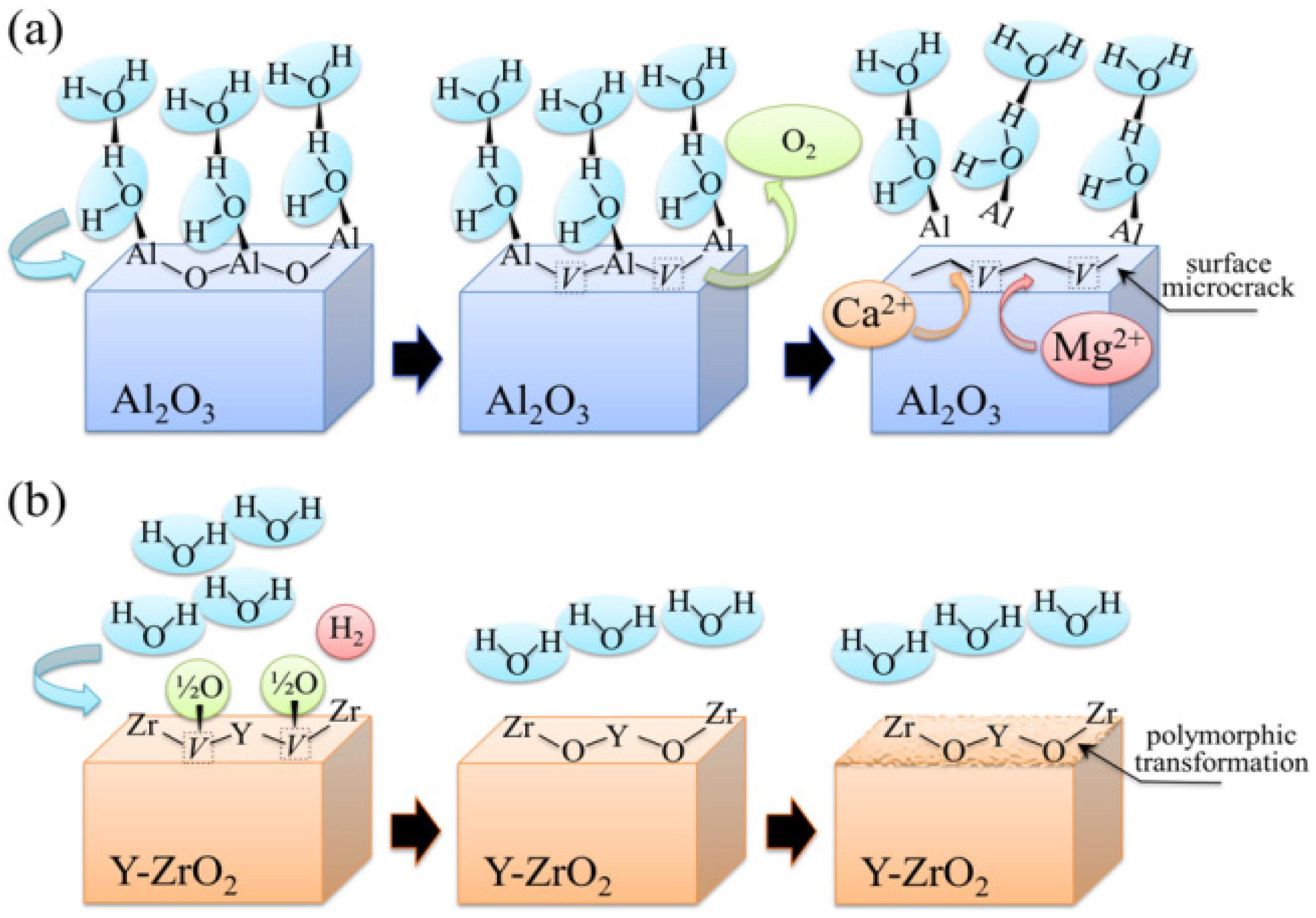
3.1.1. Al2O3 Bioceramics
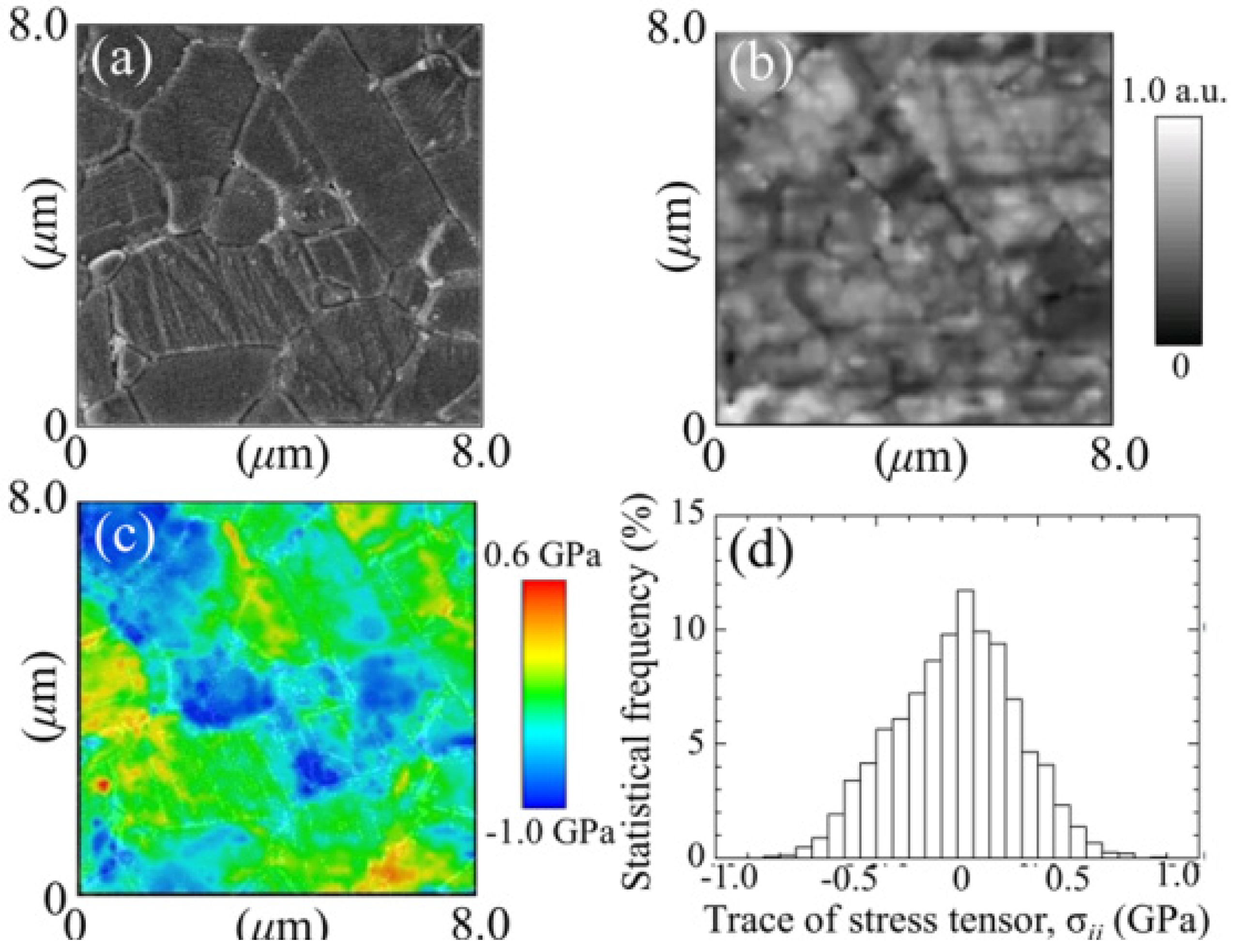
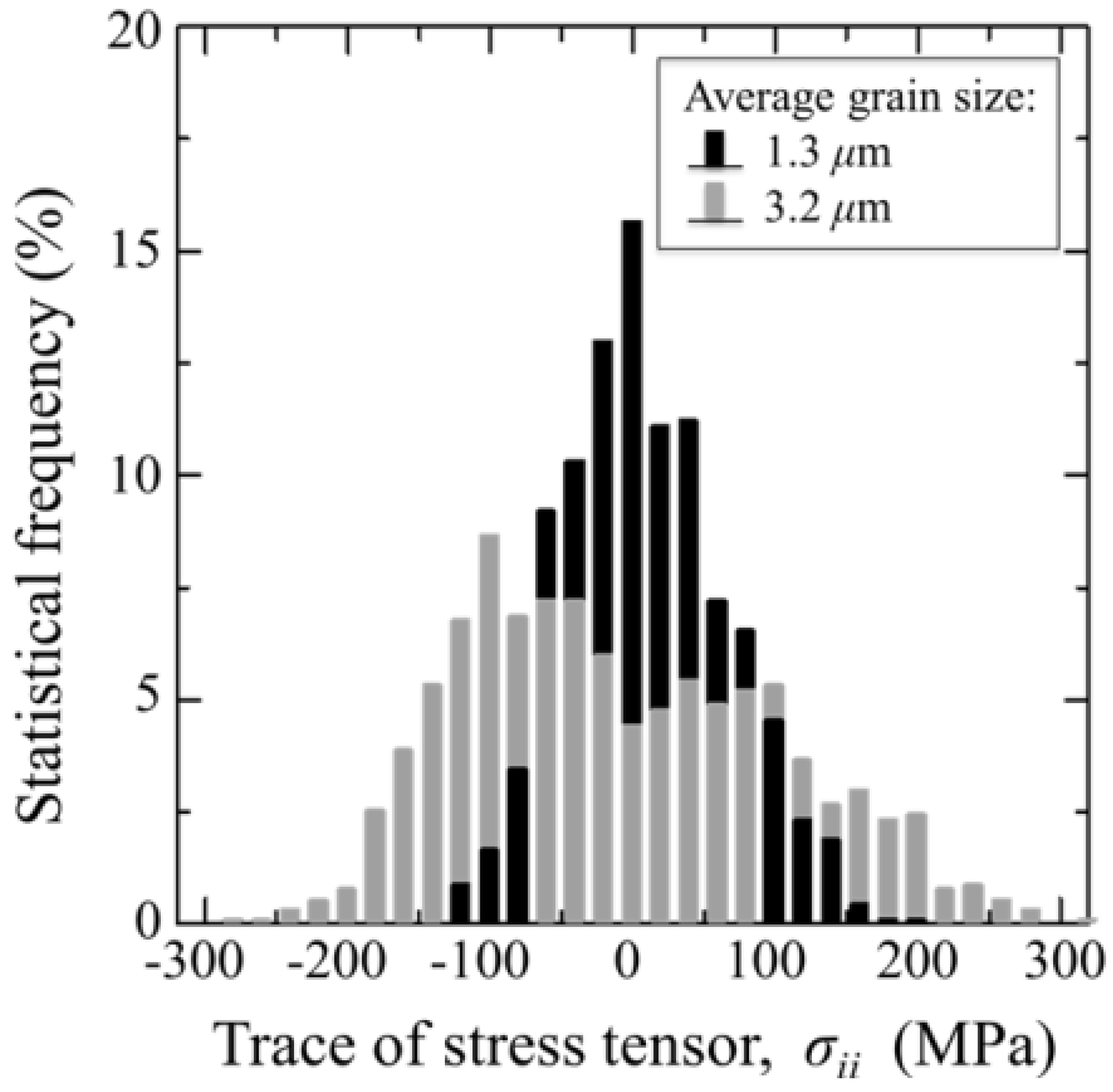
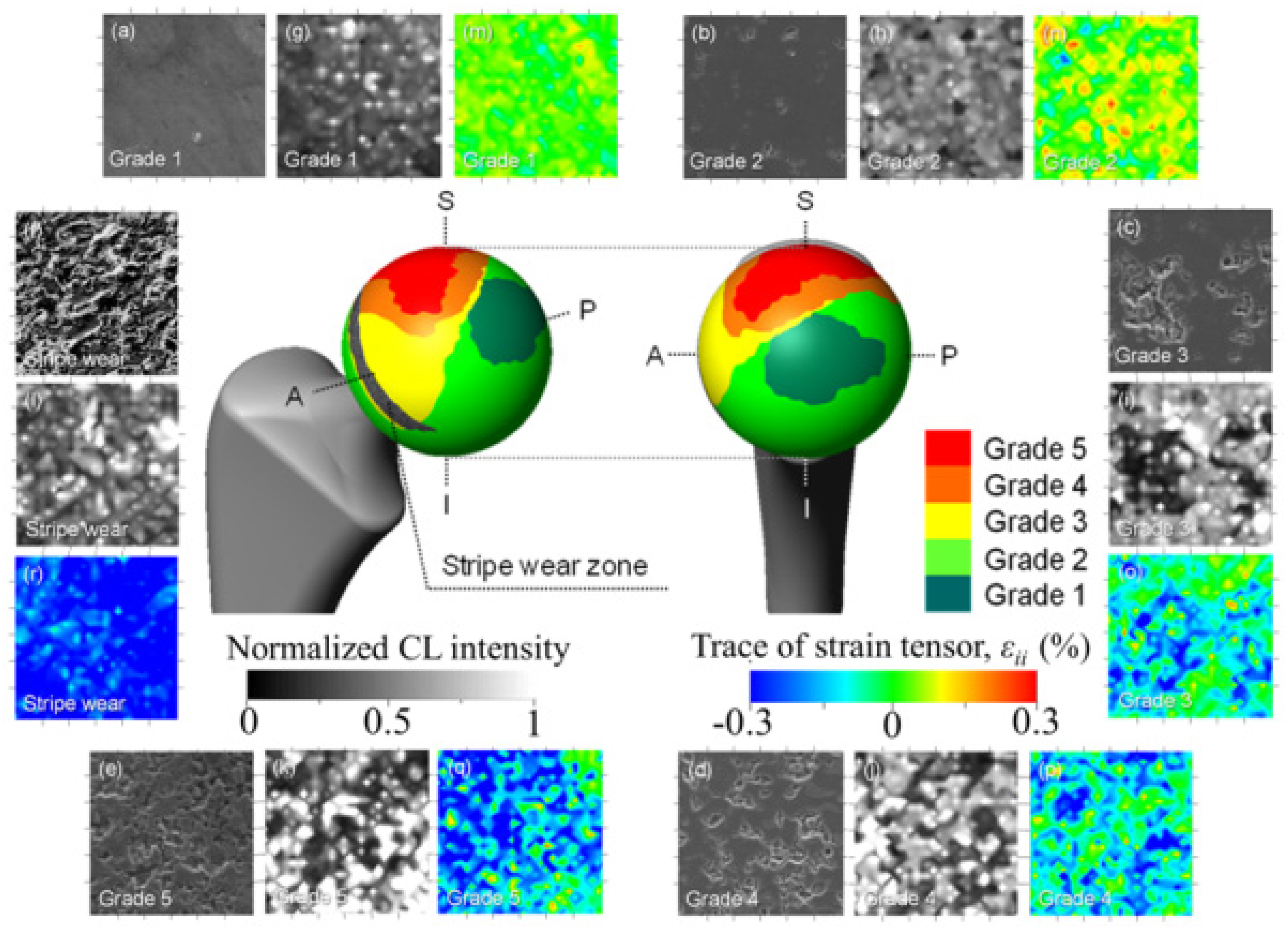
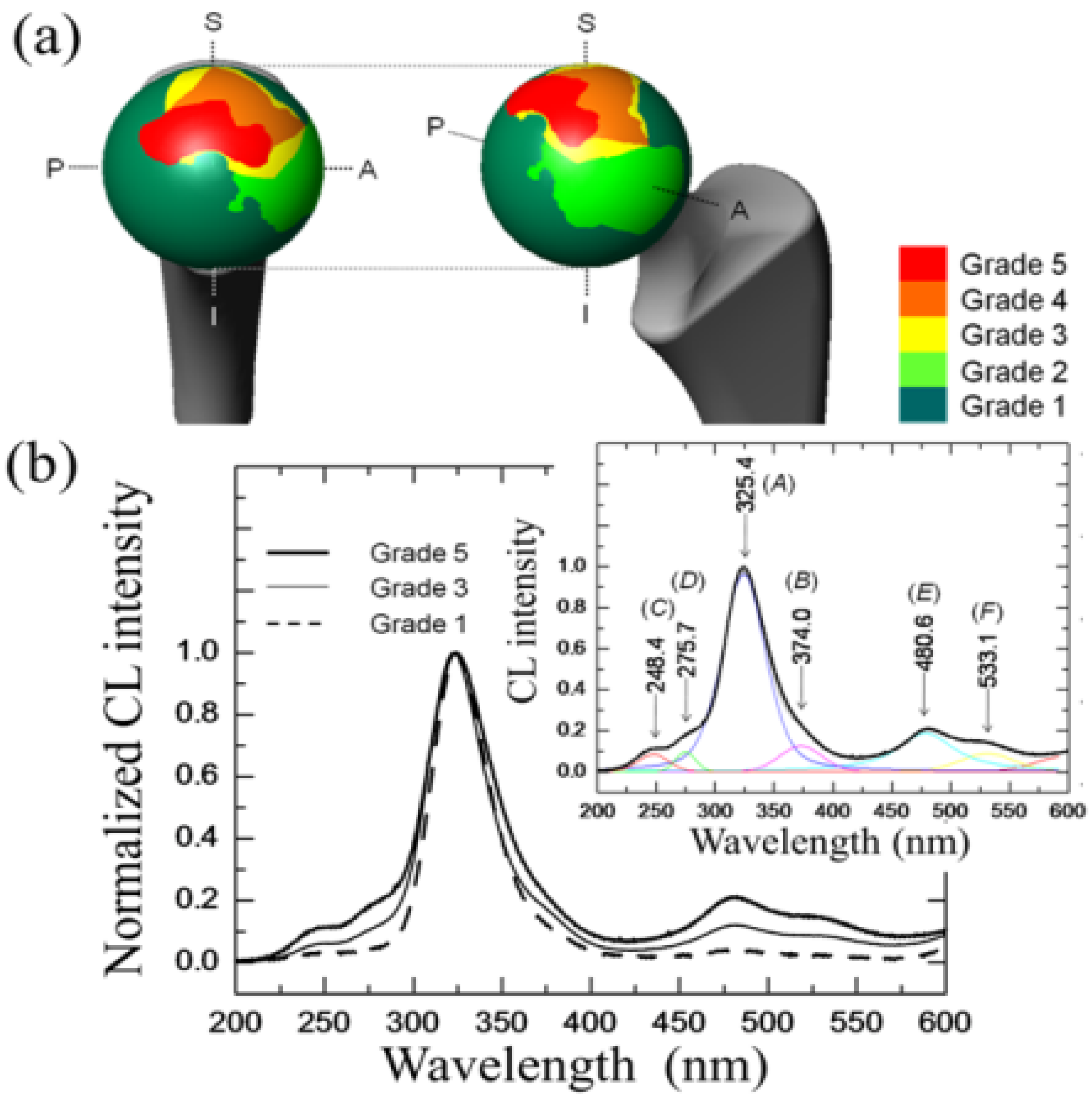
3.1.2. 3Y-TZP Bioceramics
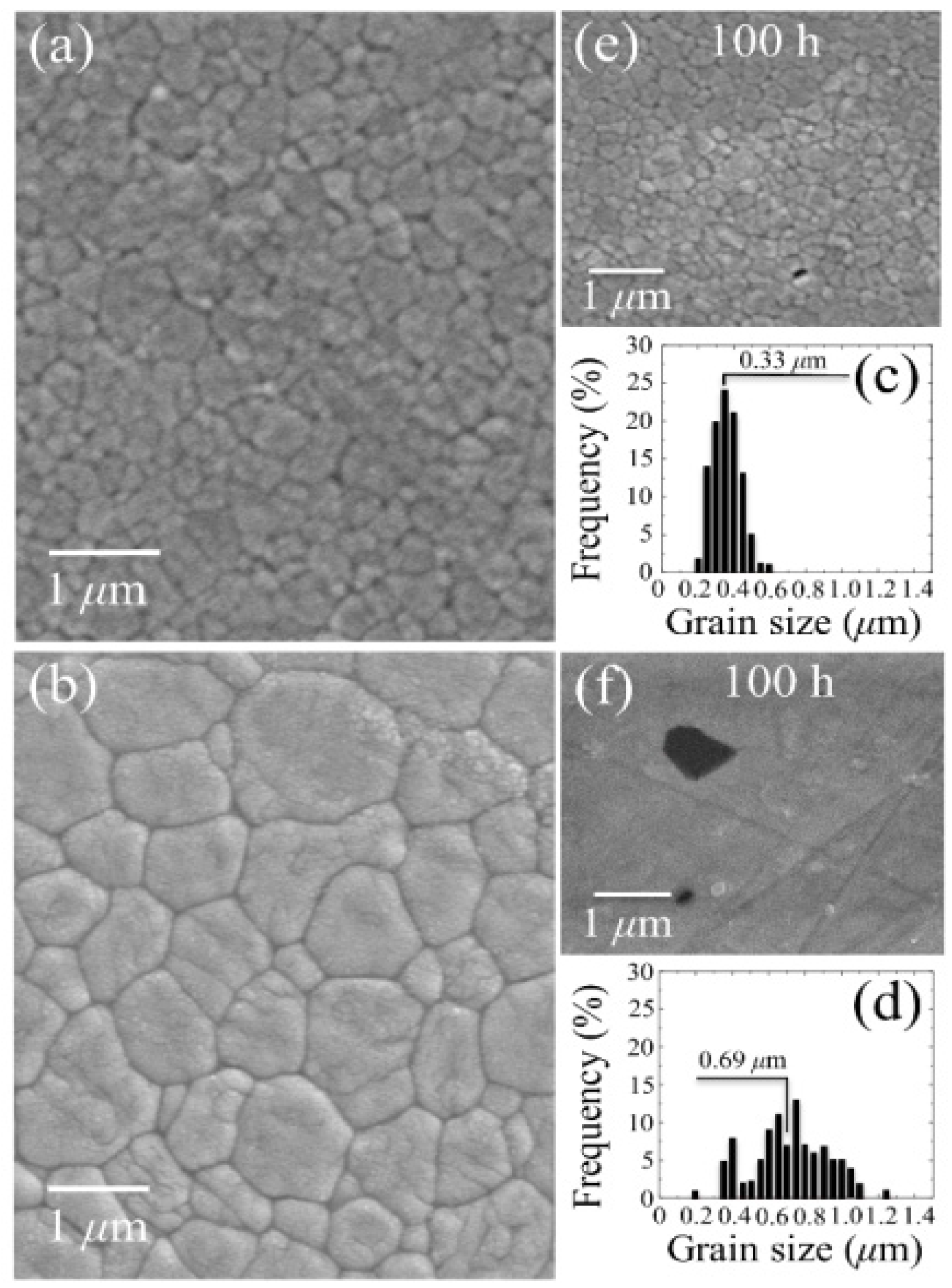


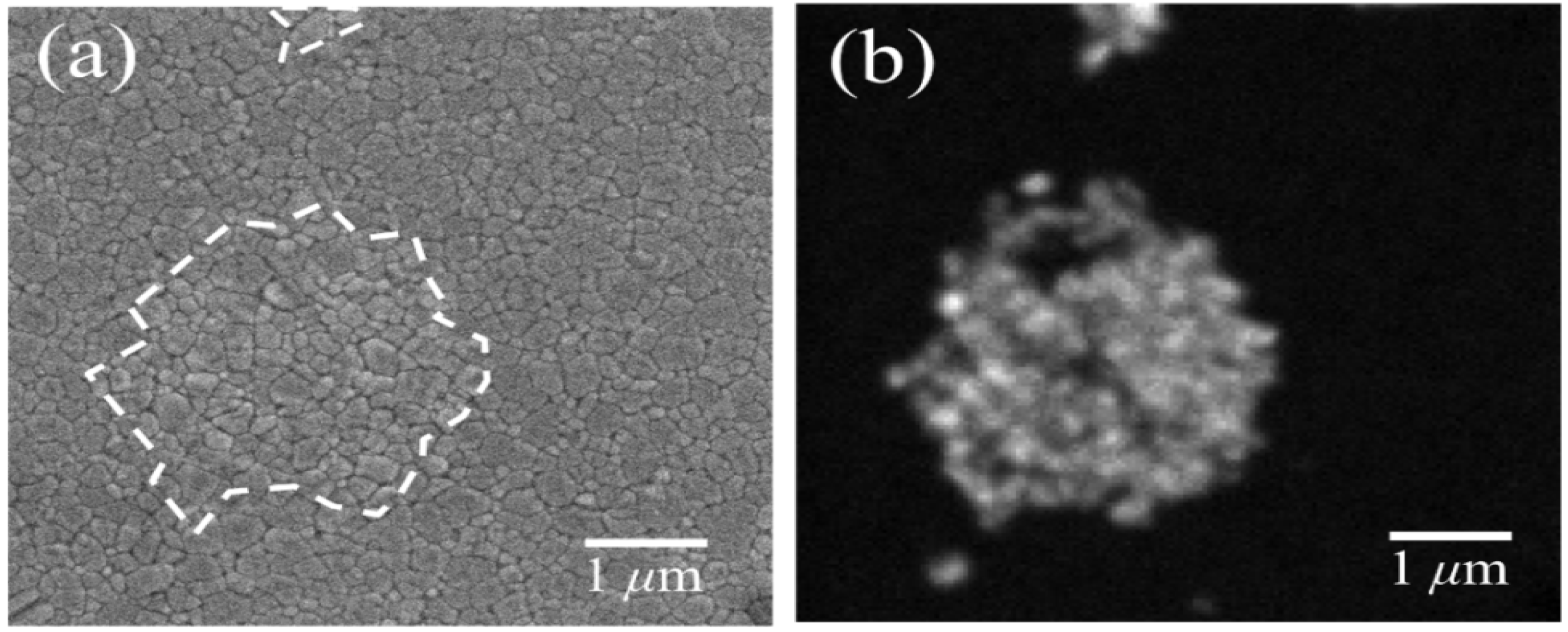
3.2. Stoichiometry Matters
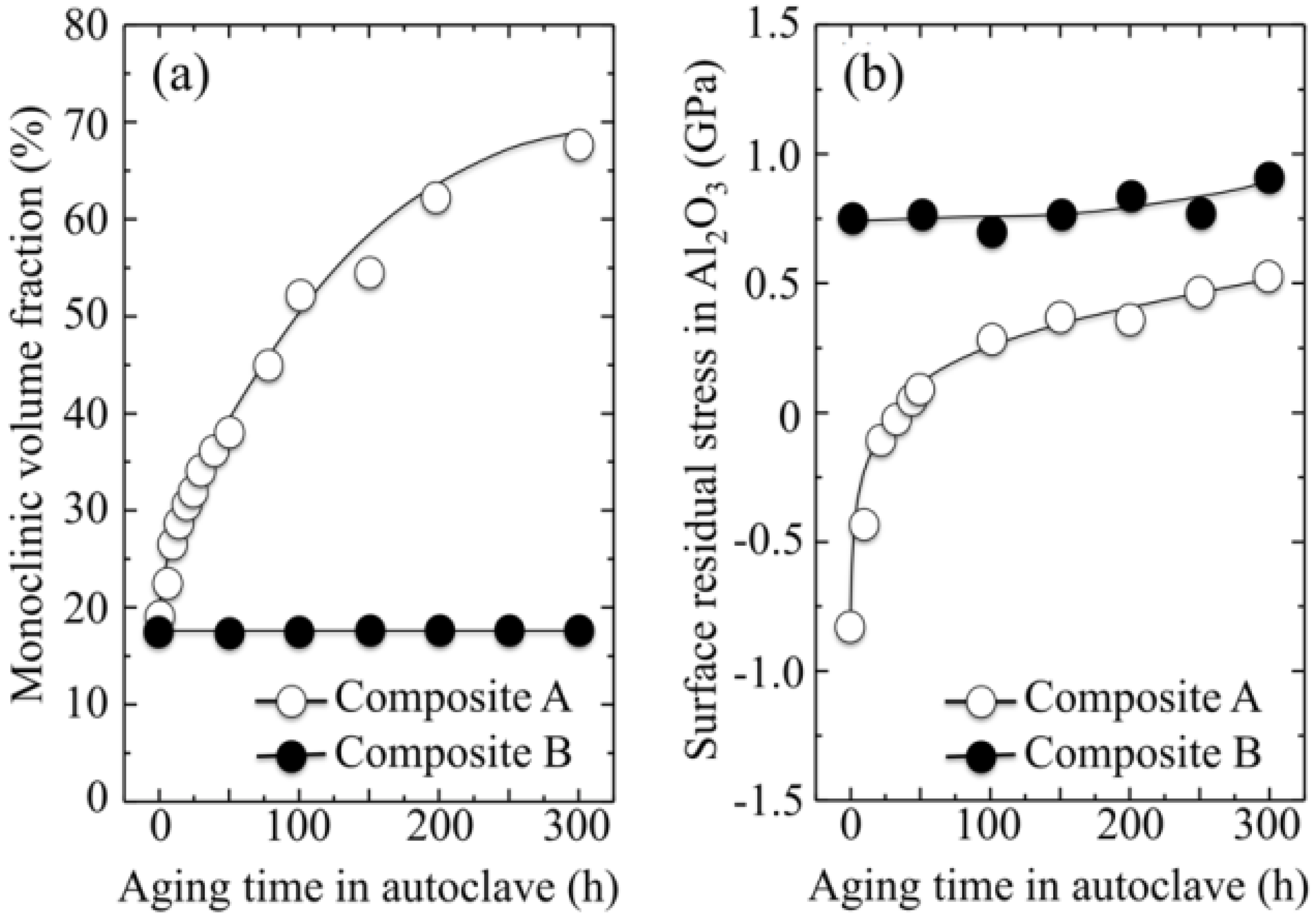
- (i)
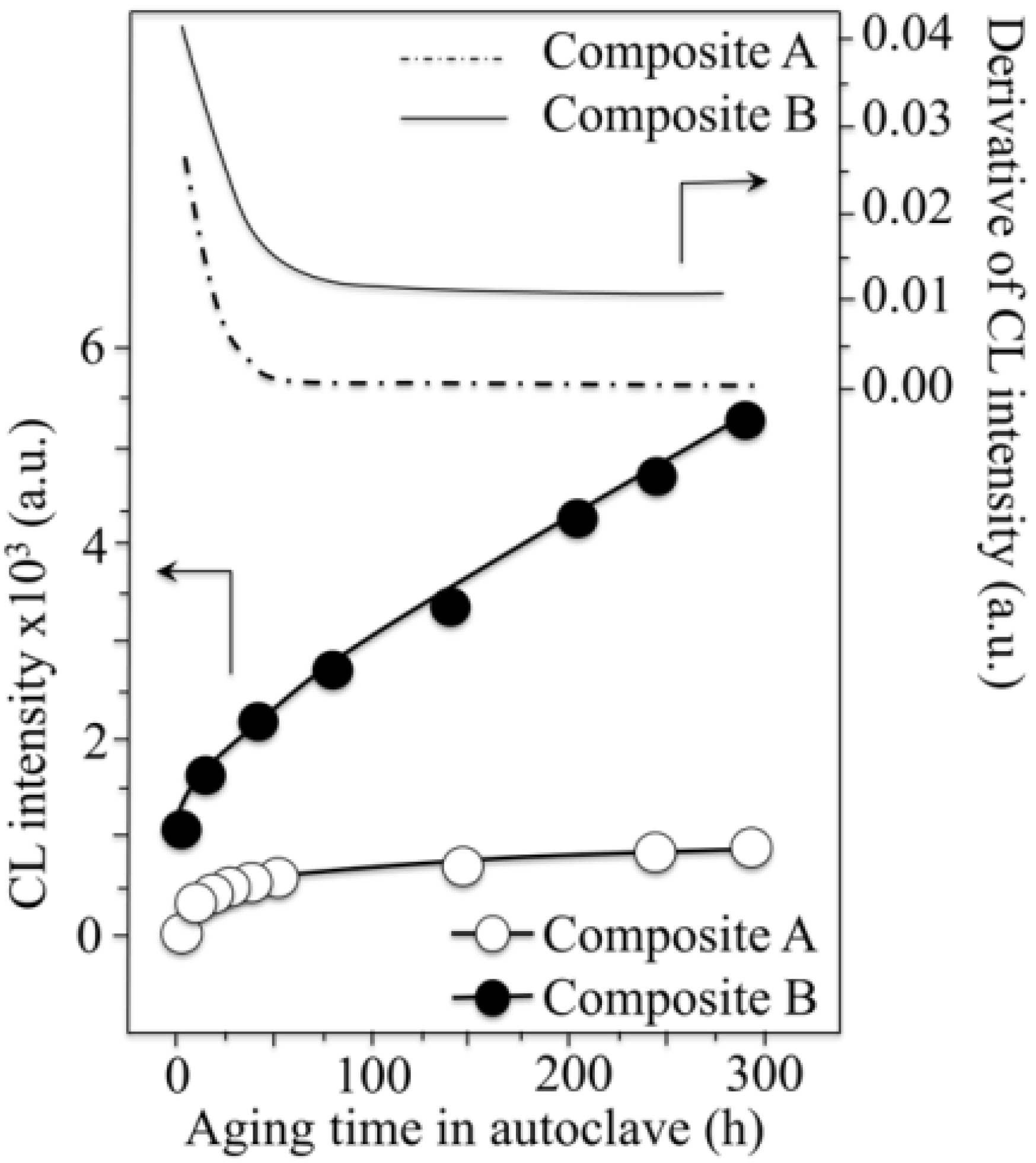
- The intensity of the F+-center emission (at 325 nm) from oxygen vacancies in the Al2O3 lattice was quite low in the as-sintered Composite A as compared to Composite B, although the intensity of this spectroscopic band consistently increased in both composites with increasing exposure time in autoclave. This means that oxygen vacancies were increasingly (and similarly) created on the surface of both alumina matrices by the effect of hydrothermal treatment. However, such increase in concentration started from quite different initial values (i.e., significantly lower in Composite A).
- (ii)
- The derivative plots in Figure 13 show that vacancy formation in the Al2O3-matrix phase of Composite A tended to saturate (derivative approaching zero), while in Composite B vacancy concentration continuously increased at a constant rate after an initially (mild) exponential rising.
- (iii)
- It was also found that the cumulative intensity of the CL emission from F+ and
centers in ZrO2 (not shown here) strongly increased upon autoclaving Composite A, but remained almost constant (at high intensity) in Composite B up to 300 h exposure. Reminding that the intensity of these CL bands is representative of the concentration of oxygen vacancies annihilated in the ZrO2 lattice, one could deduce that the tetragonal ZrO2 phase in Composite A was initially rich in vacancies (due to the presence of Y3+ in its lattice), but underwent vacancy annihilation upon hydrothermal exposure. On the other hand, the as-received Composite B possessed a lower amount of oxygen vacancies in its ZrO2 phase, due to a lack of stabilizing phase. But, such low fraction was stably retained even after quite extended environmental exposures. We know from our studies of monolithic Al2O3 that the increasing trend for the F+ emission from the Al2O3 lattice is due to surface hydroxylation followed by the formation of oxygen vacancies, in order to maintain electrical equilibrium on the material surface [27]. However, owing to the presence of traces of different dopants in solid solution in the corundum structure, there are fundamental differences between the processes of vacancy formation occurring in the Al2O3 matrices of the two studied composites. The very low initial amount of oxygen vacancies in the Al2O3 phase of Composite A (cf. CL intensity at 0 h in Figure 13) can be explained by considering that the Cr3+ isovalent cation behaves as a protective element against oxygen defect formation [128]. On the other hand, Ti3+ ions do not possess such property, besides their isovalent incorporation into the Al2O3 lattice should be similarly possible without alteration of the native oxygen sub-lattice [129]. Despite the steep vacancy-formation rate experienced in the first 50 h of autoclave exposure, the intensity of CL emission from the Al2O3-F+ centers in Composite A always remained distinctly below that recorded for Composite B. In other words, there was a systematically higher oxygen vacancy concentration in the latter biomaterial. Being a donor-type dopant, Ti3+ sites should tend to annihilate through enhancing the incorporation of oxygen excess in the crystal lattice (i.e., with providing the electrons that are required for trapping O2− from the environment). Oxygen vacancies might then gain the outer electron of Ti3+ sites creating (Ti4+-F+) spatially correlated complexes in the Al2O3 lattice.

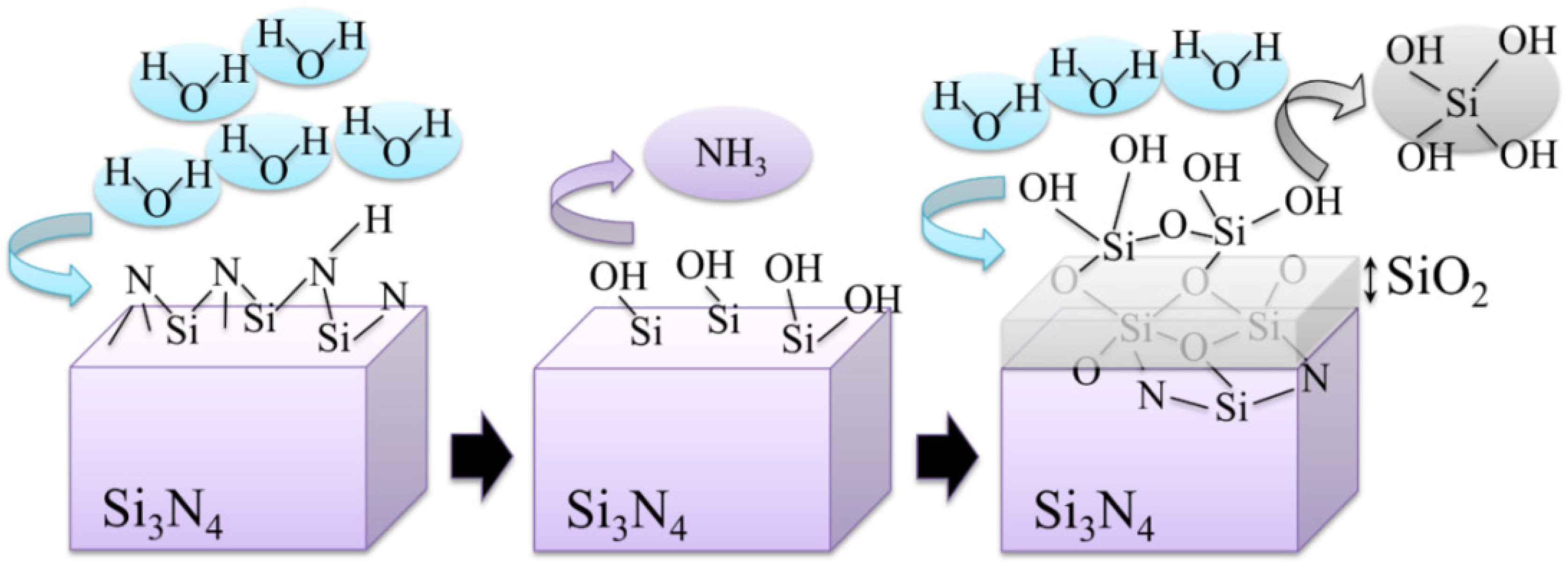
3.3. Ionic or Covalent?
4. Conclusions
Conflicts of Interest
References
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Courpied, J.P.; Caton, J. Total hip arthroplasty, state of the art for the 21st century. Int. Orthop. 2011, 35, 149–150. [Google Scholar]
- Räsänen, P.; Paavolainen, P.; Sintonen, H.; Koivisto, A.M.; Blom, M.; Ryynänen, O.P.; Roine, R.P. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop. 2007, 78, 108–115. [Google Scholar]
- Stea, S.; Bordini, B.; Sudanese, A.; Toni, A. Registration of hip prostheses at the Rizzoli Institute: 11 years’ experience. Acta Orthop. Scand. Suppl. 2002, 73, 40–44. [Google Scholar]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Okita, S.; Hasegawa, M.; Takahashi, Y.; Puppulin, L.; Sudo, A.; Pezzotti, G. Failure analysis of sandwich-type ceramic-on-ceramic hip joints: A spectroscopic investigation into the role of the polyethylene shell component. J. Mech. Behav. Biomed. Mater. 2014, 31, 55–67. [Google Scholar] [CrossRef]
- Pezzotti, G.; Sakakura, S.; Porporati, A.A.; Clarke, I.C.; Green, D.D. Phase transformation and residual stresses in retrieved zirconia hip implant—a Raman microprobe spectroscopy study. In Bioceramics: Materials and Applications IV; Sundar, V., Rusin, R.P., Rutiser, C.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Volume 147, pp. 141–154. [Google Scholar]
- Pezzotti, G. Advanced Materials for Joint Implants, 1st ed.; Pan Stanford Publ Pte Ltd.: Singapore, Republic of Singapore, 2013. [Google Scholar]
- Pezzotti, G.; Yamamoto, K. Artificial hip joints: The biomaterials challenge. J. Mech. Behav. Biomed. Mater. 2014, 31, 3–20. [Google Scholar] [CrossRef]
- Kim, T.I.; Jung, Y.H.; Chung, H.J.; Yu, K.J.; Ahmed, N.; Corcoran, C.J.; Park, J.S.; Jin, S.H.; Rogers, J.A. Deterministic assembly of releasable single crystal silicon-metal oxide field-effect devices formed from bulk wafers. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Pezzotti, G. Stress microscopy and confocal Raman imaging of load-bearing surfaces in artificial joints. Expert Rev. Med. Devices 2007, 4, 165–189. [Google Scholar] [CrossRef]
- Pezzotti, G.; Munisso, M.C.; Porporati, A.A.; Lessnau, K. On the role of oxygen vacancies and lattice strain in the tetragonal to monoclinic transformation in alumina/zirconia composites and improved environmental stability. Biomater 2010, 31, 6901–6908. [Google Scholar]
- Pezzotti, G.; Yamada, K.; Sakakura, S.; Pitto, R.P. Raman spectroscopic analysis of advanced ceramic composite for hip prosthesis. J. Am. Ceram. Soc. 2008, 91, 1199–1206. [Google Scholar] [CrossRef]
- Takahashi, Y.; Zhu, W.; Sugano, N.; Pezzotti, G. On the role of oxygen vacancies, aliovalent ions and lattice strain in the in vivo wear behavior of alumina hip joints. J. Mech. Behav. Biomed. Mater. 2011, 4, 993–1003. [Google Scholar] [CrossRef]
- Fukatsu, K.; Leto, A.; Zhu, W.; Sugano, N.; Pezzotti, G. Kinetics and the role of off-stoichiometry in the environmentally driven phase transformation of commercially available zirconia femoral heads. Acta Biomater. 2012, 8, 1639–1647. [Google Scholar]
- Leto, A.; Zhu, W.; Matsubara, M.; Pezzotti, G. Bioinertness and fracture toughness evaluation of the monoclinic zirconia surface film of OxiniumTM femoral head by Raman and cathodoluminescence spectroscopy. J. Mech. Behav. Biomed. Mater. 2014, 21, 135–144. [Google Scholar]
- Zhu, W.; Sugano, N.; Pezzotti, G. Nondestructive inspection of phase transformation in zirconia-containing hip joints by confocal Raman spectroscopy. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef]
- Pezzotti, G.; Kumakura, T.; Yamada, K.; Tateiwa, T.; Puppulin, L.; Zhu, W.; Yamamoto, K. Confocal Raman spectroscopic analysis of cross-linked ultra-high molecular weight polyethylene for application in artificial hip joints. J. Biomed. Opt. 2007, 12. [Google Scholar] [CrossRef]
- Pezzotti, G.; Takahashi, Y.; Takamatsu, S.; Puppulin, L.; Nishii, T.; Miki, H.; Sugano, N. Non-destructively differentiating the roles of creep, wear and oxidation in long-ter, in vivo exposed polyethylene cups. J. Biomater. Sci. Polym. Ed. 2011, 22, 2165–2184. [Google Scholar]
- Takahashi, Y.; Puppulin, L.; Zhu, W.; Pezzotti, G. Raman tensor analysis of ultra-high molecular weight polyethylene and its application to study retrieved hip joint components. Acta Biomater. 2010, 6, 3583–3594. [Google Scholar] [CrossRef]
- Schlenker, B.R. Introduction to Materials Science: SI Edition; John Wiley & Sons: Australasia Pty: Sydney, Australia, 1974. [Google Scholar]
- Basu, B.; Kalin, M. Tribology of Ceramics and Composites: Materials Science Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Williams, D.F. Bioinertness: An outdated principle. In Tissue Engineering of Prosthetic Vascular Graft; Zilla, P., Greisler, H.P., Eds.; R.G. Landes Co.: Georgetown, TX, USA, 1999. [Google Scholar]
- Jeng, M.C.; Yan, L.Y. Environmental effects on wear behavior of alumina. Wear 1993, 161, 111–119. [Google Scholar] [CrossRef]
- Mori, S. The lubrication of ceramics—Adsorption and chemical reaction. Tribol. J. Jpn. Soc. Tribol. 1991, 36, 130–134. [Google Scholar]
- Shapovalov, V.; Truong, T.N. Ab initio study of water adsorption on α-Al2O3 (0001) crystal surface. J. Phys. Chem. B 2000, 104, 9859–9866. [Google Scholar] [CrossRef]
- Fernandez, E.M.; Eglitis, R.I.; Borstel, G.; Balbas, L.C. Ab initio calculations of H2O and O2 adsorption on Al2O3 substrates. Comput. Mater. Sci. 2007, 39, 587–592. [Google Scholar] [CrossRef]
- Freund, H.J.; Kuhlenbeck, H.; Staemmler, V. Oxide surfaces. Rep. Prog. Phys. 1996, 59, 283–347. [Google Scholar] [CrossRef]
- Lippens, B.C.; Steggerda, J.J. Active alumina. In Physical and Chemical Aspects of Adsorbents and Catalysts; Linsen, B.G., Ed.; Academic: New York, NY, USA, 1970. [Google Scholar]
- Knözinger, H.; Ratnasamy, P. Catalytic aluminas: Surface models and characterization of surface sites. Catal. Rev.Sci. Eng. 1978, 17, 31–70. [Google Scholar] [CrossRef]
- Morterra, C.; Ghiotti, G.; Garrone, E.; Boccuzzi, F. Infrared spectroscopic characterization of the γ-alumina surface. J. Chem. Soc.Faraday Trans. 1976, 72, 2722–2734. [Google Scholar] [CrossRef]
- Morterra, C.; Magnacca, G.; Cerrato, G.; Del Favero, N.; Filippi, F.; Folonari, C.V. X-ray diffraction, high-resolution transmission electron microscopy and Fourier-transform infrared study of Ca-doped Al2O3. J. Chem. Soc. Faraday Trans. 1993, 89, 135–150. [Google Scholar] [CrossRef]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Mardilovich, P.P. Structure of alumina surfaces. J. Chem. Soc. Faraday Trans. 1996, 92, 4843–4852. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Mirnov, K.S.S.; Rzhevskij, A.M.; Mardilovich, P.P. Infrared spectroscopic evidence for the structural OH− groups of spinel alumina modifications. Mater. Chem. Phys. 1990, 26, 35–46. [Google Scholar] [CrossRef]
- Liu, X.; Truitt, R.E. DRFT-IR studies of the surface of γ-alumina. J. Am. Chem. Soc. 1997, 119, 9856–9860. [Google Scholar] [CrossRef]
- Ahn, J.; Rabalais, J.W. Composition and structure of the Al2O3{0001}-(1×1) surface. Surf. Sci. 1997, 388, 121–131. [Google Scholar] [CrossRef]
- Nygren, M.A.; Gay, D.H.; Catlow, C.R.A. Hydroxylation of the surface of the corundum basal plane. Surf. Sci. 1997, 380, 113–123. [Google Scholar] [CrossRef]
- Wang, X.G.; Chaka, A.; Scheffler, M. Effect of the environment on α-Al2O3 (0001) surface structures. Phys. Rev. Lett. 2000, 86, 3650–3653. [Google Scholar]
- Elam, J.W.; Nelson, C.E.; Cameron, M.A.; Tolbert, M.A.; George, S.M. Adsorption of H2O on a single-crystal α-Al2O3 (0001) surface. J. Phys. Chem. B 1998, 102, 7008–7015. [Google Scholar] [CrossRef]
- Wade, W.H.; Hackerman, N. Heats of immersion. IV. The alumina-water system-variations with particle size and outgassing temperature. J. Phys. Chem. 1960, 64, 1196–1199. [Google Scholar] [CrossRef]
- Venable, R.I.; Wade, W.H.; Hackerman, N. Heats of immersion. VIII. Differential heats of adsorption as a function of particle size for the alumina-water system. J. Phys. Chem. 1965, 69, 317–321. [Google Scholar]
- Peri, J.B.; Hannan, R.B. Surface hydroxyl groups on γ-alumina. J. Phys. Chem. 1960, 64, 1526–1530. [Google Scholar] [CrossRef]
- Peri, J.B. Infrared and gravimetric study of the surface hydration of γ-alumina. J. Phys. Chem. 1965, 69, 211–219. [Google Scholar] [CrossRef]
- Yu Yao, Y.-F. Adsorption of polar molecules on metal oxide single crystals. J. Phys. Chem. 1965, 69, 3930–3941. [Google Scholar] [CrossRef]
- Hendriksen, B.A.; Pearce, D.R.; Rudham, R. Heats of adsorption of water on α- and γ-alumina. J. Catal. 1972, 24, 82–87. [Google Scholar] [CrossRef]
- Della Gatta, G.; Fubini, B.; Stradella, L. Energies of different surface rehydration processes on “eta”, “theta” and “alpha” aluminas. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1997, 73, 1040–1049. [Google Scholar]
- Chen, J.G.; Crowell, J.E.; Yates, J.T., Jr. Assignment of a surface vibrational mode by chemical means: Modification of the lattice modes of aluminum oxide by a surface reaction with water. J. Chem. Phys. 1986, 84, 5906–5909. [Google Scholar] [CrossRef]
- Thiel, P.A.; Madey, T.E. The interaction of water with solid surfaces: fundamental aspects. Surf. Sci. Rep. 1987, 7, 211–385. [Google Scholar] [CrossRef]
- Frederick, B.G.; Apai, G.; Rhodin, T.N. Electronic and vibrational properties of hydroxylated and dehydroxylated thin alumina films. Surf. Sci. 1991, 244, 67–80. [Google Scholar] [CrossRef]
- Rossi, P.F.; Oliveri, G.; Bassoli, M. Heat of water chemisorption on α-Al2O3 at 200–400 °C. J. Chem. Soc. Faraday Trans. 1994, 90, 363–366. [Google Scholar] [CrossRef]
- McHale, J.M.; Navrotsky, A.; Perrotta, A.J. Effects of increased surface area and chemisorbed H2O on the relative stability of nanocrystalline γ-Al2O3 and α-Al2O3. J. Phys. Chem. B 1997, 101, 603–613. [Google Scholar] [CrossRef]
- McHale, J.M.; Auroux, A.; Perrotta, A.J.; Navrotsky, A. Surface energies and thermodynamic stability in nanocrystalline alumina. Science 1997, 277, 788–791. [Google Scholar] [CrossRef]
- Hass, K.C.; Schneider, W.F.; Curioni, A.; Andreoni, W. The chemistry of water on alumina surfaces: Reaction dynamics from first principles. Science 1998, 282, 265–268. [Google Scholar] [CrossRef]
- Nevelos, J.E.; Ingham, E.; Doyle, C.; Streicher, R.; Nevelos, A.B.; Walter, W.; Fisher, J. Microseparation of the centers of alumina-alumina artificial hip joints during simulator testing produces clinically relevant wear rates and patterns. J. Arthropl. 2000, 15, 793–795. [Google Scholar] [CrossRef]
- Manaka, M.; Clarke, I.C.; Yamamoto, K.; Shishido, T.; Gustafson, A.; Imakiire, A. Stripe wear rates in alumina THR—Comparison of microseparation simulator study with retrieved implants. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 15, 149–157. [Google Scholar]
- Zeng, H.; Chittur, K.K.; Lacefield, W.R. Analysis of bovine serum albumin adsorption on calcium phosphate and titanium surfaces. Biomater 1999, 20, 377–384. [Google Scholar] [CrossRef]
- Garrett, Q.; Griesser, H.J.; Milthorpe, B.K.; Garrett, R.W. Irreversible adsorption of human serum albumin to hydrogel contact lenses: A study using electron spin resonance spectroscopy. Biomater 1999, 20, 1345–1356. [Google Scholar] [CrossRef]
- Mishina, M.; Kojima, M. Changes in human serum albumin on arthroplasty frictional surfaces. Wear 2008, 265, 655–663. [Google Scholar] [CrossRef]
- Nevelos, J.E.; Prudhommeaux, F.; Hamadouche, M.; Doyle, C.; Ingham, E.; Meunier, A.; Nevelos, A.B.; Sedel, L.; Fisher, J. Comparative analysis of two different types of alumina-alumina hip prosthesis retrieved for aseptic loosening. J. Bone Joint Surg. 2001, 83B, 598–603. [Google Scholar]
- Deckman, D.E.; Jahanmir, S. Wear mechanisms of alpha alumina lubricated with a paraffin oil. Wear 1991, 149, 155–168. [Google Scholar] [CrossRef]
- Dahl, L.E.; Dahl, I.M.; Engström-Laurent, A.; Granath, K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann. Rheum. Dis. 1985, 44, 817–822. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the concentrations of imperfections in solids. J. Phys. Chem. Solids 1958, 5, 208–223. [Google Scholar] [CrossRef]
- Morikawa, H.; Shimizugawa, Y.; Murumo, F.; Harashawa, T.; Ikawa, H.; Tohji, K.; Udagawa, Y. Local structures around Y atoms in Y2O3-stabilized tetragonal ZrO2. J. Jpn. Ceram. Soc. 1988, 96, 253–258. [Google Scholar] [CrossRef]
- Guo, X. Low temperature degradation mechanism of tetragonal zirconia ceramics in water: Role of oxygen vacancies. Solid State Ion. 1998, 112, 113–116. [Google Scholar] [CrossRef]
- Clarke, I.C.; Manaka, M.; Green, D.D.; Williams, P.; Pezzotti, G.; Kim, Y.H.; Ries, M.; Sugano, N.; Sedel, L.; Delauney, C.; et al. Current status of zirconia used in total hip implants. J. Bone Joint Surg. 2003, 85A, 73–84. [Google Scholar]
- Clarke, I.C.; Green, D.D.; Pezzotti, G.; Sakakura, S.; Ben-Nissan, B. The bio-lubrication phenomena: Proteins may control the wear performance of zirconia hip joints. In Bioceramics: Materials and Applications IV; Sundar, V., Rusin, R.P., Rutiser, C.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Volume 147. [Google Scholar]
- Lee, K.H.; Crawford, J.H. Luminescence of the F center in sapphire. Phys. Rev. B 1979, 19, 3217–3221. [Google Scholar] [CrossRef]
- Draeger, B.G.; Summers, G.P. Defects in unirradiated α-Al2O3. Phys. Rev. B 1979, 19, 1172–1177. [Google Scholar] [CrossRef]
- Ghamnia, M.; Jardin, C.; Bouslama, M. Luminescence centres F and F+ in α-alumina detected by cathodoluminescence technique. J. Electron Spectr. Rel. Phenom. 2003, 133, 55–63. [Google Scholar]
- Pezzotti, G.; Wan, K.; Munisso, M.C.; Zhu, W. Stress dependence of F+-center cathodoluminescence of sappire. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Munisso, M.C.; Zhu, W.; Leto, A.; Pezzotti, G. Stress dependence of sapphire cathodoluminescence from optically active oxygen defects as a function of crystallographic orientation. J. Phys. Chem. A 2007, 111, 3526–3533. [Google Scholar] [CrossRef]
- Brandt, G.; Mikus, M. An electron microprobe and cathodoluminescence study of chemical reactions between tool and workpiece when turning steel with alumina-based ceramics. Wear 1987, 115, 243–263. [Google Scholar] [CrossRef]
- Petrik, N.G.; Taylor, D.P.; Orlando, T.M. Laser-stimulated luminescence of yttria-stabilized cubic zirconia crystals. J. Appl. Phys. 1999, 85, 6770–6776. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.W.; Penner-Hahn, J.E. X-ray-absorption studies of zirconia polymorphs. I. Characteristic local structures. Phys. Rev. B 1993, 48, 10063–10073. [Google Scholar]
- Smits, K.; Grigorjeva, L.; Qojkowski, W.; Fidelus, J.D. Luminescence of oxygen related defects in zirconia nanocrystals. Phys. Stat. Solidi C 2007, 4, 770–773. [Google Scholar]
- Smits, K. Luminescence of Zirconia Nanocrystals. Ph.D. Thesis, Faculty of Physics and Mathematics, University of Latvia, Riga, Latvia, May 2010. [Google Scholar]
- Gholinia, A.; Leach, C. Cathodoluminescence microscopy of impurity phases in ZrO2/Ni nano-composites. J. Mater. Sci. 1997, 32, 6625–6628. [Google Scholar] [CrossRef]
- Pezzotti, G.; Munisso, M.C.; Lessnau, K.; Zhu, W. Quantitative assessments of residual stress fields at the surface of alumina hip joints. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95B, 250–262. [Google Scholar] [CrossRef]
- Zhu, W.; Munisso, M.C.; Matsutani, A.; Ge, W.; Pezzotti, G. Stress perturbation method for the assessment of cathodoluminescence probe response function. Appl. Spectr. 2009, 63, 185–191. [Google Scholar] [CrossRef]
- Evans, A.G. Microfracture from thermal expansion anisotropy—I. Single phase systems. Acta Metall. 1978, 26, 1845–1853. [Google Scholar]
- Dillon, S.; Miller, H.; Harmer, M.P.; Rohrer, G.S. Grain boundary plane distributions in aluminas evolving by normal and abnormal grain growth and displaying different complexions. Int.J. Mat. Res. 2010, 101, 1–7. [Google Scholar]
- West, G.D.; Perkins, J.M.; Lewis, M.H. The effect of rare earth dopants on grain boundary coesion in alumina. J. Eur. Ceram. Soc. 2007, 27, 1913–1918. [Google Scholar]
- Buban, J.P.; Matsunaga, K.; Chen, J.; Shibata, N.; Ching, W.Y.; Yamamoto, T.; Ikuhara, Y. Grain boundary strengthening in alumina by rare earth impurities. Science 2006, 311, 212–215. [Google Scholar]
- Zeghad, A.; N’Guyen, F.; Forest, S.; Gourgues, A.F.; Bouaziz, O. Ensemble averaging stress-strain fields in polycrystalline aggregates with a constrained surface microstructure—Part 1: Anisotropic elastic behavior. Phil. Mag. 2007, 87, 1401–1424. [Google Scholar]
- Ma, Q.; Pompe, W.; Clarke, D.R. Residual stress in Al2O3-ZrO2 composites: A test of stochastic stress models. Acta Metall. Mater. 1994, 42, 1673–1681. [Google Scholar] [CrossRef]
- Ma, Q.; Clarke, D.R. Piezo-spectroscopic determination of residual stresses in polycrystalline alumina. J. Am. Ceram. Soc. 1994, 77, 298–302. [Google Scholar] [CrossRef]
- Willmann, G. Ceramic femoral head retrieval data. Clin. Orthop. Rel. Res. 2000, 379, 22–28. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sugano, N.; Zhu, W.; Nishii, T.; Sakai, T.; Takao, M.; Pezzotti, G. Wear degradation of long-term in vivo exposed alumina-on-alumina hip joint: Linking nanometer-scale phenomena to macroscopic joint design. J. Mater. Sci. Mater. Med. 2012, 23, 591–603. [Google Scholar] [CrossRef]
- Shishido, T.; Clarke, I.C.; Williams, P.; Boehler, M.; Asano, T.; Shoji, H.; Masaoka, T.; Yamamoto, K.; Imakiire, A. Clinical and simulator wear study of alumina ceramic THR to 17 years and beyond. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 638–647. [Google Scholar]
- Larché, F.; Cahn, J.W. The interactions of composition and stress in crystalline solids. Acta Metall. 1985, 33, 331–357. [Google Scholar] [CrossRef]
- Pezzotti, G.; Takahashi, Y.; Zhu, W.; Sugano, N. In-depth profiling of elastic residual stress and the in vivo wear mechanism of self-mating alumina hip joints. Wear 2012, 284–285, 91–97. [Google Scholar] [CrossRef]
- Oonishi, H.; Clarke, I.C.; Good, V.; Amino, H.; Ueno, M. Alumina hip joints characterized by run-in wear and steady state wear to 14 million cycles in hip-simulator model. J. Biomed. Mater. Res. Part A 2004, 70, 523–532. [Google Scholar]
- Rainforth, W.M. The wear behaviour of oxide ceramics—A Review. J. Mater. Sci. 2004, 39, 6705–6721. [Google Scholar] [CrossRef]
- Barceinas-Sanchez, O.; Rainforth, W.M. On the role of plastic deformation during the mild wear of alumina. Acta Mater. 1998, 46, 6475–6483. [Google Scholar] [CrossRef]
- Kalin, M.; Novak, S.; Vižintin, J. Wear and friction behavior of alumina ceramics in acqueous solutions with different pH. Wear 2003, 254, 1141–1146. [Google Scholar] [CrossRef]
- Castaing, J.; Kronenberg, A.K.; Kirby, S.H.; Mitchell, T.E. Hydrogen defects in α-Al2O3 and water weakening of sapphire and alumina ceramics between 600 °C and 1000 °C—II. Mechanical properties. Acta Mater. 2000, 48, 1495–1504. [Google Scholar] [CrossRef]
- Rieth, P.H.; Reed, J.S.; Naumann, A.W. Fabrication and flexural strength of ultra-fine grained yttria-stabilised zirconia. Bull. Am. Ceram. Soc. 1976, 55, 717–721. [Google Scholar]
- Christel, P.; Meunier, A.; Heller, M.; Torre, J.P.; Peille, C.N. Mechanical properties and short-term in vivo evaluation of yttrium-oxide partially-stabilized zirconia. J. Biomed. Mater. Res. 1989, 23, 45–61. [Google Scholar]
- Tateishi, T.; Yunoki, H. Research and development of alumina and zirconia artificial hip joint. Clin. Mater. 1993, 12, 219–225. [Google Scholar] [CrossRef]
- Piconi, C.; Burger, W.; Richter, H.G.; Cittadini, A.; Maccauro, G.; Covacci, V.; Bruzzese, N.; Ricci, G.A.; Marmo, E. Y-TZP ceramics for artificial joint replacements. Biomater 1998, 19, 1489–1494. [Google Scholar]
- Samaras, D.; Dimitroulias, A.; Varitimidis, S.E.; Malizos, K.N. Delayed fracture of a zirconium head after total hip arthroplasty. Am. J. Orthop. 2011, 40, E14–E16. [Google Scholar]
- Medical Devices Agency-Adverse Incident Centre. Zirconia Ceramic Heads for Modular Total Hip Femoral Components: Advice to Users on Resterilization; Safety Notice MDA SN 9617; Medical Devices Agency: London, UK, 1996. [Google Scholar]
- Kawanabe, K.; Liang, B.; Ise, K.; Nakamura, T. Alumina and zirconia heads in cemented total hip arthroplasty. In Bioceramics and Alternative Bearings in Joint Arthroplasty; Chang, J.D., Billau, K., Eds.; Steinkopff Verlag: Heidelberg, Germany, 2007; pp. 83–87. [Google Scholar]
- Guo, X. Roles of alumina in zirconia for functional applications. J. Am. Ceram. Soc. 2003, 86, 1867–1873. [Google Scholar] [CrossRef]
- Guo, X. On the degradation of zirconia ceramic during low- temperature annealing in water or water vapor. J. Phys. Chem. Solids 1999, 60, 539–546. [Google Scholar] [CrossRef]
- Matsui, K.; Ohmichi, N.; Ohgai, M.; Enomoto, N.; Hojo, J. Sintering kinetics at constant rates of heating: Effect of Al2O3 on the initial sintering stage of fine zirconia powder. J. Am. Ceram. Soc. 2005, 88, 3346–3352. [Google Scholar]
- Matsui, K.; Yamakawa, T.; Uehara, M.; Enomoto, N.; Hojo, J. Mechanism of alumina-enhanced sintering of fine zirconia powder: Influence of alumina concentration on the initial stage sintering. J. Am. Ceram. Soc. 2008, 91, 1888–1897. [Google Scholar] [CrossRef]
- Li, J.F.; Watanabe, R. Influence of a small amount of Al2O3 addition on the transformation of Y2O3-partially stabilized ZrO2 during annealing. J. Mater. Sci. 1997, 32, 1149–1153. [Google Scholar] [CrossRef]
- Mullens, S.; Cooymans, J.; Smolders, C.; Luyten, J. Processing oxide-based nanocomposites. Am. Ceram. Soc. Bull. 2005, 84, 9101–9103. [Google Scholar]
- Nasu, N.; Hirota, D.; Inoue, K.; Hashimoto, T.; Ishihara, A. Luminescent properties of amorphous Al2O3 prepared by sol-gel method. J. Ceram. Soc. Jpn. 2008, 116, 835–836. [Google Scholar] [CrossRef]
- Adiga, S.P.; Zapol, P.; Curtiss, L.A. Structure and morphology of hydroxylated amorphous alumina surfaces. J. Phys. Chem. C 2007, 111, 7422–7429. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Burger, W.; Richter, H.G. High strength and toughness alumina matrix composites by transformation toughening and “in situ“ platelet reinforcement (ZPTA). Key Eng. Mater. 2001, 192–195, 545–548. [Google Scholar] [CrossRef]
- Nakanishi, T.; Sasaki, M.; Ikeda, J.; Miyaji, F.; Kondo, M. Mechanical and phase stability of zirconia toughened alumina. Key Eng. Mater. 2007, 330–332, 1267–1270. [Google Scholar] [CrossRef]
- Ikeda, J.; Pezzotti, G.; Nakanishi, T. Phase stability of zirconia toughened alumina composite for artificial joints. Key Eng. Mater. 2006, 309–311, 1243–1246. [Google Scholar] [CrossRef]
- Richter, H.G.; Willmann, G. Reliability of ceramic components for total hip prostheses. Br. Ceram. Trans. 1999, 98, 29–34. [Google Scholar] [CrossRef]
- Kuntz, M. Validation of a new high performance alumina matrix composite for use in total joint replacement. Semin. Arthropl. 2006, 17, 141–145. [Google Scholar] [CrossRef]
- Insley, G.M.; Streicher, R.M. Next generation ceramics based on zirconia toughened alumina for hip joint prostheses. Key Eng. Mater. 2004, 254–256, 675–678. [Google Scholar] [CrossRef]
- Chevalier, J.; Grandjean, S.; Kuntz, M.; Pezzotti, G. On the kinetics and impact of tetragonal to monoclinic transformation in an alumina/zirconia composite for arthroplasty applications. Biomater. 2009, 30, 5279–5282. [Google Scholar] [CrossRef]
- Schubert, H.; Frey, F. Stability of Y-TZP during hydrothermal treatment: neutron experiments and stability considerations. J. Eur. Ceram. Soc. 2005, 25, 1597–1602. [Google Scholar] [CrossRef]
- Kreher, W.; Pompe, W. Internal Stresses in Heterogeneous Solids; Akademie Verlag: Berlin, Germany, 1988. [Google Scholar]
- Gregori, G.; Burger, W.; Sergo, V. Piezo-spectroscopic analysis of the residual stresses in zirconia-toughened alumina ceramics: The influence of the tetragonal-to-monoclinic transformation. Mater. Sci. Eng. A 1999, 271, 401–406. [Google Scholar] [CrossRef]
- Scardi, P.; Leoni, M.; Bertamini, L. Residual stresses in plasma sprayed partially stabilized zirconia TBCs: Influence of the deposition temperature. Thin Solid Films 1996, 278, 96–103. [Google Scholar] [CrossRef]
- Lushchik, A.; Lushchik, C.; Kirm, M.; Nagirnyi, V.; Savikhin, F.; Vasil’chenko, E. Defect creation caused by the decay of cation excitons and hot electron-hole recombination in wide-gap dielectrics. Nucl. Instr. Meth. B 2006, 250, 330–336. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Kröger, F.A. Defect structure of α-Al2O3 doped with titanium. J. Am. Ceram. Soc. 1977, 60, 381–387. [Google Scholar] [CrossRef]
- Zhu, W.; Puppulin, L.; Leto, A.; Takahashi, Y.; Sugano, N.; Pezzotti, G. In situ measurements of local temperature and contact stress magnitude during wear of ceramic-on-ceramic hip joints. J. Mech. Behav. Biomed. Mater. 2014, 31, 68–76. [Google Scholar]
- Puppulin, L.; Leto, A.; Zhu, W.; Sugano, N.; Pezzotti, G. Innovative tribometer for in situ spectroscopic analyses of wear mechanisms and phase transformation in ceramic femoral heads. J. Mech. Behav. Biomed. Mater. 2014, 31, 45–54. [Google Scholar] [CrossRef]
- Bal, B.S.; Khandkar, A.; Lakshminarayanan, A.; Clarke, I.C.; Hoffman, A.A.; Rahaman, M.N. Testing of silicon nitride ceramic bearings for total hip arthroplasty. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 447–454. [Google Scholar]
- Mazzocchi, M.; Gardini, D.; Traverso, P.L.; Faga, M.G.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part II: Chemical stability and wear resistance in body environment. J. Mater. Sci. Mater. Med. 2008, 19, 2889–2901. [Google Scholar] [CrossRef]
- Zhang, W.; Titze, M.; Cappi, B.; Wirtz, D.C.; Telle, R.; Fischer, H. Improved mechanical long-term reliability of hip resurfacing prostheses by using silicon nitride. J. Mater. Sci. Mater. Med. 2010, 21, 3049–3057. [Google Scholar]
- Bal, B.S.; Rahaman, M.N. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef]
- Pezzotti, G.; Yamada, K.; Porporati, A.A.; Kuntz, M.; Yamamoto, K. Fracture toughness analysis of advanced ceramic composite for hip prosthesis. J. Am. Ceram. Soc. 2009, 92, 1817–1822. [Google Scholar] [CrossRef]
- Hampshire, S. Silicon nitride ceramics—Review of structure, processing and properties. J. Achiev. Mater. Manuf. Eng. 2007, 24, 43–50. [Google Scholar]
- Ziegler, G.; Heinrich, J.; Wötting, G. Review: Relationships between processing, microstructure, and properties of dense and reaction-bonded silicon nitride. J. Mater. Sci. 2007, 22, 3041–3086. [Google Scholar] [CrossRef]
- Kleebe, H.J.; Pezzotti, G.; Ziegler, G. Microstructure and fracture toughness of Si3N4 ceramics: Combined roles of grain morphology and secondary phase chemistry. J. Am. Ceram. Soc. 1999, 82, 1857–1867. [Google Scholar] [CrossRef]
- Becher, B.F.; Sun, E.Y.; Plucknett, K.P.; Alexander, K.B.; Hsueh, C.H.; Lin, H.T.; Waters, S.B.; Westmoreland, C.G.; Kang, E.S.; Hirao, K.; et al. Microstructural design of silicon nitride with improved fracture toughness: I, Effects of grain size and shape. J. Am. Ceram. Soc. 1998, 81, 2821–2830. [Google Scholar]
- Sun, E.Y.; Becher, P.F.; Plucknett, K.P.; Hsueh, C.H.; Alexander, K.B.; Waters, S.B.; Hirao, K.; Brito, M.E. Microstructural design of silicon nitride with improved fracture toughness: II, Effects of yttria and alumina additives. J. Am. Ceram. Soc. 1998, 81, 2831–2840. [Google Scholar]
- Sun, E.Y.; Becher, P.F.; Hsueh, C.H.; Painter, G.S.; Waters, S.B.; Hwang, S.L.; Hoffmann, M.J. Debonding behavior between β-Si3N4 whiskers and oxynitride glasses with or without an epitaxial β-Sialon interfacial layer. Acta Mater. 1999, 47, 2777–2785. [Google Scholar] [CrossRef]
- Becher, P.F.; Painter, G.S.; Shibata, N.; Satet, R.L.; Hoffmann, M.J.; Pennycook, S.J. Influence of additives on anisotropic grain growth in silicon nitride ceramics. Mater. Sci. Eng. A 2006, 422, 85–91. [Google Scholar]
- Gates, R.S.; Hsu, S.M. Boundary Lubrication of Silicon Nitride; National Institute of Standards and Technology (NIST) Special Publication 876; NIST: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Dante, R.C.; Kajdas, C.K. A review and a fundamental theory of silicon nitride tribochemistry. Wear 2012, 288, 27–38. [Google Scholar] [CrossRef]
- Tomizawa, T.E.; Fischer, T.E. Friction and wear of silicon nitride and silicon carbide in water: Hydrodynamic lubrication at low sliding speed obtained by tribochemical wear. ASLE Trans. 1986, 30, 41–46. [Google Scholar] [CrossRef]
- Mizuhara, K.; Hsu, S.M. Tribochemical reaction of oxygen and water on silicon surfaces. Tribol. Series 1992, 21, 323–328. [Google Scholar] [CrossRef]
- Volante, M.; Fubini, B.; Giamello, E.; Bolis, V. Reactivity induced by grinding in silicon nitride. Mater. Sci. Lett. 1989, 8, 1076–1078. [Google Scholar] [CrossRef]
- Muratov, V.A.; Luangvaranunt, T.; Fischer, T.E. The tribochemistry of silicon nitride: Effects of friction, temperature and sliding velocity. Tribol. Int. 1998, 31, 601–611. [Google Scholar] [CrossRef]
- Jia, Z.; Shen, D.; Xu, W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydrate Res. 2001, 333, 1–6. [Google Scholar]
- Dong, X.; Jahanmir, S. Wear transition diagram for silicon nitride. Wear 1993, 165, 169–180. [Google Scholar] [CrossRef]
- Kleebe, H.J.; Cinibulk, M.K.; Cannon, R.M.; Rühle, M. Statistical analysis of the intergranular film thickness in silicon nitride ceramics. J. Am. Ceram. Soc. 1993, 76, 1969–1977. [Google Scholar] [CrossRef]
- Webster, T.J.; Patel, A.A.; Rahaman, M.N.; Sonny Bal, B. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef]
- Gorth, D.J.; Puckett, S.; Ercan, B.; Webster, T.J.; Rahaman, M.; Sonny Bal, B. Decreased bacteria activity on Si3N4 surfaces compared with PEEK or titanium. Int. J. Nanomed. 2012, 7, 4829–4840. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pezzotti, G. Bioceramics for Hip Joints: The Physical Chemistry Viewpoint. Materials 2014, 7, 4367-4410. https://doi.org/10.3390/ma7064367
Pezzotti G. Bioceramics for Hip Joints: The Physical Chemistry Viewpoint. Materials. 2014; 7(6):4367-4410. https://doi.org/10.3390/ma7064367
Chicago/Turabian StylePezzotti, Giuseppe. 2014. "Bioceramics for Hip Joints: The Physical Chemistry Viewpoint" Materials 7, no. 6: 4367-4410. https://doi.org/10.3390/ma7064367




