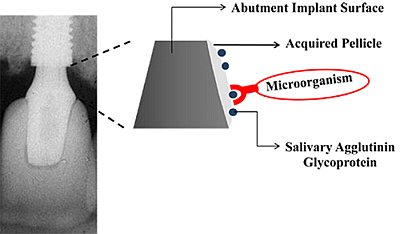
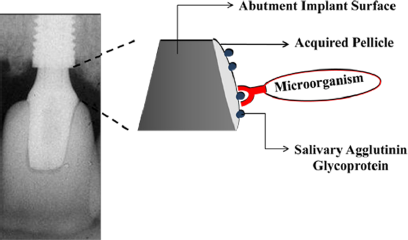
The Relationship between Biofilm and Physical-Chemical Properties of Implant Abutment Materials for Successful Dental Implants
Abstract
: The aim of this review was to investigate the relationship between biofilm and peri-implant disease, with an emphasis on the types of implant abutment surfaces. Individuals with periodontal disease typically have a large amount of pathogenic microorganisms in the periodontal pocket. If the individuals lose their teeth, these microorganisms remain viable inside the mouth and can directly influence peri-implant microbiota. Metal implants offer a suitable solution, but similarly, these remaining bacteria can adhere on abutment implant surfaces, induce peri-implantitis causing potential destruction of the alveolar bone near to the implant threads and cause the subsequent loss of the implant. Studies have demonstrated differences in biofilm formation on dental materials and these variations can be associated with both physical and chemical characteristics of the surfaces. In the case of partially edentulous patients affected by periodontal disease, the ideal type of implant abutments utilized should be one that adheres the least or negligible amounts of periodontopathogenic bacteria. Therefore, it is of clinically relevance to know how the bacteria behave on different types of surfaces in order to develop new materials and/or new types of treatment surfaces, which will reduce or inhibit adhesion of pathogenic microorganisms, and, thus, restrict the use of the abutments with indication propensity for bacterial adhesion.1. Introduction
The success of dental implants depends on the maintenance of osseointegration that is defined as a direct bone-to-implant contact without interposition of any other tissue [1]. Simultaneously, in order to preserve osseointegration around dental implants it is desirable to have no relationship between the maxillary and mandibular or parafunctional forces, mal-aligned forces of stress, peri-implantitis [2,3], absence of systemic diseases, e.g., diabetes mellitus [4], and to consider the host immune-inflammatory response to the bacterial challenge [5]. Despite the relatively high success rates of dental implant survival, reported to be higher than 90% for both partially or completely edentulous patients in longitudinal studies, some groups have demonstrated the role of putative periodontal pathogens in the etiology of peri-implantitis and their deleterious effects on hard and soft peri-implant tissues [6–10].
Late implant failure could be due to a disruption between implant and the mineralized tissues after osseointegration has been established due to overloading or microbial infection [11–13]. Whereas the main problem of osseointegration is solved by the use of high quality implants, with appropriate surface treatment and adequate surgical technique, the peri-implant tissue inflammation as a consequence of biofilms on abutments in the subgingival region is currently considered a major contributor to implant loss [14,15]. The presence of biofilms near to the implant abutments is characterized clinically by inflammation of the peri-implant mucosa progressing to subsequent destruction of the alveolar bone in contact with the implants threads. The teeth are unique structures, unlike the implants, which have the prosthetic restorations that bind to the implant body, e.g., crowns, metal structures or simple metal rods, which can lead to cracks or gaps forming between the implants and connectors. When compared to its natural non-implanted counterpart, peri-implant tissue comprises fewer fibroblasts, an increased amount of collagen fibers, blood supply, and the periosteal vascular plexus and parallel orientation of the gingival fibers [16].
In addition to these inherent factors in histopathology of peri-implant tissue, there are several differences in the designs of implants or macrostructure (screw versus cemented; one or two surgical stages), the type of surface or microstructure (commercially pure titanium, titanium alloys, titanium plasma sprayed, hydroxyapatite surfaces blasted with oxides, treated with acids, or a combination thereof) and the degree of smoothness or roughness or ultrastructure (crystallinity of the hydroxyapatite coating the implant, or nitrous acid type used), as well as different shapes and abutment materials [17]. Thus, these parameters existing between tooth and implant materials profoundly and directly influence the local microbiota.
2. Surface Characteristics of Abutments Implants
The scientific literature shows that bacterial plaque may play a prominent role as an etiologic factor responsible for implant loss after osseointegration, due to the presence of high levels of bacteria in the peri-implant sites [2,18–22]. As observed for teeth, the microorganisms need to interact with the implant abutment surface for the formation and growth of biofilm. Several studies suggest that some restorative materials have antibacterial activity, while others induce bacterial growth.
The physical and chemical characteristics of the materials will determine the type and quantity of the microbiota around these surfaces [23,24]. The non-specific physicochemical mechanisms of bacterial adhesion involve the superficial free energies and interaction surfaces theory in which adhesion is regarded as the interaction of Van der Waals forces and electrostatic phenomena [25]. Surface chemical composition, surface energy, surface water contact angle [26], and roughness are important parameters that may have a critical and fundamental influence on the interaction of biomaterial surfaces with proteins and cells. Once biomaterial surfaces have contact with biological molecules either in vitro or in vivo, the proteins present in the biological medium immediately coat the surfaces. Thereafter, salivary acquired pellicle formation takes place as the first step to biofilm formation (Figure 1).
With regard to the influence of surface roughness on biofilm formation, previous reports showed that protein adsorption and bacterial adhesion in vivo might be determined by a threshold surface roughness of 0.2 μm [27,28]. Burgers et al. [29] evaluated the initial biofilm formation, in vitro and in vivo, on different titanium surfaces and correlated these findings with different surface properties. Before biofilm formation, the authors determined the surface roughness and the surface free energy of samples and observed that the initial bacterial adhesion to differently textured titanium surfaces was primarily influenced by roughness surfaces values. This can be explained because the rough surfaces tend to entrap bacteria into micropits, protecting them from washing forces [28]. The difference in results from in vivo, in situ, and in vitro experiments is clear and the interfering factors involved are inclusion criterions established to select the patients, in relation to in vivo and in situ studies, and the number and types of bacteria used to biofilm formation, in case of in vitro study. Freitas et al. [30] in 2005 showed that a more rough surface causes an exponential increase in the number of bacterial cells, when just one kind of bacterium, Streptococcus sanguis, was utilized. However, when the study was performed upon the same type of surface, titanium, changing only the roughness value, and using a large number of bacteria species, the roughness does not act as an influential factor. In this case, no difference on bacteria adhesion can be justified by the same physical characteristic. The hydrophobicity and hydrophilic characteristic surfaces are other crucial elements that can directly influence bacterial adhesion [31]. In the case of implant surfaces, it is known that bone cells are attracted to a hydrophilic surface [32]. Recent studies have focused on the mechanism of chemical alterations within the dioxide titanium coating to enhance osteoconductivity and improve early osseointegration [33–35]. The increase in surface wettability may also have an influence on the amount of adsorbed proteins, since a very hydrophobic surface may prevent water from wetting the available surface, and, thus, further protein interaction with it. Alternatively, an increase in surface hydrophilicity may reduce the hydrophobic interaction between proteins and the surface, causing a lower adsorption affinity. Moreover, bacteria also have biomolecules in their cell wall that determine the surface properties and the adhesion dynamics [36]. In the case of gram-negative bacteria, the presence of lipopolysaccharide (LPS) in the outer membrane, tends to become more hydrophilic bacterial cell, and increase the attraction to hydrophilic surfaces too [37]. According to Husmark and Ronner, surface charge can also be influenced by the pH of the medium and consequently, change the bacteria adhered to it [38]. The relationship between surface and bacterial cell is mediated by a complex array of chemical and physical interactions, which add to the complexity of identifying the ideal surface with respect to abutment implants.
2.1. Types of Implant Abutments
In relation to the implant material types, titanium is the most commonly used material in dentistry due to its excellent physical and chemical characteristics, i.e., biocompatibility, stability and corrosion resistance [39]. To date, titanium is considered the “gold standard” and has maintained a dominant position as an abutment and implants material in long-term dental implant treatments. However, the high demand for aesthetic restorations has led to the introduction of ceramic implant abutments made from zirconium oxide stabilized with yttrium [40]. The microstructural and mechanical properties of the zirconia, as well as its excellent biocompatibility, have been well documented [41,42]. In dentistry, zirconia has been used for clinical applications in ceramic crowns, fixed partial dentures, orthodontic treatment supports, implants as well as abutments [43]. In addition, it has been shown that zirconia accumulates less plaque than titanium [42]. Despite the ceramic being used as abutment material for several years, only a limited number of related articles have been published concerning biofilm and abutment implants surfaces [44,45].
2.1.1. Microbiology of Periodontal Disease
Periodontitis is a chronic inflammatory disease, initiated by the accumulation of plaque on enamel surfaces in close proximity with the gingival tissue, in which disease expression involves intricate interactions of the biofilm with the host immune inflammatory response and subsequent alterations in bone and connective tissue homeostases [46]. With the permanence of dental plaque on the tooth surface, the population dynamics of the microbiota is changed, favoring the development of biofilm with anaerobic bacteria, in particular microorganisms of the red complex (a group of bacteria that are grouped together based on their association with severe forms of periodontal disease) [47], which are responsible for alveolar bone loss and ultimately the tooth. Among periodontopathogenic bacteria, (Porphyromonas gingivalis), a gram-negative anaerobe and one of the most important pathogens in chronic periodontitis, has the ability for co-aggregation not only with (Fusobacterium nucleatum), but also with early colonizers (such as Streptococcus gordonii) [48], which could help explain its early appearance in the development of dental plaque biofilms [49,50]. However, it is important to mention that the virulence of P. gingivalis has been attributed to a variety of potential factors associated with its cell surface: fimbriae, lipopolysaccharides, capsules, proteases, hemagglutinins, and major outer membrane proteins [51]. On the tooth surfaces, these microorganisms are detected in dental plaque samples within six hours after professional tooth cleaning [52], and their numbers increase in compromised sites. Moreover, these structures can bind with receptors of epithelial cells, invade them and initiate an inflammatory process. The increase of cytokines released by the host defense cells can cause bone resorption and, consequently, loss of teeth or even implants. Attention has also been given to F. nucleatum, a gram-negative anaerobic bacteria, commonly found in the subgingival biofilm in periodontal pockets. This organism also has an important role in biofilm maturation, acting as a bridge between the early and late colonizers, guiding biofilm architecture and, consequently, enhancing the adherence of more periodontitis-associated bacteria [53]. As well as P. gingivalis, F. nucleatum is also capable of adherence to and invasion of host epithelial cells and stimulates the host immune inflammatory response. Since the presence of these microorganisms increases and/or decreases in the presence of other primary and intermediate colonizers, the successful treatment of periodontal disease would suggest an increase of the Actinomyces spp, and simultaneously, a reduction of pathogens of the orange and red complex [54].
2.1.2. Periodontal Disease—Peri-Implant Disease
There is a philosophy that patients with periodontal disease should be considered a risk factor for peri-implantitis [55]. After partial alveolar bone loss as a consequence of periodontal disease, the periodontopathogenic microorganisms remain within periodontal pockets, and these microorganisms have the ability to colonize various implants even after osseointegration has been successfully achieved [47]. The remaining microorganisms adhere to the teeth, as well as on crowns and implants, and directly influence the peri-implant microbiota to promote the plaque development for a more subgingival microbiota [56–58]. The history of periodontitis has been associated with peri-implant disease. Marrone et al. [59] showed the prevalence of peri-implantitis in patients with active periodontitis was 57.1%. Thus, if a patient is not stable with respect to periodontitis they could have more chances to present peri-implantitis on one of their implants after >5 years duration. This finding is in agreement with a study regarding prevalence and risk variables for peri-implant disease in Brazilian subjects where those with periodontitis were more prone to develop peri-implantitis [60]. In addition, other studies have also associated a history of periodontitis with peri-implant disease [57,58,61]. Karoussis et al. [62] compared the survival rate of implants in patients with and without a history of periodontitis. They concluded that in 10 years, the implants survival rate for the group with a past history of chronic periodontitis was 90.5% while for the group with no past history of periodontitis was 96.5% [62]. Roos-Jansaker et al. [63] evaluated the long-term result of implant therapy, using implant loss as an outcome variable. The patients were called in for a complete clinical and radiographic examination, 9–14 years after implant placements. A significant relationship was observed between implant loss and periodontal bone loss of the remaining teeth at implant placement. Other authors associate the microbiota with unsuccessful healing of the implants [10,59,64–66]. What perhaps make such conclusions more difficult to interpret are the conflicting definitions of peri-implantitis found in the literature [60,67,68]. Depending on how peri-implantitis is defined, the frequencies of occurrence will considerably vary and it may become difficult for comparison between studies. Berglundh et al. [16] in a systematic review, reported frequencies of peri-implantitis ranging of 0% to 14.4%, with a weighted mean on fixed partial dentures of 6.4%. The authors observed that late implant loss (5–10 years) occurs in the range of 2.1% to 11.3%. This suggestion may be partly explain the controversial range of peri-implantitis and posterior implants loss. The bacterial colonization upon implant surfaces and in the gingival tissues may occur only minutes after implantation [69] and, after 10 days, the bacterial microbiota composition around these new implants becomes similar to microbiota around periodontally compromised teeth [70].
2.2. Biofilm Formation on Abutment Implant Surfaces
Strategies to reduce bacterial adhesion and biofilm formation on implant abutment surfaces play an important role on clinical practice and may be used to maintain soft tissue integrity or improve peri-implantitis treatment [71]. However, conflicting opinions exist on biofilm formation among different types of materials [15,72–75]. Titanium and zirconia are hydrophobic materials. Since gram-positive bacteria present hydrophobic characteristics due to a thick peptidoglycan layer, they will be attracted immediately to these materials. In contrast to gram-negative bacteria, those in direct contact will be repelled. Hydrophobic/hydrophilic interactions may explain why some reports do not show differences between biofilm formation when utilizing material surfaces of a similar chemical nature. Brakel et al. [76] compared the early bacterial colonization and the health of soft tissues adjacent to the mucosal surfaces of the titanium and zirconia abutments. Microbiological sampling and measurement of clinical parameters were performed two weeks and three months after abutment implantation. The authors concluded that there was no significant difference in bacterial adhesion in both abutments, titanium and zirconia [76]. Although titanium and zirconia are hydrophobic materials, titanium exhibits semi conductor features due to its bioactive dioxide layer [77] and this may explain controversial results in the scientific literature. Scarano et al. [15] showed that zirconia discs fixed on a device worn intraorally showed less plaque accumulation than Titanium discs, even with similar surface roughness. This was attributed to lower electrical conductivity of zirconia in comparison to titanium. Al-Ahmad et al. [75] also evaluated biofilm formation in various types of titanium and zirconia abutment surfaces in vivo and found that the oral biofilm accumulation was lower on the zirconia surface compared to the titanium surface.
It is also important to discuss, that when the implants are in contact with plasma or saliva, proteins can direct the attraction or repulsion of bacteria present on external layers since proteins have different degrees of hydrophobic to hydrophilic regions. The main salivary protein adsorbed to titanium in vivo and in vitro is albumin [78,79], and albumin adsorption to titanium occurs through calcium (Ca+2 bridges [80]. The negative charge from titanium dioxide may attract positive ions, such as Ca+2 and its presence thus increases the adhesion of some bacteria species. Hauslich et al. [81] 2012, demonstrated that pretreatment of titannium surfaces with Ca+2 ions increased the adhesion of S. mutans and F. nucleatum to the Ti surfaces, but did not influenced the P. gingivalis adhesion. F. nucleatum possesses Ca+2-dependent binding proteins on the cell surface similar to those of S. mutans [82]. These findings indicate that the divalent ion Ca+2 may serve as a bridging agent in the adhesion of bacteria to Ti surfaces.
Bacteria can detect the non-biological substrate and express different genes, probably as part of the adaptation to a new microenvironment. The differences in the depth and viability of the biofilms on the different materials are a result of physical and chemical properties that determine gene expression profiling of bacteria, regardless of film formation [75].
3. Conclusions
In the case of partially edentulous patients affected by periodontal disease, the type of abutment implant to be used requires careful consideration. In general, previous reports compare biofilm formation on different types of surfaces using few numbers of bacteria. The multiple factors involved in complex biofilm formation, such as roughness and electrostatic interactions between bacteria and surfaces and interbacterial interactions can make it difficult to characterize and determine the ideal abutment implant surface. However, understanding the influence of materials surfaces on bacterial adhesion will help future development of new materials or surface treatments, in order to reduce or inhibit adhesion of pathogenic microorganisms on them.
Acknowledgments
Avila ED was supported by scholarship from the State of Sao Paulo Research Foundation (FAPESP) # 2011/05106-6 and Coordination for the Improvement of Higher Level or Educational Personnel (CAPES).
Author Contributions
Erica Dorigatti de Avila and Rafael Scaf de Molon wrote the manuscript. All authors participated of study conception, acquisition of data, design, critical revision and corrections.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Branemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindstrom, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg 1969, 3, 81–100. [Google Scholar]
- Rosenberg, E.S.; Torosian, J.P.; Slots, J. Microbial differences in 2 clinically distinct types of failures of osseointegrated implants. Clin. Oral Implant. Res 1991, 2, 135–144. [Google Scholar]
- Montes, C.C.; Pereira, F.A.; Thome, G.; Alves, E.D.; Acedo, R.V.; de Souza, J.R.; Melo, A.C.; Trevilatto, P.C. Failing factors associated with osseointegrated dental implant loss. Implant. Dent 2007, 16, 404–412. [Google Scholar]
- De Molon, R.S.; Morais-Camilo, J.A.; Verzola, M.H.; Faeda, R.S.; Pepato, M.T.; Marcantonio, E., Jr. Impact of diabetes mellitus and metabolic control on bone healing around osseointegrated implants: Removal torque and histomorphometric analysis in rats. Clin. Oral Implant. Res 2013, 24, 831–837. [Google Scholar]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur. J. Oral Sci 1998, 106, 527–551. [Google Scholar]
- Lindquist, L.W.; Carlsson, G.E.; Jemt, T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin. Oral Implant. Res 1996, 7, 329–336. [Google Scholar]
- Chiapasco, M.; Gatti, C.; Gatti, F. Immediate loading of dental implants placed in severely resorbed edentulous mandibles reconstructed with autogenous calvarial grafts. Clin. Oral Implant. Res 2007, 18, 13–20. [Google Scholar]
- Van Winkelhoff, A.J.; Goene, R.J.; Benschop, C.; Folmer, T. Early colonization of dental implants by putative periodontal pathogens in partially edentulous patients. Clin. Oral Implant. Res 2000, 11, 511–520. [Google Scholar]
- Rutar, A.; Lang, N.P.; Buser, D.; Burgin, W.; Mombelli, A. Retrospective assessment of clinical and microbiological factors affecting periimplant tissue conditions. Clin. Oral Implant. Res 2001, 12, 189–195. [Google Scholar]
- Quirynen, M.; De Soete, M.; van Steenberghe, D. Infectious risks for oral implants: A review of the literature. Clin. Oral Implant. Res 2002, 13, 1–19. [Google Scholar]
- Isidor, F. Loss of osseointegration caused by occlusal load of oral implants. A clinical and radiographic study in monkeys. Clin. Oral Implant. Res 1996, 7, 143–152. [Google Scholar]
- Esposito, M.; Thomsen, P.; Ericson, L.E.; Lekholm, U. Histopathologic observations on early oral implant failures. Int. J. Oral Maxillofac. Implant 1999, 14, 798–810. [Google Scholar]
- Piattelli, A.; Vrespa, G.; Petrone, G.; Iezzi, G.; Annibali, S.; Scarano, A. Role of the microgap between implant and abutment: A retrospective histologic evaluation in monkeys. J. Periodontol 2003, 74, 346–352. [Google Scholar]
- Elter, C.; Heuer, W.; Demling, A.; Hannig, M.; Heidenblut, T.; Bach, F.W.; Stiesch-Scholz, M. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int. J. Oral Maxillofac. Implant 2008, 23, 327–334. [Google Scholar]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol 2004, 75, 292–296. [Google Scholar]
- Berglundh, T.; Lindhe, J.; Jonsson, K.; Ericsson, I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J. Clin. Periodontol 1994, 21, 189–193. [Google Scholar]
- Tesmer, M.; Wallet, S.; Koutouzis, T.; Lundgren, T. Bacterial colonization of the dental implant fixture-abutment interface: An in vitro study. J. Periodontol 2009, 80, 1991–1997. [Google Scholar]
- Leonhardt, A.; Berglundh, T.; Ericsson, I.; Dahlen, G. Putative periodontal pathogens on titanium implants and teeth in experimental gingivitis and periodontitis in beagle dogs. Clin. Oral Implant. Res 1992, 3, 112–119. [Google Scholar]
- Koka, S.; Razzoog, M.E.; Bloem, T.J.; Syed, S. Microbial colonization of dental implants in partially edentulous subjects. J. Prosthet. Dent 1993, 70, 141–144. [Google Scholar]
- Botero, J.E.; Gonzalez, A.M.; Mercado, R.A.; Olave, G.; Contreras, A. Subgingival microbiota in peri-implant mucosa lesions and adjacent teeth in partially edentulous patients. J. Periodontol 2005, 76, 1490–1495. [Google Scholar]
- Salvi, G.E.; Furst, M.M.; Lang, N.P.; Persson, G.R. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin. Oral Implant. Res 2008, 19, 242–248. [Google Scholar]
- Pye, A.D.; Lockhart, D.E.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect 2009, 72, 104–110. [Google Scholar]
- Groessner-Schreiber, B.; Hannig, M.; Duck, A.; Griepentrog, M.; Wenderoth, D.F. Do different implant surfaces exposed in the oral cavity of humans show different biofilm compositions and activities? Eur. J. Oral Sci 2004, 112, 516–522. [Google Scholar]
- De Avila, E.D.; de Molon, R.S.; Spolidorio, D.M.P.; Mollo, F.D. Implications of surface and bulk properties of abutment implants and their degradation in the health of periodontal tissue. Materials 2013, 6, 5951–5966. [Google Scholar]
- Bakker, D.P.; Postmus, B.R.; Busscher, H.J.; van der Mei, H.C. Bacterial strains isolated from different niches can exhibit different patterns of adhesion to substrata. Appl. Environ. Microbiol 2004, 70, 3758–3760. [Google Scholar]
- Singh, A.V.; Vyas, V.; Salve, T.S.; Cortelli, D.; Dellasega, D.; Podesta, A.; Milani, P.; Gade, W.N. Biofilm formation on nanostructured titanium oxide surfaces and a micro/nanofabrication-based preventive strategy using colloidal lithography. Biofabrication 2012, 4, 025001:1–025001:2. [Google Scholar]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol 1995, 22, 1–14. [Google Scholar]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater 1997, 13, 258–269. [Google Scholar]
- Burgers, R.; Eidt, A.; Frankenberger, R.; Rosentritt, M.; Schweikl, H.; Handel, G.; Hahnel, S. The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch. Oral Biol 2009, 54, 595–601. [Google Scholar]
- De Freitas, M.M.; da Silva, C.H.; Groisman, M.; Vidigal, G.M., Jr. Comparative analysis of microorganism species succession on three implant surfaces with different roughness: An in vivo study. Implant. Dent 2011, 20, e14–e23. [Google Scholar]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res 2006, 17, 68–81. [Google Scholar]
- Hirota, K.; Yumoto, H.; Miyamoto, K.; Yamamoto, N.; Murakami, K.; Hoshino, Y.; Matsuo, T.; Miyake, Y. MPC-polymer reduces adherence and biofilm formation by oral bacteria. J. Dent. Res 2011, 90, 900–905. [Google Scholar]
- Tsukimura, N.; Yamada, M.; Iwasa, F.; Minamikawa, H.; Att, W.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Synergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titanium. Biomaterials 2011, 32, 4358–4368. [Google Scholar]
- Almaguer-Flores, A.; Olivares-Navarrete, R.; Wieland, M.; Ximenez-Fyvie, L.A.; Schwartz, Z.; Boyan, B.D. Influence of topography and hydrophilicity on initial oral biofilm formation on microstructured titanium surfaces in vitro. Clin. Oral Implant. Res 2012, 23, 301–307. [Google Scholar]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J. Biomed. Mater. Res. A 2013. [Google Scholar] [CrossRef]
- Strevett, K.A.; Chen, G. Microbial surface thermodynamics and applications. Res. Microbiol 2003, 154, 329–335. [Google Scholar]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol 2007, 34, 577–588. [Google Scholar]
- Husmark, U.; Ronner, U. Forces involved in adhesion of Bacillus cereus spores to solid surfaces under different environmental conditions. J. Appl. Bacteriol 1990, 69, 557–562. [Google Scholar]
- Andersson, B.; Odman, P.; Lindvall, A.M.; Lithner, B. Single-tooth restorations supported by osseointegrated implants: Results and experiences from a prospective study after 2 to 3 years. Int. J. Oral Maxillofac. Implant 1995, 10, 702–711. [Google Scholar]
- Holst, S.; Blatz, M.B.; Hegenbarth, E.; Wichmann, M.; Eitner, S. Prosthodontic considerations for predictable single-implant esthetics in the anterior maxilla. J. Oral Maxillofac. Surg 2005, 63, 89–96. [Google Scholar]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater 2008, 24, 299–307. [Google Scholar]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Zirconia: Established facts and perspectives for a biomaterial in dental implantology. J. Biomed. Mater. Res. B Appl. Biomater 2009, 88, 519–529. [Google Scholar]
- Molin, M.K.; Karlsson, S.L. Five-year clinical prospective evaluation of zirconia-based Denzir 3-unit FPDs. Int. J. Prosthodont 2008, 21, 223–227. [Google Scholar]
- Brodbeck, U. The ZiReal Post: A new ceramic implant abutment. J. Esthet. Restor. Dent 2003, 15, 10–23. [Google Scholar]
- Kohal, R.J.; Bachle, M.; Emmerich, D.; Beschnidt, S.M.; Strub, J.R. Hard tissue reaction to dual acid-etched titanium implants: Influence of plaque accumulation. A histological study in humans. Clin. Oral Implant. Res 2003, 14, 381–390. [Google Scholar]
- Taubman, M.A.; Kawai, T.; Han, X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J. Clin. Periodontol 2007, 34, 367–369. [Google Scholar]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol 1998, 25, 134–144. [Google Scholar]
- Xie, H.; Cook, G.S.; Costerton, J.W.; Bruce, G.; Rose, T.M.; Lamont, R.J. Intergeneric communication in dental plaque biofilms. J. Bacteriol 2000, 182, 7067–7069. [Google Scholar]
- Nagata, H.; Tazaki, K.; Amano, A.; Hanioka, T.; Tamagawa, H.; Shizukuishi, S. Characterization of coaggregation and fibrinogen-binding by Porphyromonas gingivalis. J. Osaka Univ. Dent. Sch 1994, 34, 37–44. [Google Scholar]
- Periasamy, S.; Kolenbrander, P.E. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol 2009, 191, 6804–6811. [Google Scholar]
- Holt, S.C.; Kesavalu, L.; Walker, S.; Genco, C.A. Virulence factors of Porphyromonas gingivalis. Periodontol 2000 1999, 20, 168–238. [Google Scholar]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J., Jr.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol 2006, 72, 2837–2848. [Google Scholar]
- Kolenbrander, P.E. Surface recognition among oral bacteria: Multigeneric coaggregations and their mediators. Crit. Rev. Microbiol 1989, 17, 137–159. [Google Scholar]
- Faveri, M.; Figueiredo, L.C.; Duarte, P.M.; Mestnik, M.J.; Mayer, M.P.; Feres, M. Microbiological profile of untreated subjects with localized aggressive periodontitis. J. Clin. Periodontol 2009, 36, 739–749. [Google Scholar]
- Meijndert, L.; van der Reijden, W.A.; Raghoebar, G.M.; Meijer, H.J.; Vissink, A. Microbiota around teeth and dental implants in periodontally healthy, partially edentulous patients: Is pre-implant microbiological testing relevant? Eur. J. Oral Sci 2010, 118, 357–363. [Google Scholar]
- Lee, K.H.; Maiden, M.F.; Tanner, A.C.; Weber, H.P. Microbiota of successful osseointegrated dental implants. J. Periodontol 1999, 70, 131–138. [Google Scholar]
- Klinge, B.; Norlund, A. A socio-economic perspective on periodontal diseases: A systematic review. J. Clin. Periodontol 2005, 32, 314–325. [Google Scholar]
- Ong, C.T.; Ivanovski, S.; Needleman, I.G.; Retzepi, M.; Moles, D.R.; Tonetti, M.S.; Donos, N. Systematic review of implant outcomes in treated periodontitis subjects. J. Clin. Periodontol 2008, 35, 438–462. [Google Scholar]
- Marrone, A.; Lasserre, J.; Bercy, P.; Brecx, M.C. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin. Oral Implant. Res 2013, 24, 934–940. [Google Scholar]
- Ferreira, S.D.; Silva, G.L.; Cortelli, J.R.; Costa, J.E.; Costa, F.O. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J. Clin. Periodontol 2006, 33, 929–935. [Google Scholar]
- Tabanella, G.; Nowzari, H.; Slots, J. Clinical and microbiological determinants of ailing dental implants. Clin. Implant. Dent. Relat. Res 2009, 11, 24–36. [Google Scholar]
- Karoussis, I.K.; Salvi, G.E.; Heitz-Mayfield, L.J.; Bragger, U.; Hammerle, C.H.; Lang, N.P. Long-term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI Dental Implant System. Clin. Oral Implant. Res 2003, 14, 329–339. [Google Scholar]
- Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part II: Presence of peri-implant lesions. J. Clin. Periodontol 2006, 33, 290–295. [Google Scholar]
- Rams, T.E.; Link, C.C., Jr. Microbiology of failing dental implants in humans: Electron microscopic observations. J. Oral Implantol 1983, 11, 93–100. [Google Scholar]
- Alcoforado, G.A.; Rams, T.E.; Feik, D.; Slots, J. Microbial aspects of failing osseointegrated dental implants in humans. J. Parodontol 1991, 10, 11–18. [Google Scholar]
- Mattheos, N.; Schittek Janda, M.; Zampelis, A.; Chronopoulos, V. Reversible, non-plaque-induced loss of osseointegration of successfully loaded dental implants. Clin. Oral Implant. Res 2013, 24, 347–354. [Google Scholar]
- Behneke, A.; Behneke, N.; d’Hoedt, B. A 5-year longitudinal study of the clinical effectiveness of ITI solid-screw implants in the treatment of mandibular edentulism. Int. J. Oral Maxillofac. Implant 2002, 17, 799–810. [Google Scholar]
- Ekelund, J.A.; Lindquist, L.W.; Carlsson, G.E.; Jemt, T. Implant treatment in the edentulous mandible: A prospective study on Branemark system implants over more than 20 years. Int. J. Prosthodont 2003, 16, 602–608. [Google Scholar]
- Furst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res 2007, 18, 501–508. [Google Scholar]
- Quirynen, M.; Vogels, R.; Peeters, W.; van Steenberghe, D.; Naert, I.; Haffajee, A. Dynamics of initial subgingival colonization of ‘pristine’ peri-implant pockets. Clin. Oral Implant. Res 2006, 17, 25–37. [Google Scholar]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol 2004, 2, 95–108. [Google Scholar]
- Nascimento, C.; Pita, M.S.; Fernandes, F.H.; Pedrazzi, V.; de Albuquerque, R.F., Jr.; Ribeiro, R.F. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin. Oral Implant. Res 2014, 25, 337–343. [Google Scholar]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. J. Oral Maxillofac. Implant 2002, 17, 793–798. [Google Scholar]
- Scotti, R.; Kantorski, K.Z.; Monaco, C.; Valandro, L.F.; Ciocca, L.; Bottino, M.A. SEM evaluation of in situ early bacterial colonization on a Y-TZP ceramic: A pilot study. Int. J. Prosthodont 2007, 20, 419–422. [Google Scholar]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Faust, J.; Bachle, M.; Follo, M.; Wolkewitz, M.; Hannig, C.; Hellwig, E.; Carvalho, C.; Kohal, R. Biofilm formation and composition on different implant materials in vivo. J. Biomed. Mater. Res. B Appl. Biomater 2010, 95, 101–109. [Google Scholar]
- Van Brakel, R.; Cune, M.S.; van Winkelhoff, A.J.; de Putter, C.; Verhoeven, J.W.; van der Reijden, W. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: An in vivo study in man. Clin. Oral Implant. Res 2011, 22, 571–577. [Google Scholar]
- Han, Y.; Chen, D.; Sun, J.; Zhang, Y.; Xu, K. UV-enhanced bioactivity and cell response of micro-arc oxidized titania coatings. Acta Biomater 2008, 4, 1518–1529. [Google Scholar]
- Kohavi, D.; Klinger, A.; Steinberg, D.; Mann, E.; Sela, N.M. Alpha-Amylase and salivary albumin adsorption onto titanium, enamel and dentin: An in vivo study. Biomaterials 1997, 18, 903–906. [Google Scholar]
- Steinberg, D.; Klinger, A.; Kohavi, D.; Sela, M.N. Adsorption of human salivary proteins to titanium powder. I. Adsorption of human salivary albumin. Biomaterials 1995, 16, 1339–1343. [Google Scholar]
- Klinger, A.; Steinberg, D.; Kohavi, D.; Sela, M.N. Mechanism of adsorption of human albumin to titanium in vitro. J. Biomed. Mater. Res 1997, 36, 387–392. [Google Scholar]
- Badihi Hauslich, L.; Sela, M.N.; Steinberg, D.; Rosen, G.; Kohavi, D. The adhesion of oral bacteria to modified titanium surfaces: Role of plasma proteins and electrostatic forces. Clin. Oral Implant. Res 2013, 24, 49–56. [Google Scholar]
- Murray, P.A.; Kern, D.G.; Winkler, J.R. Identification of a galactose-binding lectin on Fusobacterium nucleatum FN-2. Infect. Immun 1988, 56, 1314–1319. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Avila, E.D.; De Molon, R.S.; Vergani, C.E.; De Assis Mollo, Jr., F.; Salih, V. The Relationship between Biofilm and Physical-Chemical Properties of Implant Abutment Materials for Successful Dental Implants. Materials 2014, 7, 3651-3662. https://doi.org/10.3390/ma7053651
De Avila ED, De Molon RS, Vergani CE, De Assis Mollo, Jr. F, Salih V. The Relationship between Biofilm and Physical-Chemical Properties of Implant Abutment Materials for Successful Dental Implants. Materials. 2014; 7(5):3651-3662. https://doi.org/10.3390/ma7053651
Chicago/Turabian StyleDe Avila, Erica Dorigatti, Rafael Scaf De Molon, Carlos Eduardo Vergani, Francisco De Assis Mollo, Jr., and Vehid Salih. 2014. "The Relationship between Biofilm and Physical-Chemical Properties of Implant Abutment Materials for Successful Dental Implants" Materials 7, no. 5: 3651-3662. https://doi.org/10.3390/ma7053651