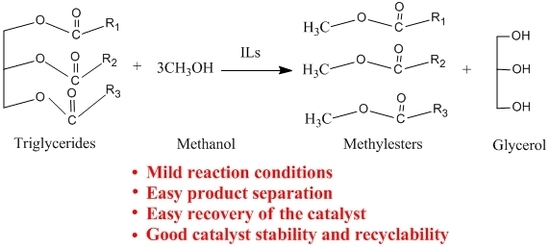
Preparation of Biodiesel from Soybean Catalyzed by Basic Ionic Liquids [Hnmm]OH
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
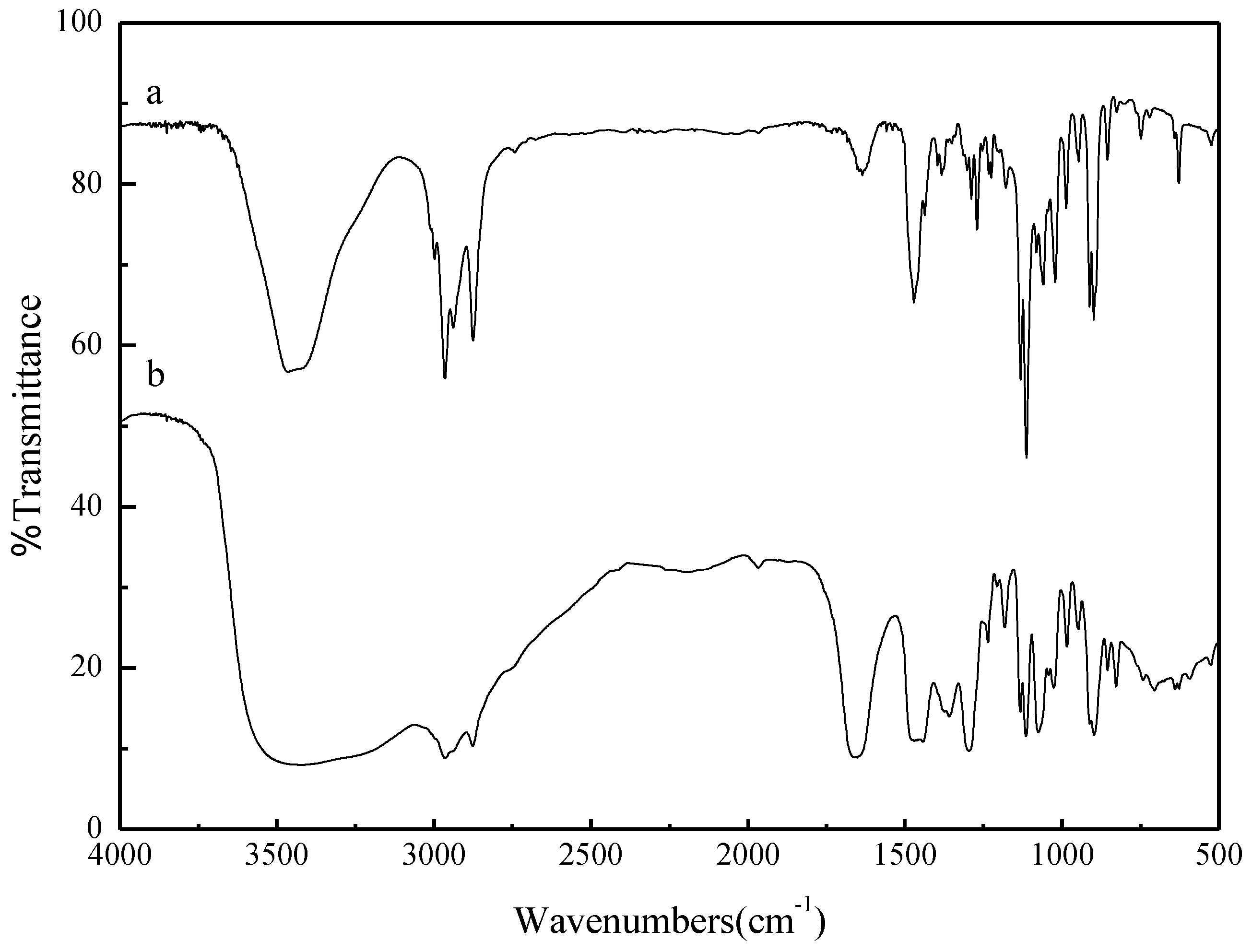
2.2. The Basicity and the Thermal Stability of the Ionic Liquids
| Solution concentration (g·mL−1) | [Hnmm]OH, pH | KOH, pH |
|---|---|---|
| 0.005 | 11.981 | 12.970 |
| 0.01 | 12.350 | 13.244 |
| 0.05 | 13.044 | 13.900 |
| 0.1 | 13.336 | 14.196 |

2.3. Effect of [Hnmm]OH Concentration

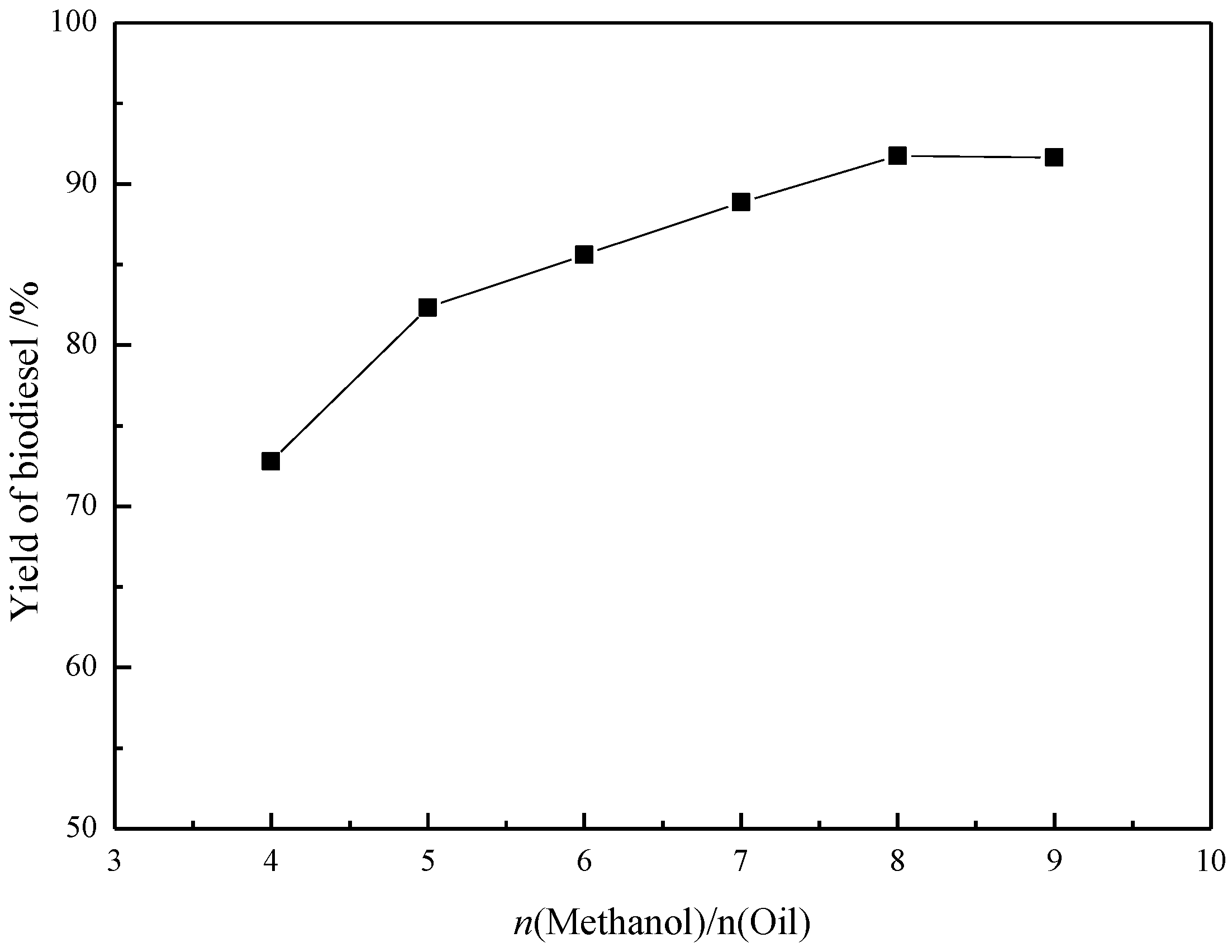
2.4. Effect of the Methanol/Soybean Oil Molar Ratio
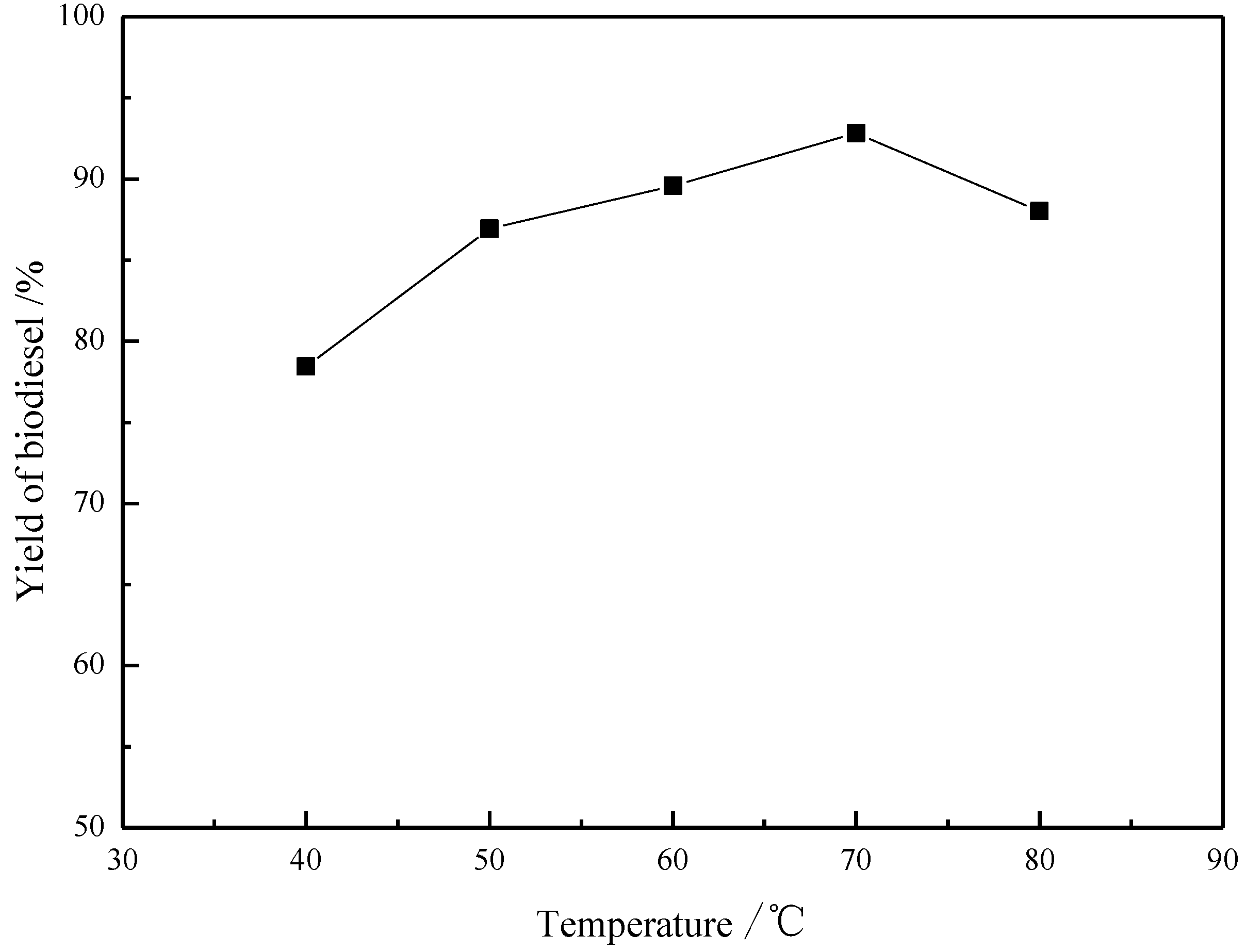
2.5. Effect of Reaction Temperature
2.6. Effect of Reaction Time

2.7. Recycling of the [Hnmm]OH
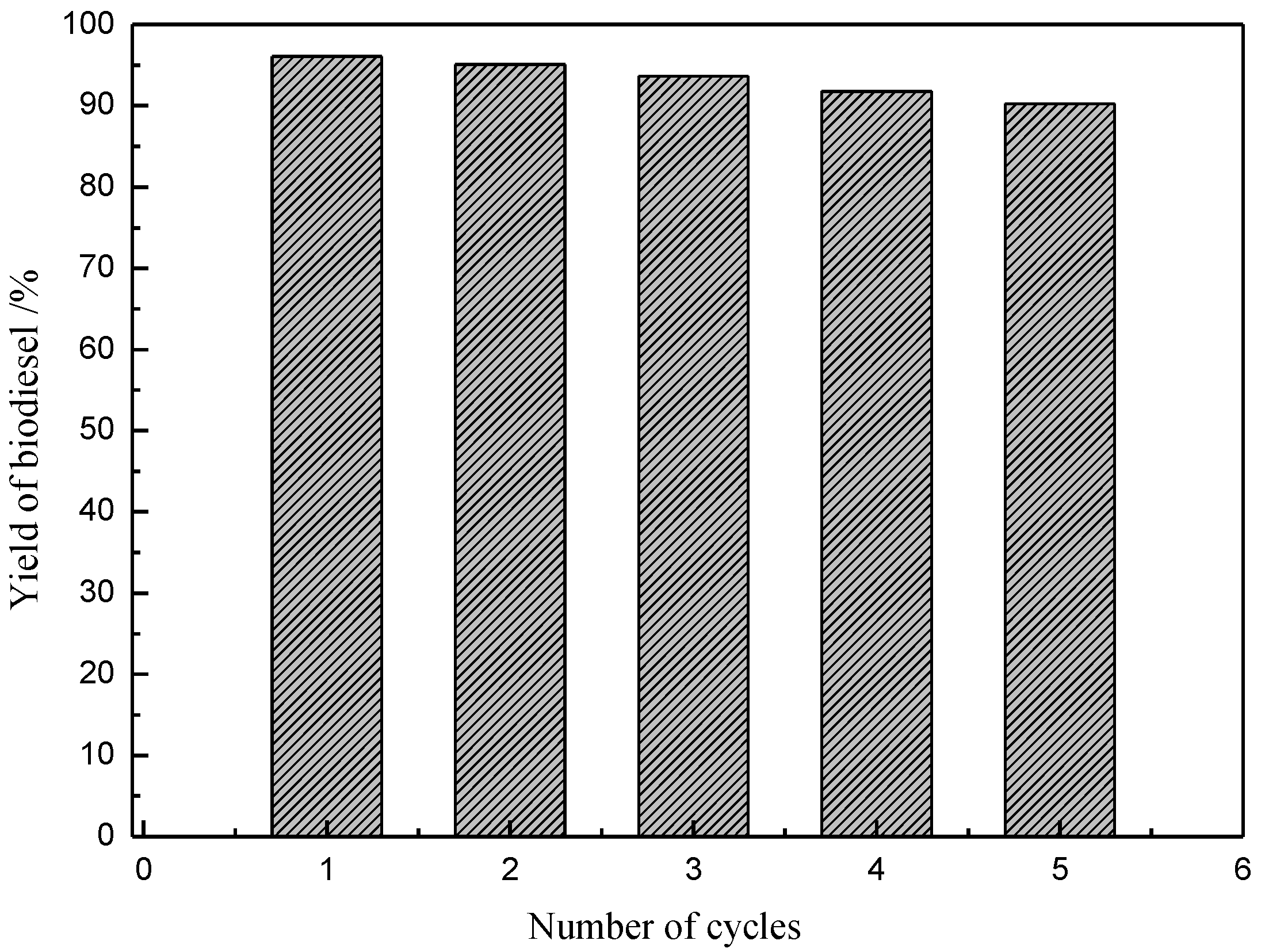
2.8. Comparative Study on the Catalytic Activities in Different Catalyst
| Catalyst | Yield of biodiesel/% |
|---|---|
| KOH | 97.8 |
| [Hnmm]OH | 97.0 |
| [Bmim]Im [16] | 95 |
2.9. Comparison of the Properties of the as Prepared Biodiesel with Specifications
| Properties | Biodiesel | Specification | Standard method |
|---|---|---|---|
| density (20 °C)/(kg/m3) | 875.2 | 820–900 | GB/T2540 |
| viscosity (40 °C)/(mm2/s) | 4.36 | 1.9–6.0 | GB/T265 |
| flash point (close cup)/°C | 171 | >130 | GB/T261 |
| cold filter pour point/°C | 3 | report | SH/T0248 |
| pour point/°C | 0 | report | SH/T0248 |
| sulfur content/(mass)/% | 0.0078 | ≤0.05 | SH/T0689 |
| Carbon res idual(mass)/% | 0.25 | ≤0.3 | GB/T17144 |
| ash content/%(mass) | 0.01 | ≤0.020 | GB/T2433 |
| water content/(Vol)/% | trace | ≤0.05 | SH/T0246 |
| Cu Corrosion(50 °C, 3 h)/Grade | 1 | ≤1 | GB/T5096 |
| centane number | 51 | ≥49 | GB/T386 |
| oxidative stability(110 °C)/h | 6.5 | ≥6.0 | EN14112 |
| acidity value/mg KOH (g·oil)−1 | 0.48 | ≤0.80 | GB/T264 |
| Free Glycerol content (mass)/% | 0.015 | ≤0.02 | ASTMD6584 |
| Total Glycerol content(mass)/% | 0.21 | ≤0.25 | ASTMD6584 |
| distillation range (90%)/°C | 348 | ≤360 | GB/T6536 |
3. Experimental Section
3.1. Materials
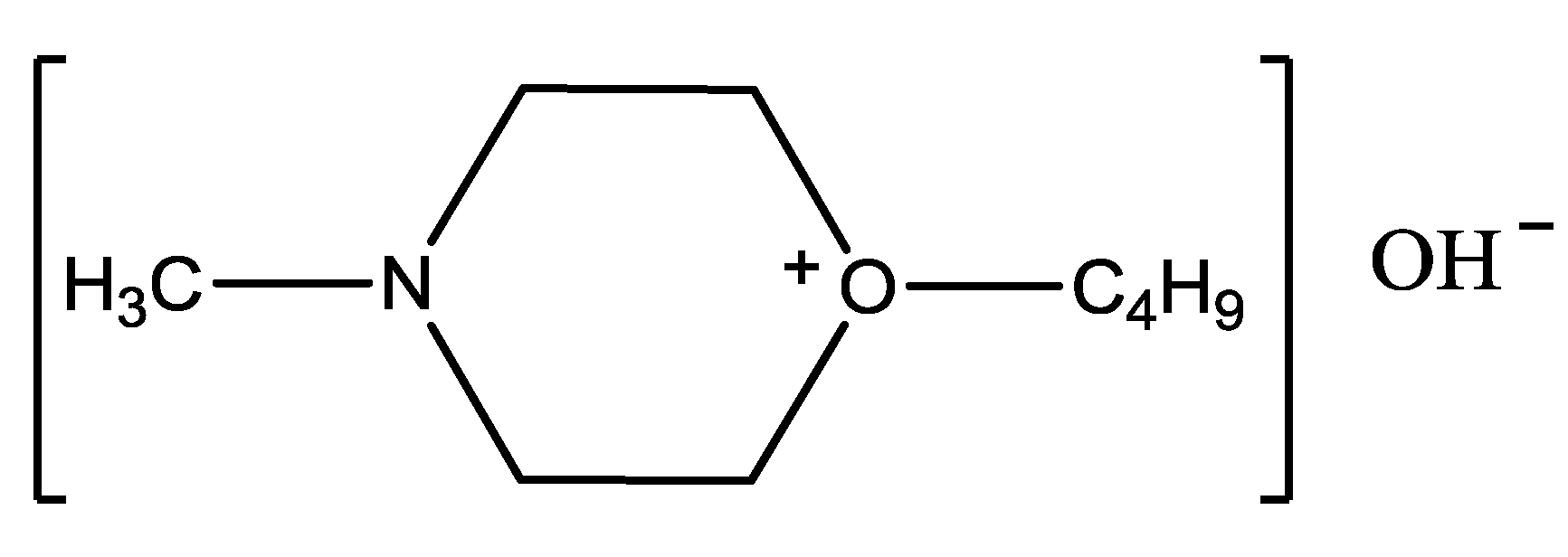
3.2. Preparation of the Alkaline Ionic Liquid [Hnmm]OH
3.3. Synthesis of Biodiesel

3.4. Analytical Methods
3.4.1. Preliminary Physical and Chemical Properties of Soybean Oil
3.4.2. The Determination of the Yield of Biodiesel
3.4.3.The Structure Analysis of Ionic Liquids
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hayyan, M.; Mjalli, F.S.; Hashim, M.A.; AINashef, I.M. A novel technique for separating glycerine from palm oil-based biodiesel using ionic liquids. Fuel Process. Technol. 2010, 91, 116–120. [Google Scholar] [CrossRef]
- Liang, J.H.; Ren, X.Q.; Wang, J.T.; Jiang, M.; Li, Z.J. Preparation of biodiesel by transesterification from cottonseed oil using the basic dication ionic liquids as catalysts. J. Fuel Chem. Technol. 2010, 38, 275–280. [Google Scholar] [CrossRef]
- Ghiaci, M.; Aghabarari, B.; Gil, A. Production of biodiesel by esterification of natural fatty acids over modified organoclay catalysts. Fuel 2011, 90, 3382–3389. [Google Scholar] [CrossRef]
- Wu, Q.; Wan, H.; Li, H.; Song, H.; Chu, T.H. Bifunctional temperature-sensitive amphiphilic acidic ionic liquids for preparation of biodiesel. Catal. Today 2013, 200, 74–79. [Google Scholar] [CrossRef]
- Elsheikh, Y.A. Preparation of Citrullus colocynthis biodiesel via dual-step catalyzed process using functionalized imidazolium and pyrazolium ionic liquids for esterification step. Ind. Crops Prod. 2013, 49, 822–829. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energ. Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Guo, W.L.; Li, H.L.; Ji, G.L.; Zhang, G.Y. Ultrasound-assisted production of biodiesel from soybean oil using Brønsted acidic ionic liquid as catalyst. Bioresour. Technol. 2012, 125, 332–334. [Google Scholar] [CrossRef]
- Liang, X.Z.; Gong, G.Z.; Wu, H.H.; Yang, J.G. Highly efficient procedure for the synthesis of biodiesel from soybean oil using chloroaluminate ionic liquid as catalyst. Fuel 2009, 88, 613–616. [Google Scholar] [CrossRef]
- Yan, H.Y.; Shi, K.Y.; Shi, H.B. Study on properties of biodiesel synthesized by catalytic esterification with sulfuric acid. J. Chem. Ind. Eng. 2011, 32, 17–19. [Google Scholar]
- Chakraborty, R.; Bepari, S.; Banerjee, A. Transesterification of soybean oil catalyzed by fly ash and egg shell derived solid catalysts. Chem. Eng. J. 2010, 165, 798–805. [Google Scholar] [CrossRef]
- Fauzi, A.H.M.; Amin, N.A.S. An overview of ionic liquids as solvents in biodiesel synthesis. Renew. Sust. Energ. Rev. 2012, 16, 5770–5786. [Google Scholar] [CrossRef]
- Judeh, Z.M.A.; Shen, H.-Y.; Chi, B.C.; Feng, L.-C.; Selvasothi, S. A facile and efficient nucleophilic displacement reaction at room temperature in ionic liquids. Tetrahedron Lett. 2002, 43, 9381–9384. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic liquids for clean technology. J. Chem. Technol. Biotechnlol. 1997, 68, 351–356. [Google Scholar]
- Claudio, A.F.M.; Ferreira, A.M.; Shahriari, S.; Freire, M.G.; Coutinho, J.A.P. Critical assessment of the formation of ionic-liquid-based aqueous two-phase systems in acidic media. J. Phys. Chem. B 2011, 115, 11145–11153. [Google Scholar] [CrossRef]
- Zanin, F.G.; Macedo, A.; Archilha, M.V.L.R.; Wendler, E.P.; Santos, A.A.D. A one-pot glycerol-based additive-blended ethyl biodiesel production: A green process. Bioresour. Technol. 2013, 143, 126–130. [Google Scholar] [CrossRef]
- Luo, H.; Fan, W.Y.; Li, Y.; Nan, G.Z. Biodiesel production using alkaline ionic liquid and adopted as lubricity additive for low-sulfur diesel fuel. Bioresour. Technol. 2013, 140, 337–341. [Google Scholar] [CrossRef]
- Zhang, L.; Xian, M.; He, Y.C.; Li, L.Z.; Yang, J.M.; Yu, S.T.; Xu, X. A Brønsted acidic ionic liquid as an efficient and environmentally benign catalyst for biodiesel synthesis from free fatty acids and alcohols. Bioresour. Technol. 2009, 100, 4368–4373. [Google Scholar] [CrossRef]
- Han, M.H.; Yi, W.L.; Wu, Q.; Liu, Y.; Hong, Y.C.; Wang, D.Z. Preparation of biodiesel from waste oils catalyzed by a Brønsted acidic ionic liquid. Bioresour. Technol. 2009, 100, 2308–2310. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z.; Tian, X.F.; Long, Y.D.; Jiang, L.Q. One-step production of biodiesel from Jatropha oil with high-acid value in ionic liquids. Bioresour. Technol. 2011, 102, 6469–6472. [Google Scholar] [CrossRef]
- Andreani, L.; Rocha, J.D. Use of ionic liquids in biodiesel production: A review. Br. J. Chem. Eng. 2012, 29, 1–13. [Google Scholar] [CrossRef]
- Arshadi, M.; Ghiaci, M.; Gil, A. Schiff base ligands immobilized on a nanosized SiO2-Al2O3 mixed oxide as adsorbents for heavy metals. Ind. Eng. Chem. Res. 2011, 50, 13628–13635. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Y.; Zhao, H.; Li, C.H. Synthesis of a new basic ionic liquid and its application in catalyzing preparation of biodiesel. Petroleum Prog. Sect. 2012, 28, 201–206. [Google Scholar]
- Li, C.Z.; Zhang, A.H.; Xiao, Z.H.; Jiang, L.J.; Li, P.W. Preparation of biodiesel from cornus wilsoniana fruit oil with basic ionic liquids as catalyst. J. Cent. South. Univ. For. Technol. 2011, 31, 38–43. [Google Scholar]
- Zhang, A.H.; Zhang, Y.J.; Li, C.Z.; Li, P.W.; Chen, J.L.; Xiao, Z.H. Synthesis of biodiesel using new basic ionic liquid as catalyst. Appl. Chem. Ind. 2009, 38, 168–177. [Google Scholar]
- Han, S.; Luo, M.; Zhou, X.Z.; He, Z.; Xiong, L.P. Synthesis of dipentyl carbonate by transesterification using basic ionic liquid [BmIm]OH catalyst. Ind. Eng. Chem. Res. 2012, 51, 5433–5437. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Q.; Zuo, T.; Pan, J.; Chen, C.; Li, W. Preparation of Biodiesel from Soybean Catalyzed by Basic Ionic Liquids [Hnmm]OH. Materials 2014, 7, 8012-8023. https://doi.org/10.3390/ma7128012
Ren Q, Zuo T, Pan J, Chen C, Li W. Preparation of Biodiesel from Soybean Catalyzed by Basic Ionic Liquids [Hnmm]OH. Materials. 2014; 7(12):8012-8023. https://doi.org/10.3390/ma7128012
Chicago/Turabian StyleRen, Qinggong, Tongmei Zuo, Jingjing Pan, Changle Chen, and Weimin Li. 2014. "Preparation of Biodiesel from Soybean Catalyzed by Basic Ionic Liquids [Hnmm]OH" Materials 7, no. 12: 8012-8023. https://doi.org/10.3390/ma7128012