Nanosize Control on Porous β-MnO2 and Their Catalytic Activity in CO Oxidation and N2O Decomposition
Abstract
: A major challenge in the synthesis of porous metal oxides is the control of pore size and/or wall thickness that may affect the performance of these materials. Herein, nanoporous β-MnO2 samples were prepared using different hard templates, e.g., ordered mesoporous silica SBA-15 and KIT-6, disordered mesoporous silica, and colloidal silica. These samples were characterized by Powder X-Ray Diffraction (PXRD), Transmission Electron Microscopy (TEM), and N2 adsorption-desorption. The pore size distribution of β-MnO2 was tuned by the different hard templates and their preparation details. Catalytic activities in CO oxidation and N2O decomposition were tested and the mesoporous β-MnO2 samples demonstrated superior catalytic activities compared with their bulk counterpart.1. Introduction
The 21st century has already presented human society with many challenges: greenhouse gas emission control, energy conservation, cleaner chemical processing, etc. Mesoporous transition metal oxides possess d-shell electrons confined to nanosized walls, redox active internal surfaces, and ordered pore networks, thus generating a great deal of interest for catalysis [1–3], separation or storage of ions/molecules [4–8], and energy conversion and storage [9,10]. While it is possible to synthesize mesoporous transition metal oxides exhibiting highly ordered pore structures with a variety of symmetries, tailoring the pore size/wall thickness to particular values is challenging [11]. However, being able to do so is key to the functionality of porous solids [12–15].
The use of hard templates, e.g., mesoporous silicas (from which transition metal oxide replicas may be cast), has delivered the combination of highly ordered pore stuctures with crystalline walls. Since the walls of the template define the pores of the mesoporous transition metal oxide, it is necessary to control the thickness of the template walls in order to prepare a variety of pore sizes for the target material. The pore size and wall thickness of mesoporous silicas change with the hydrothermal synthesis conditions and this has been used to prepare mesoporous silicas with different pore sizes [16–19]. The pore size and wall thickness of mesoporous silicas can also be tuned by varying the calcination temperature of these materials [20,21], and the pore sizes and wall thicknesses of casted mesoporous transition metal oxides can also be changed by using these mesoporous silicas as hard templates [22]. Due to the limitation of controlled size range, other templates, such as colloidal silica or alkaline ions, have also been employed to prepare mesoporous metal oxides with pore size extending to 30 nm or above [14,23]. Here we present results demonstrating the textural properties control over mesoporous manganese oxides, β-MnO2, which can be prepared with pore sizes in the range from 3.3 to 28 nm and wall thicknesses ranging from 5 to 30 nm. The catalytic performance of these catalysts was studied using CO oxidation and N2O decompostion as probe reactions.
2. Results and Discussion
2.1. Textural Properties’ Control over Mesoporous β-MnO2
Recently, 3D mesoporous β-MnO2 samples have been prepared with pore sizes ranging from 3.4 to 28 nm in diameter, with wall thicknesses from 4.7 to 30 nm; the influence of size on the rate of Li-intercalation (as the cathode for Li-ion batteries) has therefore been studied [14]. Here we re-prepared and re-characterized some mesoporous β-MnO2 samples and studied the possible correlation between their textural properties and the catalytic reaction (CO oxidation and N2O decomposition). In the current contribution, there are three categories of mesoporous β-MnO2: 3D ordered mesoporous β-MnO2 templated by KIT-6 silica (β-MnO2-X, X stands for the hydrothermal treatment temperature of KIT-6 templates, see Experimental Section); 1D ordered mesoporous β-MnO2 templated by SBA-15 silica (β-MnO2-1D-100); and 3D disordered mesoporous β-MnO2 (β-MnO2-d4 and β-MnO2-d30, here d stands for “disordered”) templated by disordered mesoporous silica and colloidal silica. The detailed preparation procedures are described in Section 3.
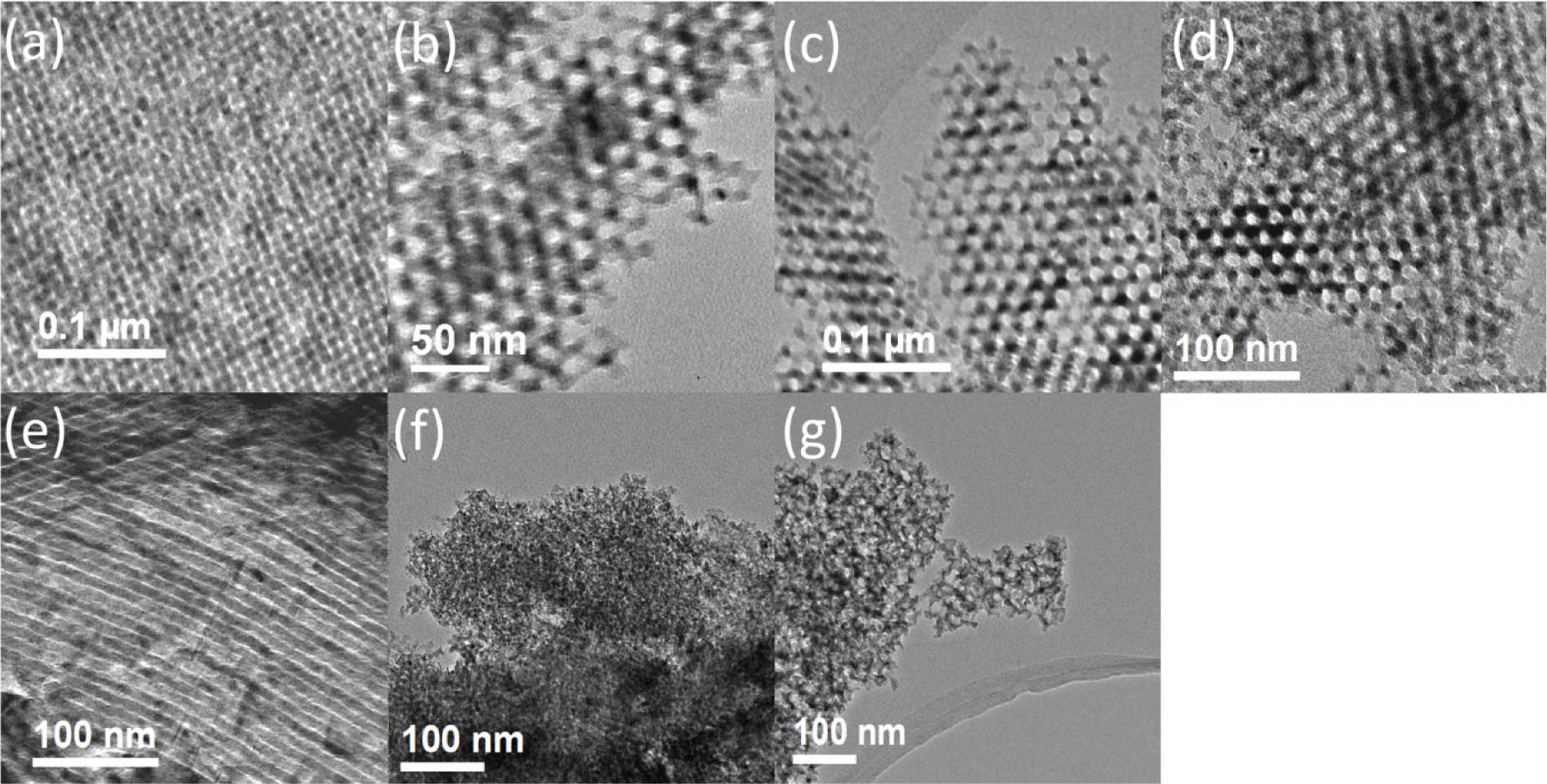
TEM data for the mesoporous β-MnO2 are presented in Figure 1. In each case, many regions of the sample were examined and the TEM images presented are representative of the materials as a whole. 3D ordered mesoporous β-MnO2 of β-MnO2-60, β-MnO2-80, β-MnO2-100, and β-MnO2-100B have ordered mesostrucutre with Ia3d symmetry (Figure 1a–d), while β-MnO2-1D-100 demonstrates the P6mm structure (Figure 1e). The other two mesoporous β-MnO2, β-MnO2-d4 and β-MnO2-d30, have disordered mesostructures (Figure 1f,g): the former has a worm-like mesostructure, while the latter has the hole mesostructure, resulting from the colloidal silica (Ludox AS-40) template.
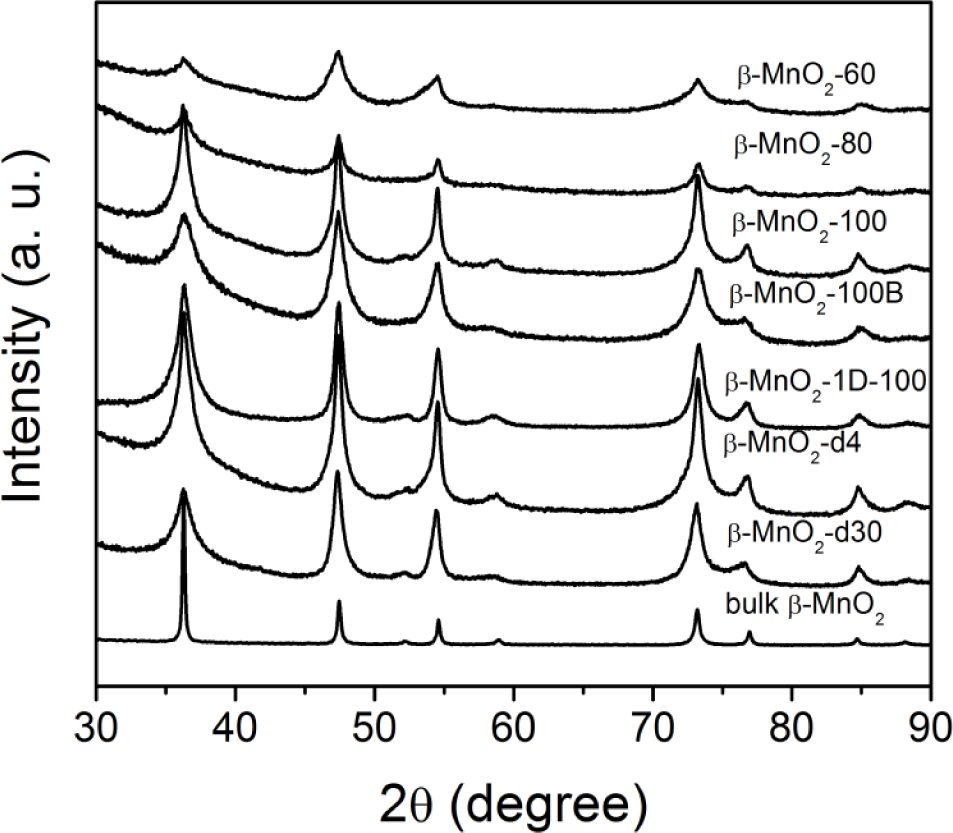
The wide angle PXRD data for different mesoporous β-MnO2 are shown in Figure 2. The data confirm the β-MnO2 phase (pyrusite, ICDD 00-024-0735), in good agreement with the bulk one (Aldich). The breadth of the diffraction peaks from mesostructured β-MnO2 is due to the nanosized pore wall.
The N2-sorption data for mesoporous β-MnO2 are shown in Figure 3. The adsorption-desorption isotherms for each mesoporous β-MnO2 are shown in Figure 3a. They correspond to a type IV isotherm with a H1 hysteresis loop [24]. Pore sizes, wall thicknesses, pore volumes, and surface areas are presented in Table 1. The BET surface area of this series of mesoporous β-MnO2 is relatively small compared with those reported previously [14]. The wall thickness of the ordered mesoporous β-MnO2, β-MnO2-60, β-MnO2-80, and β-MnO2-100 increase when increasing the hydrothermal temperature of mesoporous SiO2. The pore diameters and surface area of the ordered mesoporous β-MnO2 virtually do not change with the corresponding hydrothermal temperature of mesoporous SiO2. Considering the pore size distribution, Figure 3b, mesoporous β-MnO2 samples templated by KIT-6 (β-MnO2-60, β-MnO2-80, β-MnO2-100, and β-MnO2-100B) exhibit two peaks, and the pore volume of the larger mesopore increases in proportion with the decrease in the hydrothermal temperature. For instance, the pore volume ratio of 12.8 nm pore to 3.4 nm pore is 0.80 and 0.75 for β-MnO2-60 and β-MnO2-80, respectively, larger than that of β-MnO2-100 (0.43). It is known that the larger mesopore (12–14 nm) arises when the microporous bridges linking the two sets of pores in KIT-6 are broken, resulting in the filling of one or other set of pores—but not both simultaneously [11,14,23,25,26]. We conclude that the lower hydrothermal temperarture must reduce the micro-bridges, resulting in the appearance of the larger pores in the replica β-MnO2 structure. Meanwhile, decreasing Mn(NO3)2/KIT-6 ratio during the impregnation can also facilitate the formation of large mesopores [14].
2.2. CO Oxidation and N2O Decomposition over Mesoporous Manganese Oxide Catalysts
Different mesoporous β-MnO2 samples were tested in CO oxidation, and a commercial β-MnO2 (bulk β-MnO2) was also used for comparison. As shown in Figure 4, bulk β-MnO2 is not particularly active in CO oxidation, achieving 50% CO conversion at 400 °C. On the other hand, other mesoporous β-MnO2 samples start to show CO conversions above 50 °C and reach complete conversion at 200 °C. The T50 (temperature requiured for 50% conversion) values of mesoporous β-MnO2 samples are in the range of 102–134 °C. β-MnO2-d30 is the most active, whereas β-MnO2-100 is the least active for CO oxidation. The high activity of mesoporous β-MnO2 samples is ascribed to the higher surface area (30–135 m2/g) of these samples than that of bulk β-MnO2 (0.5 m2/g).
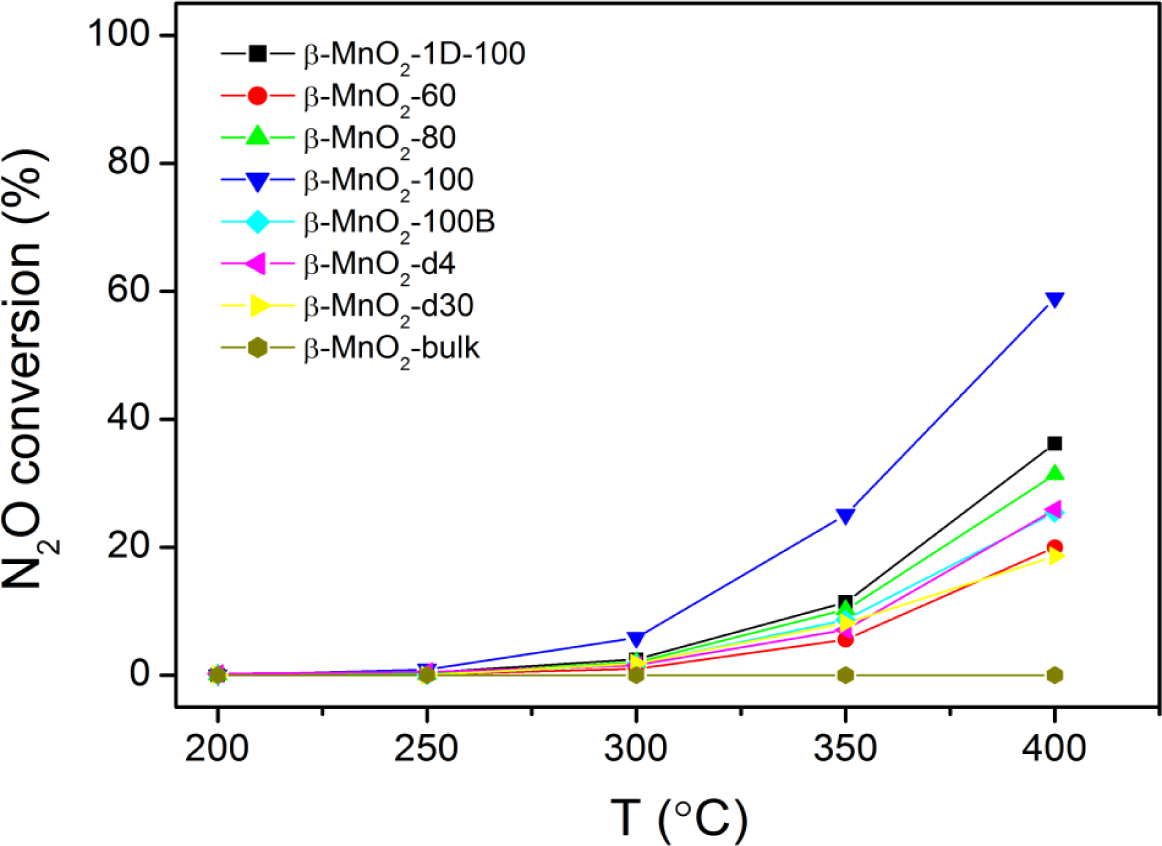
The conversion of N2O over different mesoporous β-MnO2 is shown in Figure 5. Bulk β-MnO2 is not active at all even at 400 °C, whereas mesoporous β-MnO2 samples are active at temperatures higher than 250 °C, achieving conversions between 15% and 60% at 400 °C. The trend is consistent with the trend observed in CO oxidation, i.e., mesoporous β-MnO2 samples are more active than bulk β-MnO2. However, for mesoporous β-MnO2 samples, the activities in N2O decomposition followed the sequence of β-MnO2-d30~β-MnO2-60 < β-MnO2-d4~β-MnO2-100B < β-MnO2-80 < β-MnO2-1D-100 < β-MnO2-100. The activity sequence is almost the reversal of the trend seen in CO oxidation. This could be because CO oxidation needs the adsorption of O2 on the catalyst surface, whereas N2O needs the desorption of O2 from the catalyst surface.
3. Experimental Section
3.1. Materials
Mn(NO3)2·4H2O (98%), bulk β-MnO2 (micron sized, 99.9%), concentrated HCl (37%), NaOH (99.3%), Pluronic P123 (Mn = 5800), 1-butanol (99.4%), absolute ethanol (99.9%), Ludox AS-40 colloid silica (40%), and tetraethyl orthosilicate (98%) were all purchased from Sigma-Aldrich (St Andrews, UK).
3.2. Preparation
The synthesis of the mesoporous silica KIT-6 was based on the procedure described previously [17,18]. The mesoporous silicas with different pore size prepared here are denoted as KIT-X, where X corresponds to the hydrothermal treatment temperature. The preparation of disordered mesoporous silica with a pore diameter of ca. 8 nm was based on the aforementioned procedure [14].
The synthesis of the two-dimensional mesoporous silica SAB-15 was based on the procedure described previously [16]. KIT-60, KIT-80, KIT-100, SBA-15, and the disordered ~8 nm mesoporous silica were used as the hard templates to prepare crystalline mesoporous β-MnO2 following the procedure described in a previous report [14]. Typically, 30 g Mn(NO3)2·6H2O (98%) was dissolved in 20 mL water to form a saturated solution. 5 g mesoporous silica was dispersed in 200 mL dried n-hexane. After stirring at room temperature for 3 h, 5 mL of the saturated Mn(NO3)2 solution was added slowly with stirring. The mixture was stirred overnight, filtered and dried at room temperature until a completely dried powder was obtained. The sample was heated slowly to 400 °C at a rate of 1 °C/min, calcined at that temperature for 3 h, and after cooling to room temperature, the resulting material was treated twice with a 2 M hot NaOH solution, followed by washing with water several times and drying at 60 °C. The obtained mesoporous β-MnO2 was named β-MnO2-60, β-MnO2-80, β-MnO2-100, β-MnO2-1D-100, and β-MnO2-d4 (disordered β-MnO2 with pore size of ca. 4 nm) using the KIT-60, KIT-80, KIT-100, SBA-15, and the disordered ~8 nm mesoporous silica as the hard template, respectively.
The preparation of ordered mesoporous β-MnO2 with a relatively higher proportion of large mesopore (denoted as β-MnO2-100B) followed a previously reported procedure [25]. 4 g Mn(NO3)2·6H2O (98%) was dissolved in 150 mL ethanol, followed by the addition of 5 g mesoporous silica, KIT-100. After stirring at room temperature until all the solution had been absorbed, the powder was re-dispersed in 100 mL dry n-hexane under stirring in an open beaker. Once all the solvent had evaporated, the sample was heated slowly to 400 °C at a rate of 1 °C/min and calcined at that temperature for 3 h. After cooling to room temperature, the resulting sample was treated twice with a hot aqueous solution of 2 M NaOH to remove the silica template, followed by washing with water several times and then drying at 60 °C.
The preparation of disordered mesoporous β-MnO2 with a pore diameter of ca. 30 nm was as follows [14]: 100 g Ludox AS-40 colloid silica (40%) was first dried at 60 °C overnight, then impregnated with 10 mL of saturated Mn(NO3)2 solution and again dried. Following this procedure it was calcined at 400 °C for 3 h. Finally the resulting material was treated twice with a 2 M hot NaOH solution, followed by washing with water several times and drying at 60 °C. This material was named β-MnO2-d30.
3.3. Materials Characterization
TEM studies were carried out using a JEOL JEM-2011 (JEOL Ltd., Tokyo, Japan), employing a LaB6 filament as the electron source and an accelerating voltage of 200 keV. TEM images were recorded by a Gatan CCD camera in a digital format. Wide-angle powder X-ray diffraction data were collected on a Stoe STADI/P powder diffractmeter operating in transmission mode and with a small angle position sensitive detector (STOE & Cie GmbH, Darmstadt, Germany). Incident radiation was generated using a Fe Kα1 source (λ = 1.936 Å). N2 adsorption-desorption analysis was carried out using a Micromeritics Tristar 3020 (Micromeritics Instrument Corporation, Norcross, GA, USA). The typical sample weight used was 100–200 mg. The degassing condition was set to 180 min at 120 °C under vacuum and all adsorption-desorption measurements were carried out at liquid nitrogen temperature. Pore size distribution was analyzed by BJH model.
3.4. Catalytic Testing
The catalytic reaction condition could be found our previous reports [23,27,28]. Briefly, to test the performance in CO oxidation, 50 mg of catalyst was loaded in in a U-shaped quartz tube, pretreated in 8% O2–He at 400 °C for 1 h, and cooled down to 0 °C to allow for CO oxidation. The CO concentration was 1% (balance air), and the gas flow rate was 37 mL/min. The temperature in between 0 °C and room temperature was tuned by allowing a cup of ice water to warm naturally, and the temperature above room temperature was ramped using a furnace at a rate of 1 °C/min. The exiting stream was analyzed by GC.
To test the performance in N2O decomposition, 50 mg of catalyst was loaded into a U-shaped glass tube, pretreated in 20% O2–He at 400 °C for 1 h, and cooled down to near room temperature. The gas stream was switched to 0.5% N2O–He, and the flow rate was 60 mL/min. The temperature was ramped using a furnace, and kept at 100, 150, 200, 250, 300, 350, and 400 °C for 30 min at each reaction temperature. The exiting stream was analyzed by GC.
4. Conclusions
Mesoporous nanocrystalline β-MnO2 samples were successfully synthesized by nanocasting from mesoporous silica SBA-15 and KIT-6, disordered mesoporous silica, and colloidal silica. The textural properties can be tuned by varying the hydrothermal temperature of KIT-6 from 60 °C to 100 °C, or the ration of manganese nitrate to the template in the preparation. The mesoporous manganese oxide show ordered/disordered mesostructured and large surface areas. The applications in catalytic reaction of CO oxidation and decomposition of N2O demonstrated that mesoporous manganese oxides have superior catalytic activity than the bulk one.
Acknowledgments
Zhen Ma acknowledges the financial support by National Natural Science Foundation of China (Grant Nos. 21007011 and 21177028), the PhD Programs Foundation of the Ministry of Education in China (Grant No. 20100071120012), and the Overseas Returnees Start-Up Research Fund of the Ministry of Education in China. Sheng Dai acknowledges the financial support by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, US Department of Energy, under contract No. De-AC05-00OR22725 with Oak Ridge National Laboratory managed and operated by UT-Batelle, Limited-Liability Company.
Author Contributions
Yu Ren conducted all of the synthesis and most of the characterization. Zhen Ma and Sheng Dai carried out the catalytic studies. Yu Ren and Zhen Ma interpreted the results and wrote the manuscript. All contributed to the discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev 1997, 97, 2373–2419. [Google Scholar]
- Sayari, A. Catalysis by crystalline mesoporous molecular sieves. Chem. Mater 1996, 8, 1840–1852. [Google Scholar]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater 2005, 77, 1–45. [Google Scholar]
- Liu, A.M.; Hidajat, K.; Kawi, S.; Zhao, D.Y. A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions. Chem. Commun 2000, 13, 1145–1146. [Google Scholar]
- Oye, G.; Glomm, W.R.; Vralstad, T.; Volden, S.; Magnusson, H.; Stocker, M.; Sjoblom, J. Synthesis, functionalisation and characterisation of mesoporous materials and sol-gel glasses for applications in catalysis, adsorption and photonics. Adv. Colloid Interface Sci 2006, 123–126, 17–32. [Google Scholar]
- Brady, R.; Woonton, B.; Gee, M.L.; O’Connor, A.J. Hierarchical mesoporous silica materials for separation of functional food ingredients—A review. Innov. Food Sci. Emerg. Technol 2008, 9, 243–248. [Google Scholar]
- Vinu, A.; Hossain, K.Z.; Ariga, K. Recent advances in functionalization of mesoporous silica. J. Nanosci. Nanotechnol 2005, 5, 347–371. [Google Scholar]
- Kim, Y.; Yi, J. Advances in environmental technologies via the application of mesoporous materials. J. Ind. Eng. Chem 2004, 10, 41–51. [Google Scholar]
- Jiao, F.; Shaju, K.M.; Bruce, P.G. Synthesis of nanowire and mesoporous low-temperature LiCoO2 by a post-templating reaction. Angew. Chem. Int. Ed 2005, 44, 6550–6553. [Google Scholar]
- Jiao, F.; Bruce, P.G. Mesoporous crystalline β-MnO2-a reversible positive electrode for rechargeable lithium batteries. Adv. Mater 2007, 19, 657–660. [Google Scholar]
- Rumplecker, A.; Kleitz, F.; Salabas, E.L.; Schüth, F. Hard templating pathways for the synthesis of nanostructured porous Co3O4. Chem. Mater 2007, 19, 485–496. [Google Scholar]
- Rossinyol, E.; Prim, A.; Pellicer, E.; Arbiol, J.; Hernández-Ramírez, F.; Peiro, F.; Cornet, A.; Morante, J.R.; Solovyov, L.A.; Tian, B.; et al. Synthesis and characterization of Chromium-doped mesoporous tungsten oxide for gas-sensing applications. Adv. Funct. Mater 2007, 17, 1801–1806. [Google Scholar]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.D.; Tiemann, M. Ordered mesoporous In2O3: Synthesis by structure replication and application as a methane gas sensor. Adv. Funct. Mater 2009, 19, 653–661. [Google Scholar]
- Ren, Y.; Armstrong, A.R.; Jiao, F.; Bruce, P.G. Influence of size on the rate of mesoporous electrodes for lithium batteries. J. Am. Chem. Soc 2010, 132, 996–1004. [Google Scholar]
- Ren, Y.; Ma, Z.; Bruce, P.G. Ordered mesoporous metal oxides: Synthesis and applications. Chem. Soc. Rev 2012, 41, 4909–4927. [Google Scholar]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun 2003, 17, 2136–2137. [Google Scholar]
- Kim, T.W.; Kleitz, F.; Paul, B.; Ryoo, R. MCM-48-like large mesoporous silicas with tailored pore structure: Facile synthesis domain in a ternary triblock copolymer-butanol-water system. J. Am. Chem. Soc 2005, 127, 7601–7610. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar]
- Ryoo, R.; Ko, C.H.; Kruk, M.; Antochshuk, V.; Jaroniec, M. Block-copolymer-templated ordered mesoporous silica: Array of uniform mesopores or mesopore-micropore network. J. Phys. Chem. B 2000, 104, 11465–11471. [Google Scholar]
- Shin, H.J.; Ryoo, R.; Kruk, M.; Jaroniec, M. Modification of SBA-15 pore connectivity by high-temperature calcination investigated by carbon inverse replication. Chem. Commun 2001, 4, 349–350. [Google Scholar]
- Ren, Y.; Jiao, F.; Bruce, P.G. Tailoring the pore size/wall thickness of mesoporous transition metal oxides. Microporous Mesoporous Mater 2009, 121, 90–94. [Google Scholar]
- Ren, Y.; Ma, Z.; Morris, R.E.; Liu, Z.; Jiao, F.; Dai, S.; Bruce, P.G. A solid with a hierarchical tetramodal micro-meso-macro pore size distribution. Nat. Commun 2013, 4. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity. Pure Appl. Chem 1985, 57, 603–619. [Google Scholar]
- Jiao, F.; Hill, A.H.; Harrison, A.; Berko, A.; Chadwick, A.V.; Bruce, P.G. Synthesis of ordered mesoporous NiO with crystalline walls and a bimodal pore size distribution. J. Am. Chem. Soc 2008, 130, 5262–5266. [Google Scholar]
- Dickinson, C.; Zhou, W.Z.; Hodgkins, R.P.; Shi, Y.F.; Zhao, D.Y.; He, H.Y. Formation mechanism of porous single-crystal Cr2O3 and Co3O4 templated by mesoporous silica. Chem. Mater 2006, 18, 3088–3095. [Google Scholar]
- Ren, Y.; Ma, Z.; Qian, L.P.; Dai, S.; He, H.Y.; Bruce, P.G. Ordered crystalline mesoporous oxides as catalysts for CO oxidation. Catal. Lett 2009, 131, 146–154. [Google Scholar]
- Ma, Z.; Ren, Y.; Lu, Y.B.; Bruce, P.G. Catalytic decomposition of N2O on ordered crystalline metal oxides. J. Nanosci. Nanotechnol 2013, 13, 5093–5103. [Google Scholar]


| Materials | Template | SBET (m2/g) | D (nm) | V (cm3/g) | Pore Wall Thickness (nm, by TEM) | T50 of CO Oxidation (°C) |
|---|---|---|---|---|---|---|
| β-MnO2-60 | KIT-60 | 83 | 3.6/12.8(0.80) a | 0.37 | 5.0 | 119 |
| β-MnO2-80 | KIT-80 | 86 | 3.3/12.7(0.75) a | 0.33 | 6.5 | 110 |
| β-MnO2-100 | KIT-100 | 84 | 3.4/12.8(0.43) a | 0.27 | 7.5 | 134 |
| β-MnO2-100B | KIT-100 | 87 | 3.3/12.8(0.91) a | 0.30 | 7.5 | 125 |
| β-MnO2-1D-100 | SBA-15 | 68 | 3.4 | 0.26 | 8.8 | 128 |
| β-MnO2-d4 | Disordered mesoporous silica | 135 | 3.4 | 0.44 | 8–10 | 109 |
| β-MnO2-d30 | AS-40 | 30 | 28 | 0.22 | 20–30 | 102 |
| Bulk β-MnO2 | Aldrich | 0.5 | – | – | – | 400 |
aSBET, surface area calculated by the BET method; D pore diameter calculated by the BJH method (ratios of large (12.8 nm) to small (3.4 nm) pore volumes are given in parentheses); V total pore volume at P/P0 = 0.99.
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ren, Y.; Ma, Z.; Dai, S. Nanosize Control on Porous β-MnO2 and Their Catalytic Activity in CO Oxidation and N2O Decomposition. Materials 2014, 7, 3547-3556. https://doi.org/10.3390/ma7053547
Ren Y, Ma Z, Dai S. Nanosize Control on Porous β-MnO2 and Their Catalytic Activity in CO Oxidation and N2O Decomposition. Materials. 2014; 7(5):3547-3556. https://doi.org/10.3390/ma7053547
Chicago/Turabian StyleRen, Yu, Zhen Ma, and Sheng Dai. 2014. "Nanosize Control on Porous β-MnO2 and Their Catalytic Activity in CO Oxidation and N2O Decomposition" Materials 7, no. 5: 3547-3556. https://doi.org/10.3390/ma7053547