Biocompatibility of Coronary Stents
Abstract
: Cardiovascular disease is the dominant cause of mortality in developed countries, with coronary artery disease (CAD) a predominant contributor. The development of stents to treat CAD was a significant innovation, facilitating effective percutaneous coronary revascularization. Coronary stents have evolved from bare metal compositions, to incorporate advances in pharmacological therapy in what are now known as drug eluting stents (DES). Deployment of a stent overcomes some limitations of balloon angioplasty alone, but provides an acute stimulus for thrombus formation and promotes neointimal hyperplasia. First generation DES effectively reduced in-stent restenosis, but profoundly delay healing and are susceptible to late stent thrombosis, leading to significant clinical complications in the long term. This review characterizes the development of coronary stents, detailing the incremental improvements, which aim to attenuate the major clinical complications of thrombosis and restenosis. Despite these enhancements, coronary stents remain fundamentally incompatible with the vasculature, an issue which has largely gone unaddressed. We highlight the latest modifications and research directions that promise to more holistically design coronary implants that are truly biocompatible.1. Introduction
Cardiovascular disease continues to be the leading cause of mortality [1,2], with a vast majority of these deaths attributed to obstructive coronary artery disease (CAD) [3]. Depending on the severity of the disease, the main interventional options for revascularisation include angioplasty, stent deployment and in severe, diffuse occlusions (more than 70%), bypass graft surgery [3]. Narrowed coronary arteries were originally treated percutaneously with balloon angioplasty alone [4]. However, clinical complications including abrupt vessel closure from elastic recoil in the short term and significant neointimal hyperplasia, limited the applicability of this intervention. Improved results were observed following the insertion of an additional intravascular mechanical support, cylindrical metal scaffolds known as stents [5]. The first balloon expandable stents were designed from surgical grade stainless steel, and aimed to provide additional mechanical support, limiting vessel recoil and preventing acute occlusion [6]. Stents were initially evaluated in a preclinical study relative to angioplasty alone, in canine coronaries to assess efficacy prior to human trials [5,7]. The extent of endothelial damage during angioplasty is proportional to the time of balloon inflation [5]. Since the balloon is immediately deflated after maximal inflation during stent implantation and 80% of the expandable wire mesh of the stent was open surface opposing elastic recoil, the process minimized endothelial damage compared to balloon angioplasty alone [5]. The first human clinical implantations indicated a high delivery success, low incidence of perioperative complications and a thrombosis incidence controllable with the use of anticoagulants [8,9]. In the absence of antiplatelet therapy, sub-acute thrombotic closure after stent implantation, was a notable risk [8].
Despite some benefits over angioplasty alone, stent deployment still results in significant injury to the vessel wall and disruption of the endothelium [10]. Disruption of endothelial monolayer integrity induces a cascade of pro-inflammatory events resulting in monocytic infiltration and smooth muscle cell proliferation, which are key contributing factors to neointimal hyperplasia. The rate of re-endothelialization following injury is a critical determinant of vascular lesion formation and areas of injury that rapidly re-endothelialize have significantly less intimal thickening and restenosis [11], while also deterring thrombus formation [12]. In humans, bare metal stent struts are substantially endothelialized in 6–7 months, with significant coverage present after 2 months [13]. During this reformation of the endothelium over stent struts, the smooth muscle proliferation induced by injury contributes to neointimal formation and restenosis. The high rates of restenosis for bare metal stents are a significant drawback in their clinical application.
Preliminary drug-coated stents were engineered with surface anticoagulants, such as heparin or warfarin to prevent sub-acute thrombosis and bleeding complications [14]. Despite attenuating thrombosis, restenosis was unchanged, requiring a pharmacological approach for its inhibition. Drug eluting stents (DES) releasing anti-proliferative agents such as sirolimus and paclitaxel inhibit neointimal hyperplasia but also substantially delay healing and re-endothelialization [13]. Consequently, DES are not only susceptible to early thrombosis like bare metal stents (BMS), but are also prone to both late (30 days–1 year) and very late (>1 year) stent thrombosis [15]. In stable single vessel disease patients, late stent thrombosis (LST) occurs at a constant rate (0.6% per year) [16], with even higher rates reported (0.9%–3%/year) in real world studies [17]. Accordingly, the safety of DES remains in question [18]. Hence the advent of DES has further exacerbated the biocompatibility issues of coronary stent implantation. The unsatisfactory performance of both BMS and DES has led to continued investigation of novel stent modifications, focusing on improving stent biocompatibility. The innovations discussed include surface tissue engineering, endothelial regeneration mechanisms, nanotechnology, and plasma physics for the biofunctionalization of coronary stents.
2. Limitations of Bare Metal Stents (BMS)
The metal alloys used to produce bare metal stents are fundamentally incompatible with the vasculature, promoting thrombosis due to their inherent surface properties [19] while exerting no inhibitory effect on smooth muscle cell hyperproliferation. The dominant mode of early BMS failure is acute thrombosis, which can be as high as 24% in the absence of the dual anti-platelet therapy administered to stent recipients [8]. Stent thrombosis is defined as a composite 30-day endpoint, which can present as an abrupt vessel closure, large non-fatal myocardial infarction or death [20]. Deaths attributed to cardiac causes within the first 30 days of stent implantation are usually adjudicated as stent thrombosis [21]. Neointimal hyperplasia, or restenosis, is a major cause of bare metal stent failure after the early thrombosis risk has abated. In-stent restenosis is driven by an uncontrolled immune response, triggered by the disruption of the native endothelium and damage to the vessel wall. The re-modelling of the vessel post-injury is characterized by hyper-proliferative smooth muscle cells infiltrating into the vessel lumen and secreting extracellular matrix components [22].
BMS are made from surgical grade metal alloys, initially 316 L stainless steel (316 L SS), but more recently evolving to cobalt chromium and platinum alloys [23]. Stent strut thickness and alloy type play an integral role in the biological responses elicited. Changes to the metal alloy have facilitated thinner strut design while retaining sufficient radial strength, and led to the re-design of stent structures for increased deliverability. The first Palmaz-Schatz crown stent designed for flexibility has evolved significantly to the malleable S-shaped velocity-stent, currently in development [24]. Stent design has further developed to include the Multilink stents with still thinner struts, Microstents and GFX stents [25] made of sinusoidal element of stainless steel. Sub-acute thrombosis rates, post stent implantation, have greatly reduced over the course of stent evolution, although the rate of in-stent restenosis remained high [4].
3. Drug Eluting Stents (DES): An Imperfect Solution
Systemic drug administration post BMS implantation to reduce restenosis was ultimately unsuccessful due to low drug concentrations, non-specifically targeting the neointima [26]. DES locally releasing anti-proliferative agents were introduced in 2003 to reduce restenosis associated with stent implantation [27]. While DES have been highly effective in suppression of neointimal hyperplasia (up to 10-fold compared to BMS [28]) local vascular delivery of rampamycin analogues or paclitaxel is an untargeted approach, employing non-specific agents to inhibit all cell proliferation [29]. These drugs bind FK506 binding protein-12 (FKB12) which in turn blocks the cell-cycle specific kinase, mammalian target of rapamycin (mTOR), to halt mitotic progression in the juncture of G1 and S phases in all cell types [30,31]. This in turn deregulates tissue factor in endothelial cells and monocytes [32,33]. The elution of anti-proliferative agents is associated with a dramatic delay in healing and re-endothelialization at the stent deployment site; such that DES struts have less than 50% endothelial coverage at three years [13].
DES development has focused on the major failings of current devices and has included modifications to the metal alloys, coating polymers and eluted drugs [34]. For example, Abbott Vascular have developed a 2nd generation everolimus-eluting XIENCE V stent, using a different stent alloy (cobalt chromium), polymer coating (fluoropolymer) and anti-restenotic drug to its 1st generation counterparts. This resulted in enhanced endothelialization in vitro and in vivo compared to 1st generation DES [35]. In randomised clinical trials, the XIENCE V stent also exhibited improved safety outcomes compared to two iterations of first generation paclitaxel-eluting stents [17,36]. Other approaches for second generation DES include the use of biodegradable polymers and selective coating of the anti-restenotic drug solely on the abluminal surface of the stent [37]. Despite these innovations, significant rates of major adverse cardiac events persist, particularly in real world usage of DES incorporating a high proportion of patients with acute coronary syndromes [37].
The most recent innovations in DES development are combinations of existing technology; employing drug-elution from a resorbable stent platform [38], from an ultra-thin degradable polymer coating [39] or combined with endothelial cell capture [40]. These approaches are included in more detail in Section 5, below.
4. Underlying Causes of Stent Incompatibility
The compatibility of bare metal stents is due both to the stent material and design, while DES effectiveness is also affected by polymers used for coating and the anti-proliferative drugs released. Design considerations such as strut thickness, cell design and mechanical properties have been steadily optimized, while polymer coatings and drug effects remain problematic, increasing inflammation [41], delaying re-endothelialization [13] and impairing endothelial cell function [42].
4.1. Inherent Thrombogenicity
Stents are inherently foreign bodies in the vessel wall, inducing platelet adhesion and activating coagulation, leading to thrombosis. Inhibition of platelet activation is required following stent delivery. The currently low rates of early stent thrombosis (1%–2%) [43] are predicated on tolerance and adherence to dual antiplatelet therapy with aspirin and a thienopyridine. This is not feasible for an increasing number of patients with high bleeding risk, or those requiring surgery [44] and is associated with increased risk of significant morbidity including gastrointestinal bleeding [45]. There is also risk of antiplatelet hypo-responsiveness, which increases stent thrombosis [46]. In the case of DES, management of LST is additionally problematic. To reduce the incidence of late thrombotic events, extended dual anti-platelet therapy is now recommended following DES placement, though no consensus on the effectiveness of an extended regimen has been reached [47,48]. In a recent large cohort study, new generation DES (n-DES) provided a modest improvement in clinical outcomes compared to old generation DES (o-DES) [49]. Old DES classified in the study, were first generation DES with bare metal platforms and sirolimus or paclitaxel drug elution (Cypher, Cypher Select, Taxus Express, Taxus Liberté and Endeavor) [49]. New DES classified in the study diversely included; stents eluting non-inflammatory drug zotarolimus coupled with a biocompatible polymer system (bioLinx™) [50] designed to extend the duration of drug exposure in the vessel (Endeavor Resolute), multi-layer coating technology (Xience V) [51] and self-expanding stents designed for compression resistance (Promus Element) [52]. The study compared long-term outcomes of PCI with n-DES vs. o-DES and BMS to show comparatively lowered risk of restenosis, LST and mortality for n-DES, although no significant effect was observed for thrombosis [49]. The duration of recommended dual antiplatelet therapy to prevent thrombosis remains unchanged for both old and new generation DES in patients.
4.2. Delayed Re-Endothelialization
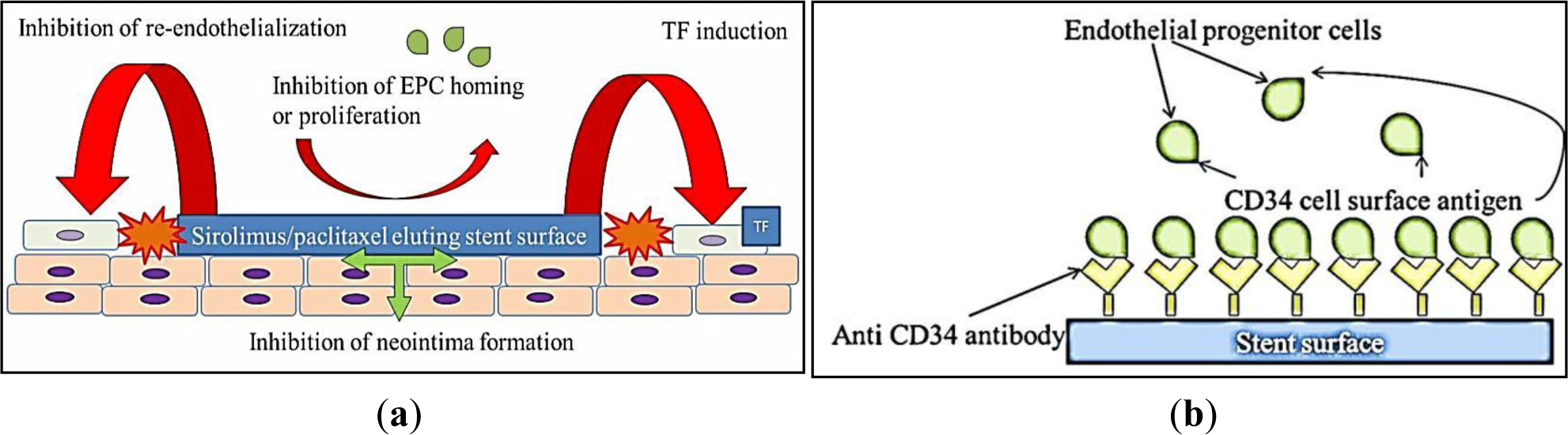
As discussed above, speed of re-endothelialization is an important predictor of clinical outcome for stents. Following vascular injury, endothelial cells migrate from intact neighboring coronary segments, or are recruited from circulating endothelial progenitor cells (EPC) [53] to re-endothelialize the injured artery. However, both rapamycin and paclitaxel actively suppress endothelial cell growth in vitro [33,54,55] and impede EPC homing and proliferation in vitro [56,57], actively impeding re-endothelialization. A morphological autopsy study conducted to compare coronary artery segments from patients after DES and BMS implantation revealed delayed arterial healing and poorer endothelialization after DES compared to BMS implantation of similar duration [13]. Within the 1st generation DES cohort, 60% of patients had evidence of LST and a 45% rate of death was reported for patients suffering DES LST [13,58]. Re-endothelialization was significantly higher in BMS compared to DES [42]. The impacts of 1st generation DES on vascular biology are schematically represented in Figure 1a.
4.3. Metal and Polymer Coating Hypersensitivity
Hypersensitivity to metal alloys such as molybdenum and nickel has been previously observed in ~10% of patients undergoing BMS implantation [61] although the inflammatory response for stainless steel, is much less pronounced in comparison [62]. Hypersensitivity towards BMS alloys is associated with restenosis in the range of 15%–20% [42], with the extent of inflammation correlating to the degree of restenosis [63]. Marked hypersensitivity reactions have also been observed to the polymers coating DES. First generation DES coated with poly-ethylene vinyl acetate polymer are demonstrably pro-inflammatory [64]. This was further verified in a preclinical study when the copolymer, used as an antigen delivery matrix elicited an inflammatory response in ~25% of rabbits [65]. The inflammatory response in patients with spontaneous coronary dissection, post DES implantation was characterized primarily with eosinophilic infiltrations in the adventitia [63]. In severe cases DES related clinical complications exhibit necrotic core prolapse, in-stent restenosis and LST, preventing arterial healing [66]. A preclinical study in a porcine model showed progressive increases in the eosinophilic, granulomatous infiltrate, post first generation sirolimus (Cypher) stent implantation, starting at 28 days, increasing to 60% at 6 months [42].
4.4. Poor Coating Integrity
Another important, often overlooked aspect of stent safety is the coating integrity after crimping and expansion. Relatively few studies have evaluated the possibility of coating delamination [67,68], despite it being widespread in commercially available DES and recognized as a safety concern by the Food and Drug Administration [69]. DES polymer coatings display widespread surface cracking, peeling and flaking at the polymer-metal interface [70–72]; exposing the underlying thrombogenic metallic substrate and contributing to chronic inflammatory and hypersensitivity reactions [41,73].
Together, these failings highlight the difficulty in simultaneously promoting re-endothelialization, while inhibiting neointimal hyperplasia and thrombosis. No current stent platform adequately achieves this goal, but the latest strategies are reviewed below.
5. Novel Stent Modifications
Coatings aimed at increasing the inertness of metallic implants have been effective at reducing thrombogenicity but have generally failed to reduce restenosis rates. Examples of these coatings include gold [74]; diamond-like carbon [75]; pyrolytic carbon [76] and phosphorylcholine (PC). PC; exemplifying the flaws of an inertness approach was observed to be non-thrombogenic in vitro [77] however; in vivo; it failed to encourage endothelialization and ultimately had no effect on the rate of stent thrombosis [78]. In parallel; enhancement of stent biocompatibility has been pursued by actively influencing the host response. These coatings have failed because they only seek to address one aspect of vascular biocompatibility (e.g., thrombogenicity alone) at the expense of other aspects. Heparin-coated stents are one such example; designed to reduce thrombosis but not neointimal hyperplasia [79]. Below, we describe some of the most recent attempts to develop biocompatible stents.
5.1. Accelerating Endothelialization
Given that re-endothelialization plays an integral role in vascular healing after stent implantation, coating stents with substances to accelerate endothelial cell coverage is an important therapeutic approach [42]. Preliminary studies designed to capture endothelial progenitor cells (EPCs) by coating stents with a polysaccharide intermediate and murine monoclonal anti-human CD34-positive antibodies showed feasibility in human clinical trials [80]. The Genous Bio-engineered R stent, similarly coated with immobilized anti-CD34 antibodies aims to enhance endothelialization by capturing circulating endothelial progenitor cells (Figure 1b). The captured CD34-positive EPCs are proposed to differentiate into a mature endothelium, but the CD34-positive markers used are non-specifically shared by haematopoietic stem cells and immune complement cells. Circulating CD34-positive mononuclear cells are also shown to differentiate into smooth muscle progenitor cells in patients with restenosis [81]. A higher rate of revascularization was observed in Genous stent compared to Taxus stent, in patients treated for coronary artery stenosis with a high risk of restenosis [82]. A recent proof-of-concept study shows some benefits for endothelialization and thrombogenicity, but leaves reduction of neointimal hyperplasia unaddressed and the platform reliant on drug-eluting technology [83]. A novel DES coated with integrin-binding cyclic Arg-Gly-Asp peptides was similarly utilized in a preclinical study to accelerate endothelialization via EPC attraction, using the same principles of EPC capture. In an initial porcine model evaluation, neointimal hyperplasia seems to be promisingly reduced [84].
5.2. Bioresorbable Stents
Bioresorbable stents are proposed to solve the problem of long-term stent biocompatibility by degrading over time [85]. The first bioabsorbable, balloon expandable stents implanted in humans were constructed from poly-L-lactic acid (PLLA) [86]. The bonds between the repeating lactide units of the bioabsorbable stent hydrolyze to produce lactic acid, metabolized to CO2 and H2O [85]. Absorption occurs via bulk erosion throughout the implant not just on the surface, allowing the stent strut to retain its shape, until the process is well advanced [86]. The Abbott Vascular bioresorbable vascular scaffold (BVS), a PLLA stent, has so far demonstrated restenosis similar to bare metal platforms, as well as late scaffold shrinkage and non-uniform vessel support, due to uneven scaffold degradation [87]. Alloys of magnesium have also been explored as bioabsorbable stent platforms [88]. Absorption by surface erosion reduces the strut thickness as the stent is absorbed, within 4 months of implantation, leading to loss of radial support [88].
The latest generation bioabsorbable stents are designed for prolonged radial support coupled with drug elution [88]. A number of different materials have been utilized to manufacture these stents ranging from metal alloys to a variety of polymers, including tyrosine-derived polycarbonate polymer, salicylate and a linker, as well as metal-cobalt chromium with n-butyl methacrylate coating [89,90]. The BioMatrix stent incorporates the thin S-stent platform with a reduced percentage metal surface area (16.3%–18.4%) to elute the anti-proliferative drug biolimus A9 [39]; a highly lipophilic semi-synthetic analogue of sirolimus. Furthermore, the stent is completely bioabsorbable degrading in vivo to lactic acid in 6–9 months post implantation [38]. The JACTAX stent (Boston Scientific Corporation, Natick, MA, USA) was designed on similar principles, coated with an ultra-thin, mixture of biodegradable PLLA and paclitaxel drug applied as microdots, per 16-mm stent [91]. The stents were comparable to the preceding paclitaxel eluting stent (TAXUS Liberté, Boston Scientific Corporation, Natick, MA, USA), although further studies are underway to evaluate their potential for improved vessel healing. As yet, none have FDA approval for humans use, but some clinical trials are underway [92]. Early indications are that the technology remains problematic. Current bioresorbable stents have markedly inferior mechanical properties in terms of device profile, flexibility, deliverability and radial strength, thereby dramatically limiting their capacity to be used for a large number of coronary lesion subsets including bifurcation lesions, calcified lesions, tortuous coronaries and long lesions [93]. As a consequence of these profound limitations, metal alloy stents will remain the mainstay for endovascular stents in the foreseeable future.
5.3. Nanotechnology for Controlled Release of Drugs and Novel Stent Design for Myocardial Reperfusion
Novel mechanisms of drug release include polymeric nanoparticles (NPs) to encapsulate pharmaceutical agents for targeted drug delivery to a tissue of interest [94–96]. For instance, d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) is used as an emulsifier in the formulation of the biomaterial matrix poly(DL-lactide-co-glycolide) (PLGA). The biodegradable PLGA/TPGS NPs deliver controlled paclitaxel release with high drug encapsulation efficiency (EE), for the treatment of restenosis. The higher drug EE improves cellular uptake and cytotoxicity against SMC proliferation, and is being considered in the development of third generation, nanoparticle coated DES [97]. The Nevo-sirolimus eluting stent is designed from L605 cobalt-chromium alloy with a drug delivery system based on PLGA. It utilizes a multi-channel reservoir system along the stent struts into which the drug-polymer (sirolimus/PLGA) matrix is loaded for elution [98] displaying superiority over traditional paclitaxel eluting stents (TAXUS Liberté) in clinical trials [99].
In patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing PCI, sub-optimal myocardial reperfusion is common. Stents have therefore been specifically designed to prevent thrombus protrusion into the lumen after PCI, in acute myocardial infarction. The potential utility of a novel polyethylene terephthalate (PET) micronet mesh-covered thin-strut metal stent (MGaurd), was evaluated in this regard for its functional design to trap and exclude thrombus and atheromatous debris to prevent distal embolization [100]. The stent showed superior rates of epicardial coronary flow and complete ST-segment resolution, compared to conventional metal stents. Larger clinical studies are required to determine improved clinical outcomes.
5.4. Plasma Polymerization
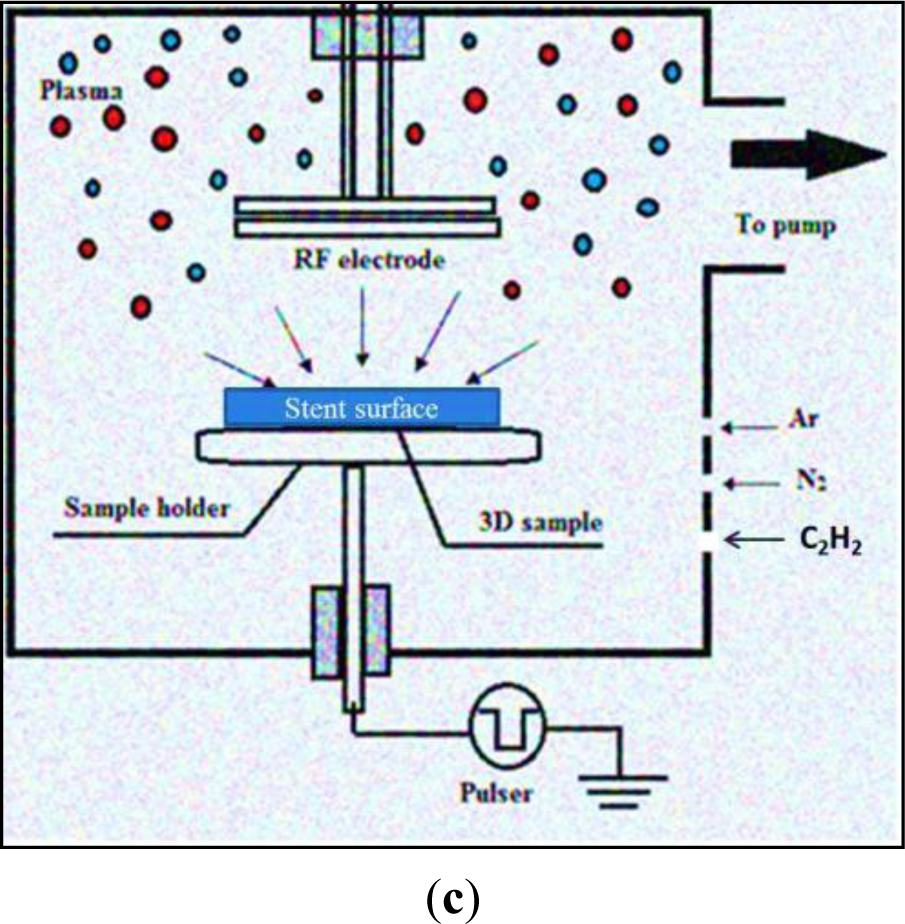
Plasma, the fourth state of matter is artificially generated when a dielectric gas ionized by free electrons is accelerated in a sufficiently strong electric field. The gas molecules separate on ion-impact, to create electrons and neutral gas atoms within a vacuum evacuated chamber. Subsequent collisions among these particles create more ions and electrons to interact with and modify the surfaces, including potential biomaterials [101,102], modifying their surface energy, charge and surface chemistry, without altering bulk properties [60]. When argon and an increasing amount of acetylene are used in a plasma deposition system, a plasma activated coating (PAC) was created on a metallic biomaterial surface (Figure 1c) suitable for stent applications. The thin carbon polymer layer on the biomaterial acts as a reservoir of free radicals, activating the surface for effective, covalent protein attachment [103,104].
Pulse biased plasma polymerization has been adapted to metallic substrates, for one step covalent biomolecule immobilization, minimising the otherwise complex process of chemical linker based biofunctionalization discussed elsewhere [105]. The relationship between protein binding and biomolecule activity on PAC strongly adhered to stainless steel, has been demonstrated by direct covalent attachment of tropoelastin, horseradish peroxidase and catalase [103,106]. Surface characterization further elucidated the importance of surface free energy for effective biomolecule attachment [60]. Recent evidence suggests that PAC on a 316 L SS stent covalently binds a dense layer of human recombinant tropoelastin, facilitating the growth of endothelial cells [107]. Plasma modified surfaces coated with tropoelastin have shown improved blood biocompatibility, significantly reduced clot formation and improved endothelialization in vitro [107]. Pre-clinical studies of PAC stents demonstrate feasibility and delivery with great potential as a carrier for local biomolecule delivery [108].
6. Conclusions and Future Perspectives
The development of coronary stents is an evolving process and a fundamental aspect of interventional therapy in the treatment of coronary artery disease. Stent biocompatibility is a multi-faceted process; having to be simultaneously hemocompatible, promote rapid re-endothelialization and suppress restenosis. BMS have evolved considerably since their first human use, but remain both thrombogenic and susceptible to restenosis. DES elute powerful cytotoxic drugs to inhibit SMC hyper-proliferation, but delay healing and induce inflammation, resulting in an increased risk of late thrombosis. We have highlighted the latest strategies that appear to be most promising, including active promotion of re-endothelialization, bioresorbable stents, nanotechnology and plasma based modification. In light of the limitations still evident for each of these approaches, further development is required, with biofunctionalized combination devices (e.g., bioresorbable drug-eluting stents) and local biomolecule delivery the most likely to have success. Overall, the many stent design innovations currently in development promise to address the underlying lack of biointegration more directly, on the path to a truly biocompatible stent.
Acknowledgments
We acknowledge funding from the National Health and Medical Research Council (APP1033079 and APP1039072).
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Mel, A.; Cousins, B.G.; Seifalian, A.M. Surface modification of biomaterials: A quest for blood compatibility. Int. J. Biomater 2012, 2012, 707863:1–707863:8. [Google Scholar]
- Allender, S.; Scarborough, P.; O’Flaherty, M.; Capewell, S. Patterns of coronary heart disease mortality over the 20th century in England and Wales: Possible plateaus in the rate of decline. BMC Public Health 2008, 8, 148. [Google Scholar]
- De Mel, A.; Jell, G.; Stevens, M.M.; Seifalian, A.M. Biofunctionalization of biomaterials for accelerated in situ endothelialization: A review. Biomacromolecules 2008, 9, 2969–2979. [Google Scholar]
- Sigwart, U.; Puel, J.; Mirkovitch, V.; Joffre, F.; Kappenberger, L. Intravascular stents to prevent occlusion and restenosis after trans-luminal angioplasty. N. Engl. J. Med 1987, 316, 701–706. [Google Scholar]
- Palmaz, J.C.; Sibbitt, R.R.; Reuter, S.R.; Tio, F.O.; Rice, W.J. Expandable intraluminal graft—A preliminary-study—Work in progress. Radiology 1985, 156, 73–77. [Google Scholar]
- Wright, K.C.; Wallace, S.; Charnsangavej, C.; Carrasco, C.H.; Gianturco, C. Percutaneous endovascular stents—An experimental evaluation. Radiology 1985, 156, 69–72. [Google Scholar]
- Palmaz, J.C.; Sibbitt, R.R.; Tio, F.O.; Reuter, S.R.; Peters, J.E.; Garcia, F. Expandable intraluminal vascular graft—A feasibility study. Surgery 1986, 99, 199–205. [Google Scholar]
- Schatz, R.A. Coronary stenting, report of the initial clinical-experience with the palmaz-schatz Tm balloon expandable stent. Dev. Cardiovasc. Med 1991, 117, 313–325. [Google Scholar]
- Schatz, R.A.; Goldberg, S.; Leon, M.; Baim, D.; Hirshfeld, J.; Cleman, M.; Topol, E. Clinical-experience with the palmaz-schatz coronary stent. J. Am. Coll Cardiol 1991, 17, B155–B159. [Google Scholar]
- Hofma, S.H.; Whelan, D.M.; van Beusekom, H.M.; Verdouw, P.D.; van der Giessen, W.J. Increasing arterial wall injury after long-term implantation of two types of stent in a porcine coronary model. Eur. Heart J 1998, 19, 601–609. [Google Scholar]
- Lau, A.K.; Leichtweis, S.B.; Hume, P.; Mashima, R.; Hou, J.Y.; Chaufour, X.; Wilkinson, B.; Hunt, N.H.; Celermajer, D.S.; Stocker, R. Probucol promotes functional reendothelialization in balloon-injured rabbit aortas. Circulation 2003, 107, 2031–2036. [Google Scholar]
- Wu, K.K.; Thiagarajan, P. Role of endothelium in thrombosis and hemostasis. Annu. Rev. Med 1996, 47, 315–331. [Google Scholar]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of drug-eluting stents in humans: Delayed healing and late thrombotic risk. J. Am. Coll. Cardiol 2006, 48, 193–202. [Google Scholar]
- Serruys, P.W.; Kay, I.P.; Disco, C.; Deshpande, N.V.; de Feyter, P.J. Periprocedural quantitative coronary angiography after Palmaz-Schatz stent implantation predicts the restenosis rate at six months: Results of a meta-analysis of the belgian netherlands stent study (BENESTENT) I, BENESTENT II pilot, BENESTENT II and MUSIC trials. J. Am. Coll. Cardiol 1999, 34, 1067–1074. [Google Scholar]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar]
- Daemen, J.; Wenaweser, P.; Tsuchida, K.; Abrecht, L.; Vaina, S.; Morger, C.; Kukreja, N.; Jüni, P.; Sianos, G.; Hellige, G.; et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: Data from a large two-institutional cohort study. Lancet 2007, 369, 667–678. [Google Scholar]
- Kedhi, E.; Joesoef, K.S.; McFadden, E.; Wassing, J.; van Mieghem, C.; Goedhart, D.; Smits, P.C. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): A randomised trial. Lancet 2010, 375, 201–209. [Google Scholar]
- Lemesle, G.; Delhaye, C.; Bonello, L.; de Labriolle, A.; Waksman, R.; Pichard, A. Stent thrombosis in 2008: Definition, predictors, prognosis and treatment. Arch. Cardiovasc. Dis 2008, 101, 769–777. [Google Scholar]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar]
- Le Feuvre, C.; Healy-Brucker, A.; Helft, G.; Monsegu, J.; Varenne, O.; Spaulding, C.; Collet, J.P.; Beygui, F.; Barthélémy, O.; Choussat, R. Long-term follow-up of patients with sirolimus-eluting stents for treatment of bare-metal in-stent restenosis. Int. J. Cardiol 2010, 140, 219–225. [Google Scholar]
- Serruys, P.W.; Kootstra, J.; Melkert, R.; de Jaegere, P.; van den Brand, M.; Morel, M. Peri-procedural PCA following Palmaz-Schatz stent implantation predicts restenosis rate at 6 months: Result of a meta-analysis of BENESTENT-I, BENESTENT-II pilot, BENESTENT-II and MUSIC. J. Am. Coll. Cardiol 1998, 31, 64. [Google Scholar]
- Price, M.J. Coronary Stenting: A Companion to Tobol’s Textbook of Interventional Cardiology; Elsevier Health Sciences: New York, NY, USA, 2013. [Google Scholar]
- Kereiakes, D.J.; Cox, D.A.; Hermiller, J.B.; Midei, M.G.; Bachinsky, W.B.; Nukta, E.D.; Leon, M.B.; Fink, S.; Marin, L.; Lansky, A.J. Usefulness of a cobalt chromium coronary stent alloy. Am. J. Cardiol 2003, 92, 463–466. [Google Scholar]
- Kiemeneij, F.; Serruys, P.W.; Macaya, C.; Rutsch, W.; Heyndrickx, G.; Albertsson, P.; Fajadet, J.; Legrand, V.; Materne, P.; Belardi, J.; et al. Continued benefit of coronary stenting versus balloon angioplasty: Five-year clinical follow-up of Benestent-I trial. J. Am. Coll. Cardiol 2001, 37, 1598–1603. [Google Scholar]
- Park, S.W.; Hong, M.K.; Lee, C.W.; Kim, J.J.; Park, H.K.; Cho, G.Y.; Kang, D.H.; Song, J.K.; Park, SJ. Immediate and late clinical and angiographic outcomes after GFX coronary stenting: Is high-pressure balloon dilatation necessary? Clin. Cardiol 2000, 23, 595–599. [Google Scholar]
- Ooya, T.; Lee, J.; Park, K. Effects of ethylene glycol-based graft, star-shaped, and dendritic polymers on solubilization and controlled release of paclitaxel. J. Control. Release 2003, 93, 121–127. [Google Scholar]
- Yan, B.P.; Ajani, A.E.; Waksman, R. Drug-eluting stents for the treatment of in-stent restenosis: A clinical review. Cardiovasc. Revascularization Med 2005, 6, 38–43. [Google Scholar]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med 2003, 349, 1315–1323. [Google Scholar]
- Lowe, H.C.; Khachigian, L.M. Coating stents with antirestenotic drugs: The blunderbuss or the magic bullet? Circulation 2002, 105, E29. [Google Scholar]
- Sehgal, S.N. Rapamune (R) (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression (reprinted from Clinical Biochemistry, 1998, Volume 31, pp. 335–340). Clin. Biochem 2006, 39, 484–489. [Google Scholar]
- Sehgal, S.N. Rapamune (R) (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin. Biochem 1998, 31, 335–340. [Google Scholar]
- Steffel, J.; Luscher, T.F.; Tanner, F.C. Tissue factor in cardiovascular diseases—Molecular mechanisms and clinical implications. Circulation 2006, 113, 722–731. [Google Scholar]
- Steffel, J.; Latini, R.A.; Akhmedov, A.; Zimmermann, D.; Zimmerling, P.; Luscher, T.F.; Tanner, F.C. Rapamycin, but not FK-506, increases endothelial tissue factor expression: Implications for drug-eluting stent design. Circulation 2005, 112, 2002–2011. [Google Scholar]
- O’Brien, B.; Carroll, W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: A review. Acta Biomater 2009, 5, 945–958. [Google Scholar]
- Joner, M.; Nakazawa, G.; Finn, A.V.; Quee, S.C.; Coleman, L.; Acampado, E.; Wilson, P.S.; Skorija, K.; Cheng, Q.; Xu, X.; et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol 2008, 52, 333–342. [Google Scholar]
- Planer, D.; Smits, P.C.; Kereiakes, D.J.; Kedhi, E.; Fahy, M.; Xu, K.; Serruys, P.W.; Stone, G.W. Comparison of everolimus- and paclitaxel-eluting stents in patients with acute and stable coronary syndromes: Pooled results from the SPIRIT (A Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (A Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice) Trials. JACC Cardiovasc. Interv 2011, 4, 1104–1115. [Google Scholar]
- Windecker, S.; Serruys, P.W.; Wandel, S.; Buszman, P.; Trznadel, S.; Linke, A.; Lenk, K.; Ischinger, T.; Klauss, V.; Eberli, F.; et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): A randomised non-inferiority trial. Lancet 2008, 372, 1163–1173. [Google Scholar]
- Costa, R.A.; Lansky, A.J.; Abizaid, A.; Mijeller, R.; Tsuchiya, Y.; Mori, K.; Cristea, E.; Leon, M.B.; Eduardo, J. Angiographic results of the first human experience with the Biolimus A9 drug-eluting stent for de novo coronary lesions. Am. J. Cardiol 2006, 98, 443–446. [Google Scholar]
- Abizaid, A.; Costa, J.R. New drug-eluting stents an overview on biodegradable and polymer-free next-generation stent systems. Circ. Cardiovasc. Interv 2010, 3, 384–393. [Google Scholar]
- Haude, M.; Lee, S.W.; Worthley, S.G.; Silber, S.; Verheye, S.; Erbs, S.; Rosli, M.A.; Botelho, R.; Meredith, I.; Sim, K.H.; et al. The REMEDEE trial a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC Cardiovasc. Interv 2013, 6, 334–343. [Google Scholar]
- Pendyala, L.; Jabara, R.; Robinson, K.; Chronos, N. Passive and active polymer coatings for intracoronary stents: Novel devices to promote arterial healing. J. Interv. Cardiol 2009, 22, 37–48. [Google Scholar]
- Luscher, T.F.; Steffel, J.; Eberli, F.R.; Joner, M.; Nakazawa, G.; Tanner, F.C.; Virmani, R. Drug-eluting stent and coronary thrombosis: Biological mechanisms and clinical implications. Circulation 2007, 115, 1051–1058. [Google Scholar]
- Urban, P.; de Benedetti, E. Thrombosis: The last frontier of coronary stenting? Lancet 2007, 369, 619–621. [Google Scholar]
- Eshaghian, S.; Kaul, S.; Amin, S.; Shah, P.K.; Diamond, G.A. Role of clopidogrel in managing atherothrombotic cardiovascular disease. Ann. Intern. Med 2007, 146, 434–441. [Google Scholar]
- Yan, B.P.; Gurvitch, R.; Ajani, A.E. Double jeopardy: Balance between bleeding and stent thrombosis with prolonged dual antiplatelet therapy after drug-eluting stent implantation. Cardiovasc. Revascularization Med 2006, 7, 155–158. [Google Scholar]
- Nebeker, J.R.; Virmani, R.; Bennett, C.L.; Hoffman, J.M.; Samore, M.H.; Alvarez, J.; Davidson, C.J.; McKoy, J.M.; Raisch, D.W.; Whisenant, B.K.; et al. Hypersensitivity cases associated with drug-eluting coronary stents—A review of available cases from the research on adverse drug events and reports (RADAR) project. J. Am. Coll. Cardiol 2006, 47, 175–181. [Google Scholar]
- Eisenstein, E.L.; Anstrom, K.J.; Kong, D.F.; Shaw, L.K.; Tuttle, R.H.; Mark, D.B.; Kramer, J.M.; Harrington, R.A.; Matchar, D.B.; Kandzari, D.E.; et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. J. Am. Med. Assoc 2007, 297, 159–168. [Google Scholar]
- Stone, G.W.; Ellis, S.G.; Colombo, A.; Grube, E.; Dawkins, K.D.; Friedman, M.; Baim, D.S. Effect of prolonged thienopyridine use after drug-eluting stent implantation (from the TAXUS landmark trials data). Am. J. Cardiol 2008, 102, 1017–1022. [Google Scholar]
- Sarno, G.; Lagerqvist, B.; Frobert, O.; Nilsson, J.; Olivecrona, G.; Omerovic, E.; Saleh, N.; Venetzanos, D.; James, S. Lower risk of stent thrombosis and restenosis with unrestricted use of onew-generation’ drug-eluting stents: A report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur. Heart J 2012, 33, 606–613. [Google Scholar]
- Galasso, G.; Piccolo, R.; Cassese, S.; Esposito, G.; Cirillo, P.; Leosco, D.; Rapacciuolo, A.; Sirico, D.; De Biase, C.; Niglio, T. Unrestricted use of endeavor resolute zotarolimus-eluting stent in daily clinical practice: A prospective registry. J. Invasive Cardiol 2012, 24, 251–255. [Google Scholar]
- Stone, G.W.; Midei, M.; Newman, W.; Sanz, M.; Hermiller, J.B.; Williams, J.; Farhat, N.; Caputo, R.; Xenopoulos, N.; Applegate, R. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents two-year clinical follow-up from the clinical evaluation of the xience V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions (SPIRIT) III trial. Circulation 2009, 119, 680–686. [Google Scholar]
- Sarno, G.; Lagerqvist, B.; Carlsson, J.; Olivecrona, G.; Nilsson, J.; Calais, F.; Gotberg, M.; Nilsson, T.; Sjogren, I.; James, S. Initial clinical experience with an everolimus eluting platinum chromium stent (Promus Element) in unselected patients from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Int. J. Cardiol 2013, 167, 146–150. [Google Scholar]
- Shi, Q.; Rafii, S.; Wu, M.H.; Wijelath, E.S.; Yu, C.; Ishida, A.; Fujita, Y.; Kothari, S.; Mohle, R.; Sauvage, L.R.; et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998, 92, 362–367. [Google Scholar]
- Marx, S.O.; Jayaraman, T.; Go, L.O.; Marks, A.R. Rapamycin-fkbp inhibits cell-cycle regulators of proliferation in vascular smooth-muscle cells. Circ. Res 1995, 76, 412–417. [Google Scholar]
- Guba, M.; von Breitenbuch, P.; Steinbauer, M.; Koehl, G.; Flegel, S.; Hornung, M.; Bruns, C.J.; Zuelke, C.; Farkas, S.; Anthuber, M.; et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat. Med 2002, 8, 128–135. [Google Scholar]
- Butzal, M.; Loges, S.; Schweizer, M.; Fischer, U.; Gehling, U.M.; Hossfeld, D.K.; Fiedler, W. Rapamycin inhibits proliferation and differentiation of human endothelial progenitor cells in vitro. Exp. Cell Res 2004, 300, 65–71. [Google Scholar]
- Chen, T.G.; Chen, J.Z.; Wang, X.X. Effects of rapamycin on number activity and eNOS of endothelial progenitor cells from peripheral blood. Cell Prolif 2006, 39, 117–125. [Google Scholar]
- Iakovou, I.; Schmidt, T.; Bonizzoni, E.; Ge, L.; Sangiorgi, G.M.; Stankovic, G.; Airoldi, F.; Chieffo, A.; Montorfano, M.; Carlino, M.; Michev, I.; Corvaja, N.; Briguori, C.; Gerckens, U.; et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005, 293, 2126–2130. [Google Scholar]
- Garg, S.; Duckers, H.J.; Serruys, P.W. Endothelial progenitor cell capture stents: Will this technology find its niche in contemporary practice? Eur. Heart J 2010, 31, 1032–1035. [Google Scholar]
- Wise, S.G.; Waterhouse, A.; Kondyurin, A.; Bilek, M.M.; Weiss, A.S. Plasma-based biofunctionalization of vascular implants. Nanomed. UK 2012, 7, 1907–1916. [Google Scholar]
- Koster, R.; Vieluf, D.; Kiehn, M.; Sommerauer, M.; Kahler, J.; Baldus, S.; Meinertz, T.; Hamm, CW. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 2000, 356, 1895–1897. [Google Scholar]
- Schofer, J.; Schluter, M.; Gershlick, A.H.; Wijns, W.; Garcia, E.; Schampaert, E.; Breithardt, G.; Investigators, E.-S. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: Double-blind, randomised controlled trial (E-SIRIUS). Lancet 2003, 362, 1093–1099. [Google Scholar]
- Virmani, R.; Guagliumi, G.; Farb, A.; Musumeci, G.; Grieco, N.; Motta, T.; Mihalcsik, L.; Tespili, M.; Valsecchi, O.; Kolodgie, F.D. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent should we be cautious? Circulation 2004, 109, 701–705. [Google Scholar]
- Finn, A.V.; Kolodgie, F.D.; Harnek, J.; Guerrero, L.J.; Acampado, E.; Tefera, K.; Skorija, K.; Weber, D.K.; Gold, H.K.; Virmani, R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 2005, 112, 270–278. [Google Scholar]
- Niemi, S.M.; Fox, J.G.; Brown, L.R.; Langer, R. Evaluation of ethylene-vinyl acetate copolymer as a noninflammatory alternative to freunds complete adjuvant in rabbits. Lab. Anim. Sci 1985, 35, 609–612. [Google Scholar]
- Farb, A.; Weber, D.K.; Kolodgie, F.D.; Burke, A.P.; Virmani, R. Morphological predictors of restenosis after coronary stenting in humans. Circulation 2002, 105, 2974–2980. [Google Scholar]
- Chen, M.-C.; Liang, H.-F.; Chiu, Y.-L.; Chang, Y.; Wei, H.-J.; Sung, H.-W. A novel drug-eluting stent spray-coated with multi-layers of collagen and sirolimus. J. Controlled Release 2005, 108, 178–189. [Google Scholar]
- Huang, Y.; Venkatraman, S.S.; Boey, F.Y.C.; Lahti, E.M.; Umashankar, P.R.; Mohanty, M.; Arumugam, S.; Khanolkar, L.; Vaishnav, S. In vitro and in vivo performance of a dual drug-eluting stent (DDES). Biomaterials 2010, 31, 4382–4391. [Google Scholar]
- Levy, Y.; Mandler, D.; Weinberger, J.; Domb, A.J. Evaluation of drug-eluting stents’ coating durability—Clinical and regulatory implications. J. Biomed. Mater. Res. Part B Appl. Biomater 2009, 91B, 441–451. [Google Scholar]
- Basalus, M.W.Z.; Ankone, M.J.K.; Houwelingen, G.V.; Man, F.H.A.F.D.; Birgelen, C.V. Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy. Eurointervention 2009, 5, 157–165. [Google Scholar]
- Otsuka, F.; Vorpahl, M.; Nakano, M.; Foerst, J.; Newell, J.B.; Sakakura, K.; Kutys, R.; Ladich, E.; Finn, A.V.; Kolodgie, F.D.; et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and Paclitaxel-eluting stents in humans. Circulation. 2014, 129, 211–23. [Google Scholar]
- Wiemer, M.; Butz, T.; Schmidt, W.; Schmitz, K.P.; Horstkotte, D.; Langer, C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: From nearly undamaged to major damaged polymers. Catheter. Cardiovasc. Interv. Off. J. Soc. Cardiac. Angiogr. Interv 2010, 75, 905–911. [Google Scholar]
- Finn, A.V.; Nakazawa, G.; Joner, M.; Kolodgie, F.D.; Mont, E.K.; Gold, H.K.; Webb, J.; Kaul, U.; Chan, C.; Thuesen, L.; et al. Vascular responses to drug eluting stents: Importance of delayed healing. Arterioscler. Thromb. Vasc. Biol 2007, 27, 1500–1510. [Google Scholar]
- Reifart, N.; Morice, M.C.; Silber, S.; Benit, E.; Hauptmann, K.E.; de Sousa, E.; Padmanaban, K.; Kuehnl, P. The NUGGET study: NIR ultra gold-gilded equivalency trial. Catheter. Cardiovasc. Interv 2004, 62, 18–25. [Google Scholar]
- Gutensohn, K.; Beythien, C.; Bau, J.; Fenner, T.; Grewe, P.; Koester, R.; Santoro, G.M.; Cerisano, G. In vitro analyses of diamond-like carbon coated stents. Reduction of metal ion release, platelet activation, and thrombogenicity. Thromb. Res 2000, 99, 577–585. [Google Scholar]
- Antoniucci, D.; Valenti, R.; Migliorini, A.; Moschi, G.; Trapani, M.; Bolognese, L.; Santoro, G.M.; Cerisano, G. Clinical and angiographic outcomes following elective implantation of the Carbostent in patients at high risk of restenosis and target vessel failure. Catheter. Cardiovasc. Interv 2001, 54, 420–426. [Google Scholar]
- Lewis, A.L. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf. B Biointerfaces 2000, 18, 261–275. [Google Scholar]
- Whelan, D.M.; van der Giessen, W.J.; Krabbendam, S.C.; van Vliet, E.A.; Verdouw, P.D.; Serruys, P.W.; Hoher, M. Biocompatibility of phosphorylcholine coated stents in normal porcine coronary arteries. Heart 2000, 83, 338–345. [Google Scholar]
- Wohrle, J.; Al-Khayer, E.; Grotzinger, U.; Schindler, C.; Kochs, M.; Hombach, V.; Baan, J.; Vis, M.M.; Scheunhage, E.; Piek, J.J.; et al. Comparison of the heparin coated vs. the uncoated Jostent—No influence on restenosis or clinical outcome. Eur. Heart J 2001, 22, 1808–1816. [Google Scholar]
- Aoki, J.; Serruys, P.W.; van Beusekom, H.; Ong, A.T.L.; McFadden, E.P.; Sianos, G.; van der Giessen, W.J.; Regar, E.; de Feyter, P.J.; Davis, H.R.; et al. Endothelial progenitor cell capture by stents coated with antibody against CD34—The HEALING-FIM (healthy endothelial accelerated lining inhibits neointimal growth-first in man) registry. J. Am. Coll. Cardiol 2005, 45, 1574–1579. [Google Scholar]
- Inoue, T.; Sata, M.; Hikichi, Y.; Sohma, R.; Fukuda, D.; Uchida, T.; Shimizu, M.; Komoda, H.; Node, K.; et al. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation—Impact on restenosis. Circulation 2007, 115, 553–561. [Google Scholar]
- Beijk, M.A.; Klomp, M.; Verouden, N.J.; van Geloven, N.; Koch, K.T.; Henriques, J.P.; Baan, J.; Vis, M.M.; Scheunhage, E.; Piek, J.J.; et al. Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: A randomized, single-centre, pilot study. Eur. Heart J 2010, 31, 1055–1064. [Google Scholar]
- Larsen, K.; Cheng, C.; Tempel, D.; Parker, S.; Yazdani, S.; den Dekker, W.K.; Houtgraaf, J.H.; de Jong, R.; Swager-ten Hoor, S.; Ligtenberg, E.; et al. Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model. Eur. Heart J 2012, 33, 120–128. [Google Scholar]
- Blindt, R.; Vogt, F.; Astafieva, I.; Fach, C.; Hristov, M.; Krott, N.; Seitz, B.; Kapurniotu, A.; Kwok, C.; Dewor, M.; et al. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J. Am. Coll. Cardiol 2006, 47, 1786–1795. [Google Scholar]
- Ormiston, J.A.; Serruys, P.W.S. Bioabsorbable coronary stents. Circ.-Cardiovasc. Interv 2009, 2, 255–260. [Google Scholar]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Uehata, H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 2000, 102, 399–404. [Google Scholar]
- Serruys, P.W.; Ong, A.T.; Piek, J.J.; Neumann, F.J.; van der Giessen, W.J.; Wiemer, M.; Zeiher, A.; Grube, E.; Haase, J.; Thuesen, L.; et al. A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: The SPIRIT first trial. EuroIntervention 2005, 1, 58–65. [Google Scholar]
- Erbel, R.; di Mario, C.; Bartunek, J.; Bonnier, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M.; et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: A prospective, non-randomised multicentre trial. Lancet 2007, 369, 1869–1875. [Google Scholar]
- Ormiston, J.A.; Webster, M.W.I.; Ruygrok, P.N.; Meredith, I.T.; Ardill, J.P.; Buller, C.E.; Ricci, D.R.; Chan, C.; Devlin, G.P.; Stewart, J.T.; et al. Six-month angiographic and 12-month clinical follow-up of multilink long (25 to 35 mm) stents for long coronary narrowings in patients with angina pectoris. Am. J. Cardiol 2002, 90, 222–226. [Google Scholar]
- Jabara, R.; Pendyala, L.; Geva, S.; Chen, J.; Chronos, N.; Robinson, K. Novel fully bioabsorbable salicylate-based sirolimus-eluting stent. Eurointervention 2009, 5, F58–F64. [Google Scholar]
- Grube, E.; Schofer, J.; Hauptmann, K.E.; Nickenig, G.; Curzen, N.; Allocco, D.J.; Dawkins, K.D. A novel paclitaxel-eluting stent with an ultrathin abluminal biodegradable polymer 9-month outcomes with the JACTAX HD stent. JACC Cardiovasc. Interv 2010, 3, 431–438. [Google Scholar]
- Onuma, Y.; Ormiston, J.; Serruys, P.W. Bioresorbable scaffold technologies. Circ. J 2011, 75, 509–520. [Google Scholar]
- Bittl, J.A. Bioresorbable stents: The next revolution. Circulation 2010, 122, 2236–2238. [Google Scholar]
- Mu, L.; Feng, S.S. PLGA/TPGS nanoparticles for controlled release of paclitaxel: Effects of the emulsifier and drug loading ratio. Pharm. Res 2003, 20, 1864–1872. [Google Scholar]
- Locatelli, E.; Franchini, M.C. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanopart Res 2012, 14, 1316. [Google Scholar]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Controlled Release 2001, 70, 1–20. [Google Scholar]
- Feng, S.S.; Zhao, L.Y.; Tang, J.T. Nanomedicine for oral chemotherapy. Nanomed.-UK 2011, 6, 407–410. [Google Scholar]
- Otake, H.; Honda, Y.; Courtney, B.K.; Shimohama, T.; Ako, J.; Waseda, K.; Macours, N.; Rogers, C.; Popma, J.J.; Abizaid, A.; et al. Intravascular ultrasound results from the NEVO ResElution-I trial: A randomized, blinded comparison of sirolimus-eluting NEVO stents with paclitaxel-eluting TAXUS Liberte stents in de novo native coronary artery lesions. Circ. Cardiovasc. Interv 2011, 4, 146–154. [Google Scholar]
- Ormiston, J.A.; Abizaid, A.; Spertus, J.; Fajadet, J.; Mauri, L.; Schofer, J.; Thuesen, L.; Dubois, C.; Hoffmann, R.; Wijns, W.; et al. Six-month results of the NEVO Res-Elution I (NEVO RES-I) trial: A randomized, multicenter comparison of the NEVO sirolimus-eluting coronary stent with the TAXUS Liberte paclitaxel-eluting stent in de novo native coronary artery lesions. Circ. Cardiovasc. Interv 2010, 3, 556–564. [Google Scholar]
- Stone, G.W.; Abizaid, A.; Silber, S.; Dizon, J.M.; Merkely, B.; Costa, R.A.; Wojdyla, R.; Maehara, A.; Dressler, O.; Brener, S.J.; et al. Prospective, randomized, multicenter evaluation of a polyethylene terephthalate micronet mesh-covered stent (MGuard) in ST-segment elevation myocardial infarction: The MASTER trial. J. Am. Coll. Cardiol 2012, 60, 1975–1984. [Google Scholar]
- Svorcik, V.; Makajova, Z.; Kasalkova-Slepickova, N.; Kolska, Z.; Bacakova, L. Plasma-modified and polyethylene glycol-grafted polymers for potential tissue engineering applications. J. Nanosci. Nanotechnol 2012, 12, 6665–6671. [Google Scholar]
- Peter, A.S. Plasma Physics: An Introduction to the Theory of Astrophysical, Geophysical and Laboratory Plasmas, 1st ed.; Peter, A.S., Ed.; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Yin, Y.B.; Bilek, M.M.M.; McKenzie, D.R.; Nosworthy, N.J.; Kondyurin, A.; Youssef, H.; Byrom, M.J.; Yang, W.R. Acetylene plasma polymerized surfaces for covalent immobilization of dense bioactive protein monolayers. Surf. Coat. Technol 2009, 203, 1310–1316. [Google Scholar]
- Bilek, M.M.M.; Bax, D.V.; Kondyurin, A.; Yin, Y.B.; Nosworthy, N.J.; Fisher, K.; Waterhouse, A.; Weiss, A.S.; dos Remedios, C.G.; McKenzie, D.R. Free radical functionalization of surfaces to prevent adverse responses to biomedical devices. Proc. Natl. Acad. Sci. USA 2011, 108, 14405–14410. [Google Scholar]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Process. Polym 2006, 3, 392–418. [Google Scholar]
- Bilek, M.M.; McKenzie, D.R. Plasma modified surfaces for covalent immobilization of functional biomolecules in the absence of chemical linkers: Towards better biosensors and a new generation of medical implants linkers. Biophys. Rev 2010, 2, 55–65. [Google Scholar]
- Waterhouse, A.; Yin, Y.B.; Wise, S.G.; Bax, D.V.; McKenzie, D.R.; Bilek, M.M.M.; Weiss, A.S.; Ng, M.K.C. The immobilization of recombinant human tropoelastin on metals using a plasma-activated coating to improve the biocompatibility of coronary stents. Biomaterials 2010, 31, 8332–8340. [Google Scholar]
- Waterhouse, A.; Wise, S.G.; Yin, Y.B.; Wu, B.C.; James, B.; Zreiqat, H.; McKenzie, D.R.; Bao, S.S.; Weiss, A.S.; Ng, M.K.C.; et al. In vivo biocompatibility of a plasma-activated, coronary stent coating. Biomaterials 2012, 33, 7984–7992. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jeewandara, T.M.; Wise, S.G.; Ng, M.K.C. Biocompatibility of Coronary Stents. Materials 2014, 7, 769-786. https://doi.org/10.3390/ma7020769
Jeewandara TM, Wise SG, Ng MKC. Biocompatibility of Coronary Stents. Materials. 2014; 7(2):769-786. https://doi.org/10.3390/ma7020769
Chicago/Turabian StyleJeewandara, Thamarasee M., Steven G. Wise, and Martin K. C. Ng. 2014. "Biocompatibility of Coronary Stents" Materials 7, no. 2: 769-786. https://doi.org/10.3390/ma7020769



