Study of Lysozyme-Loaded Poly-L-Lactide (PLLA) Porous Microparticles in a Compressed CO2 Antisolvent Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of LSZ-Loaded PLLA PMs (LSZ-PLLA PMs): Morphology, Size, Porosity and Tapped Density Investigation
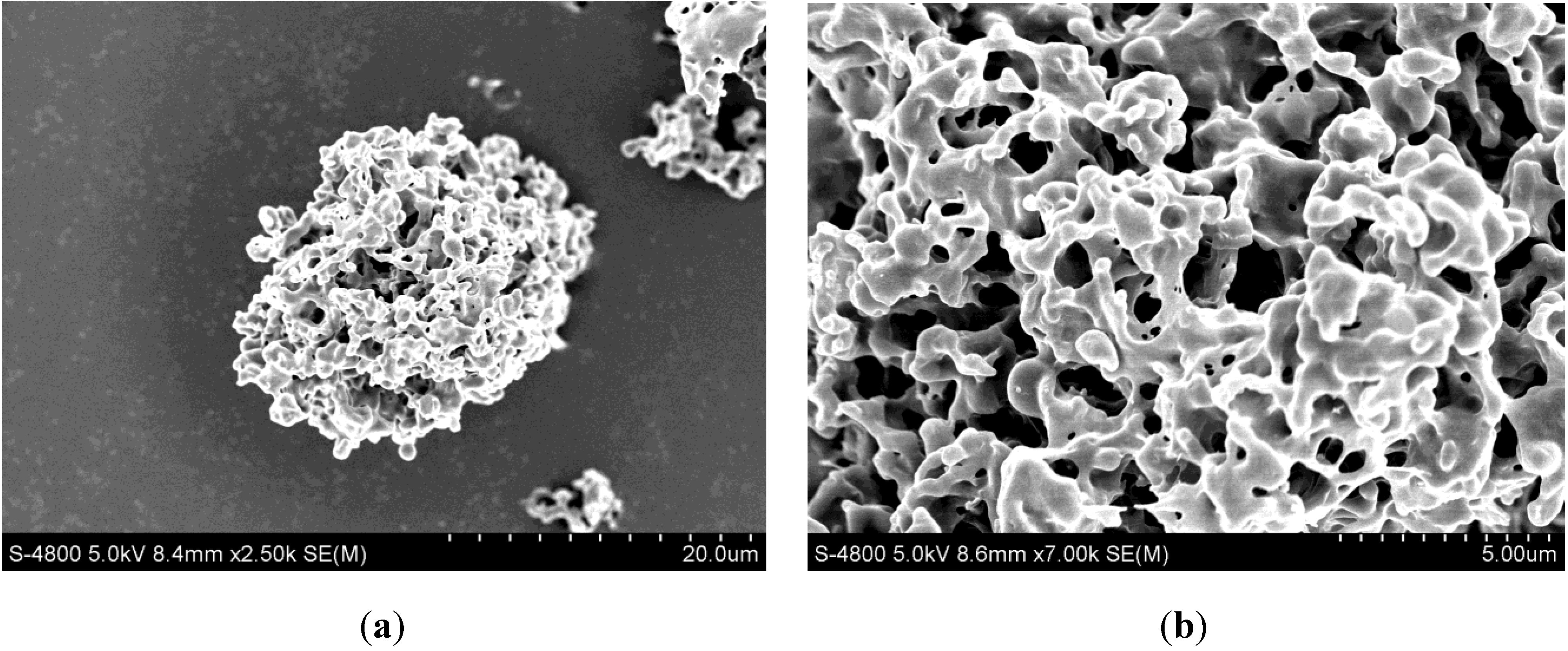
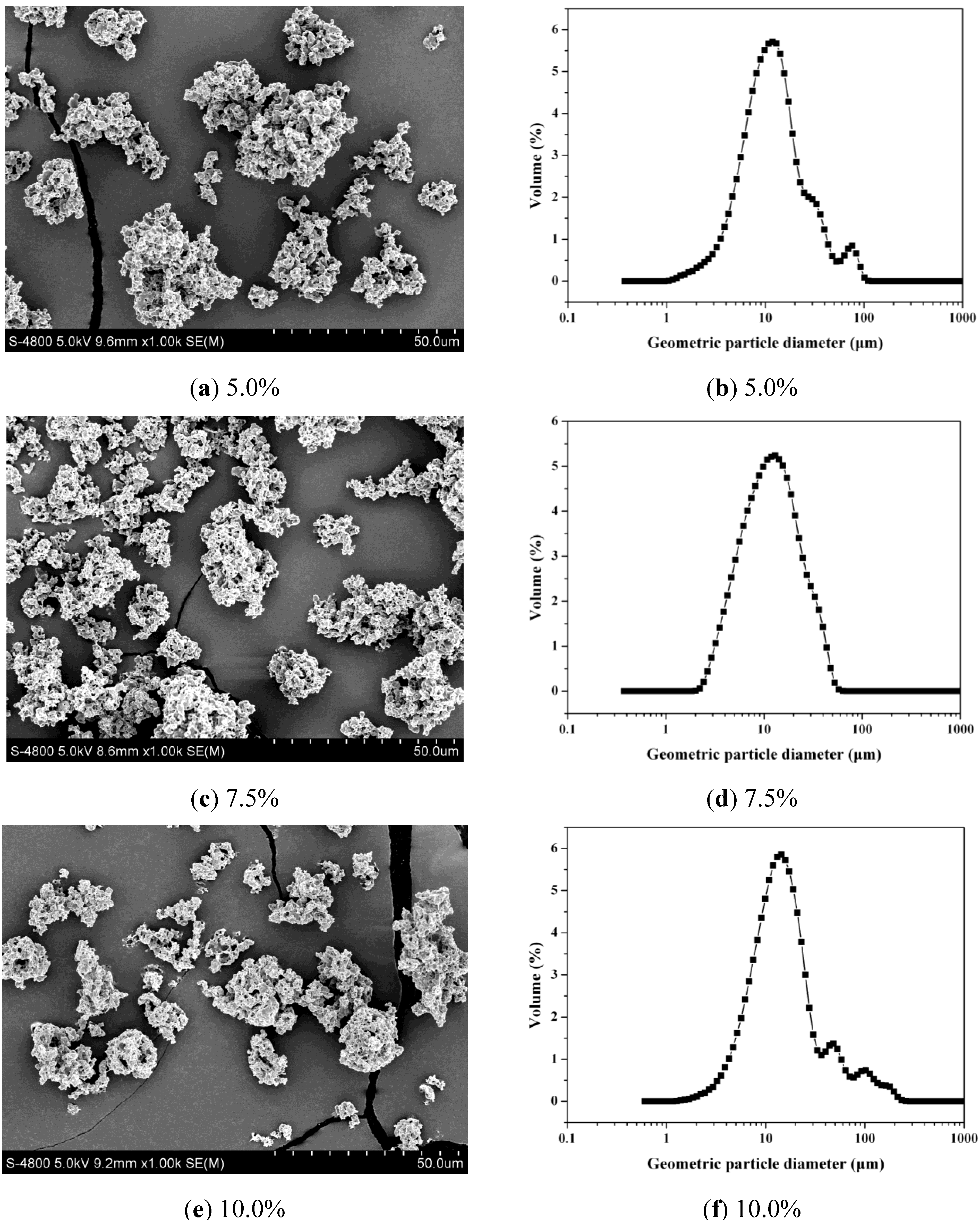
| Sample | Dg [μm] | Da [μm] | FPF [%] | Porosity [%] | Density [g·cm−3] | Aperture [μm] |
|---|---|---|---|---|---|---|
| LSZ-5.0% | 18.8 ± 2.4 | 2.6 ± 0.2 | 66.8 ± 1.5 | 86.3 | 0.09 ± 0.02 | 0.70 |
| LSZ-7.5% | 17.5 ± 0.7 | 2.7 ± 0.4 | 63.9 ± 1.4 | 81.2 | 0.07 ± 0.02 | 0.79 |
| LSZ-10.0% | 16.9 ± 1.9 | 2.8 ± 0.6 | 59.2 ± 1.9 | 78.2 | 0.10 ± 0.03 | 0.82 |
2.2. Investigation of DL, Encapsulation Efficiency (EE) and in vitro Drug Release Profiles
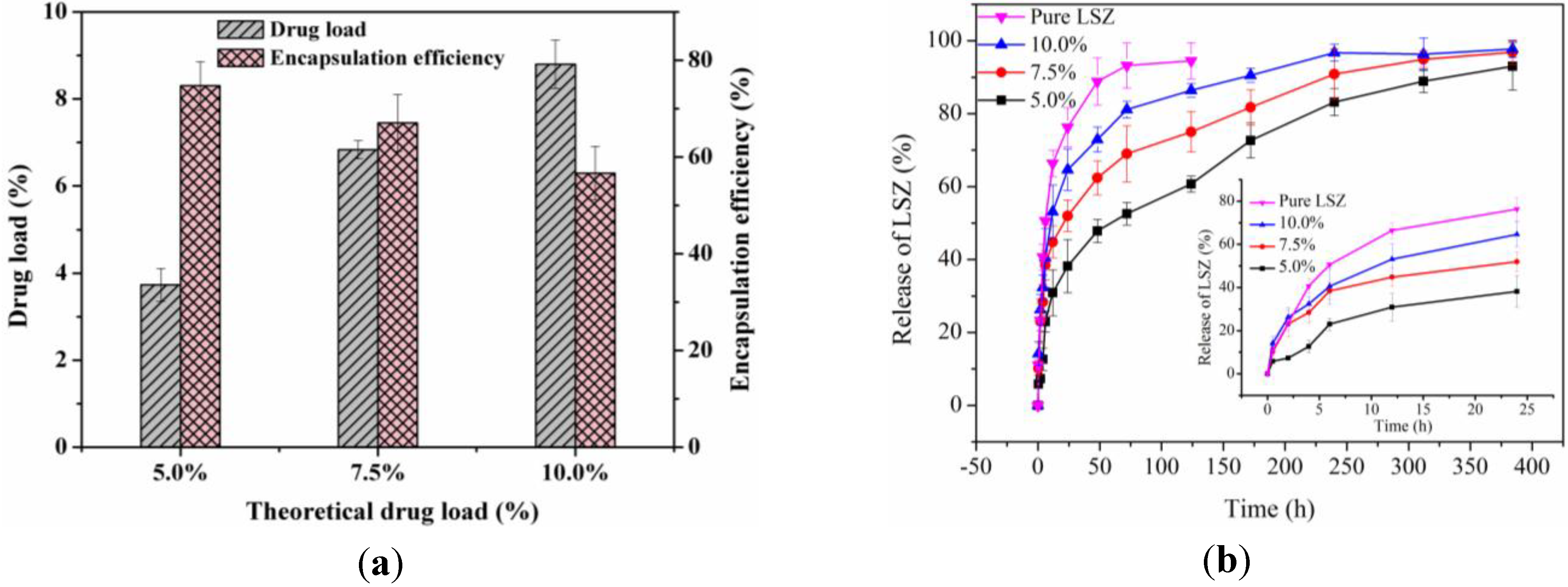
2.3. Characterization of Physicochemical Properties of LSZ-PLLA PMs
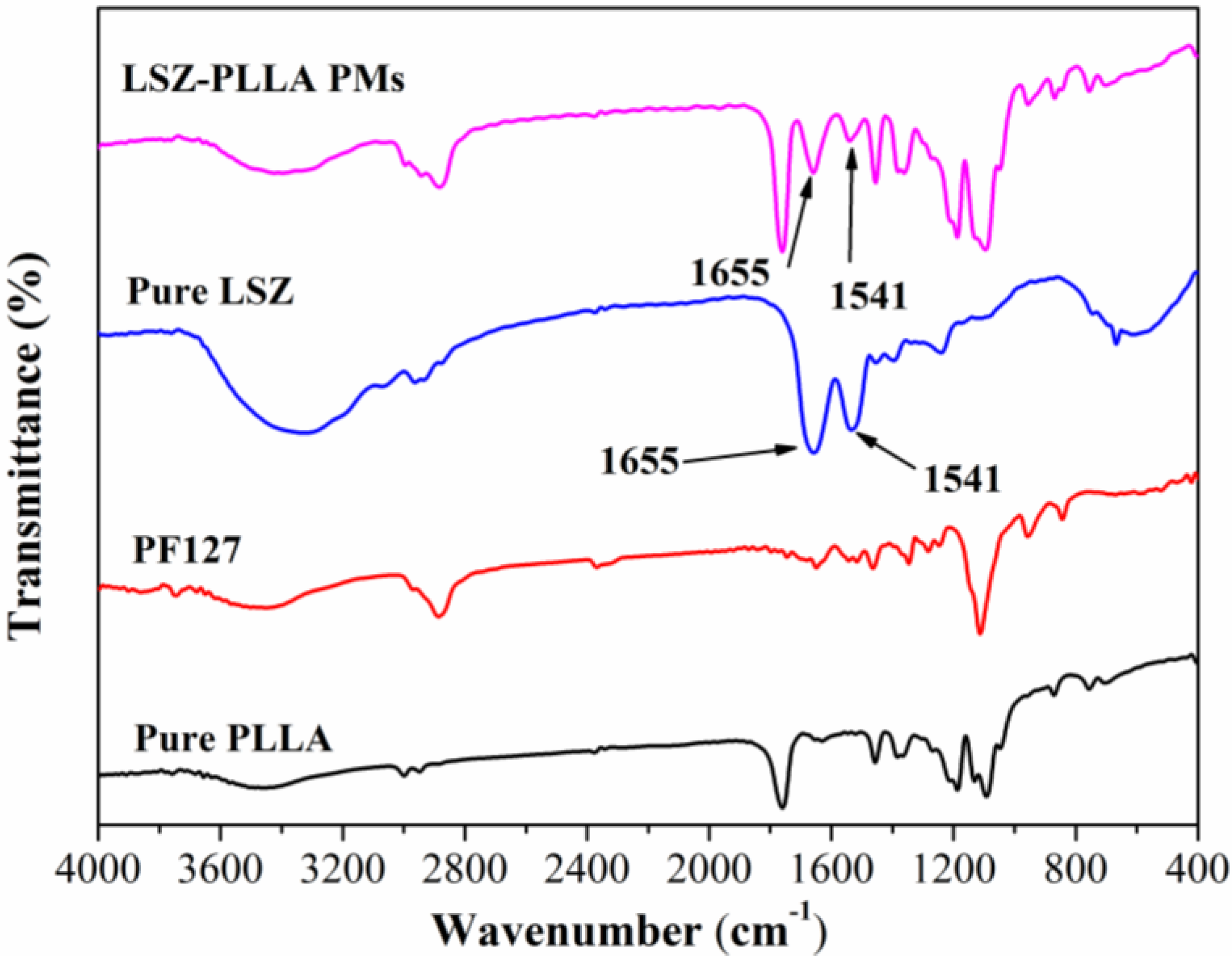
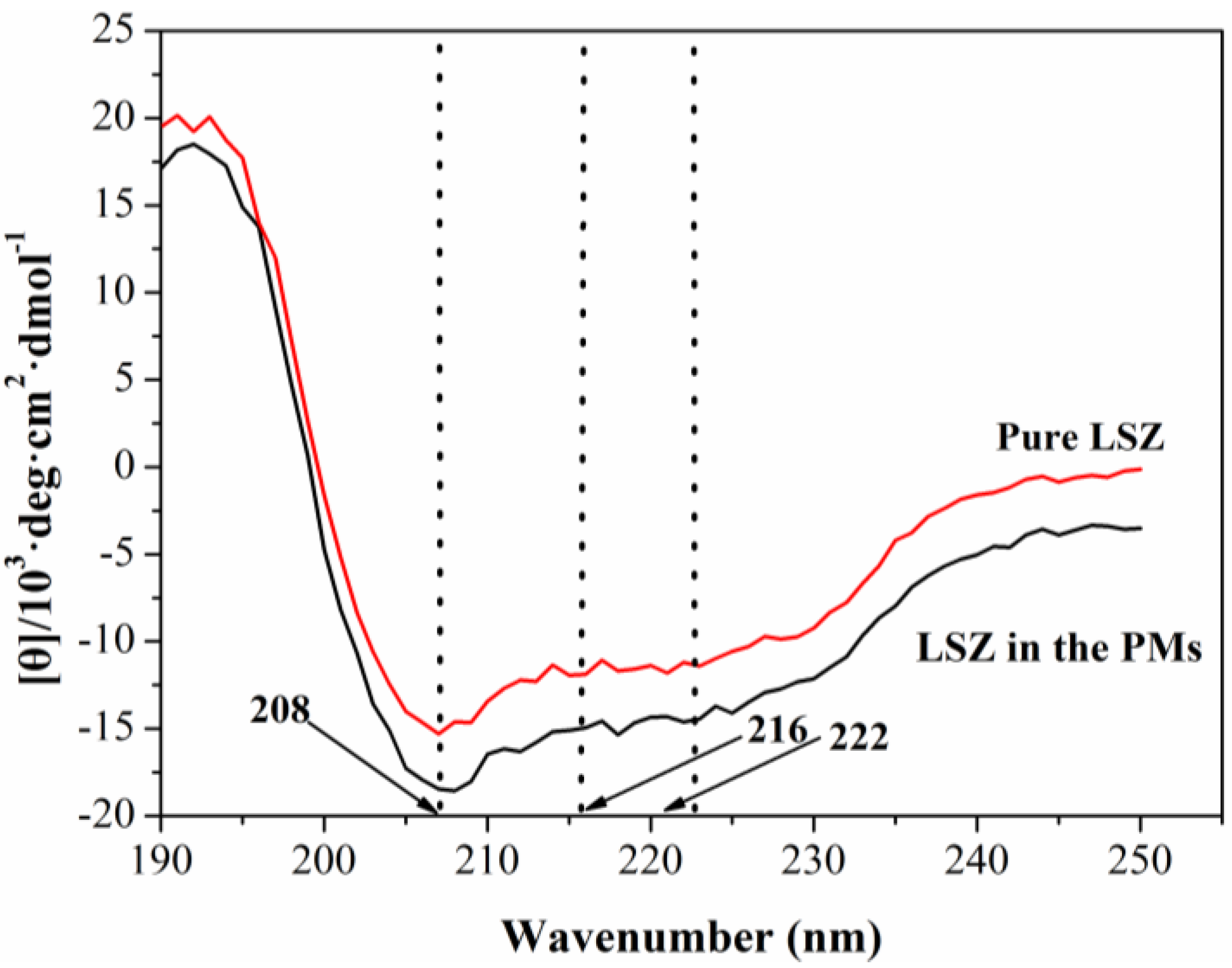
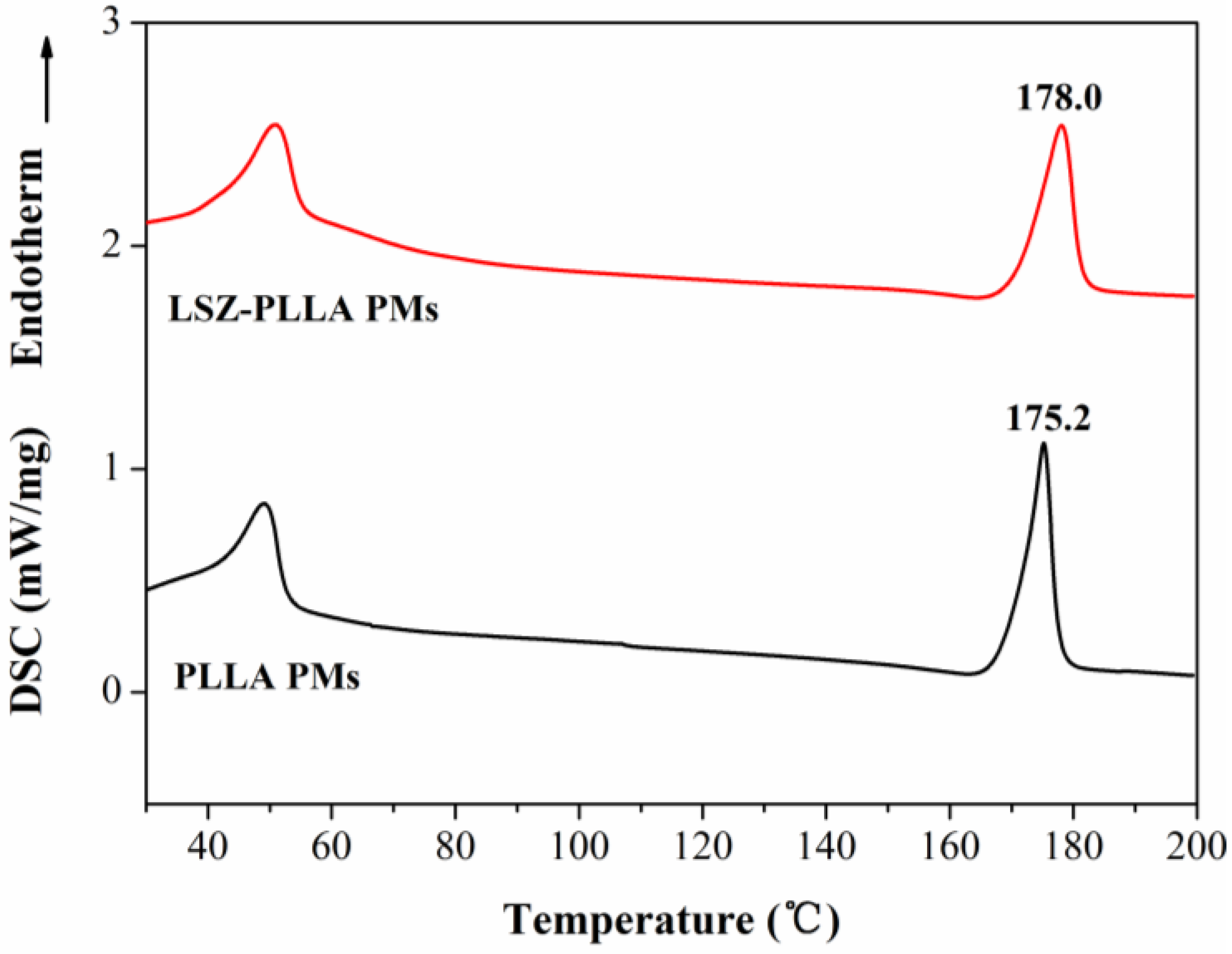
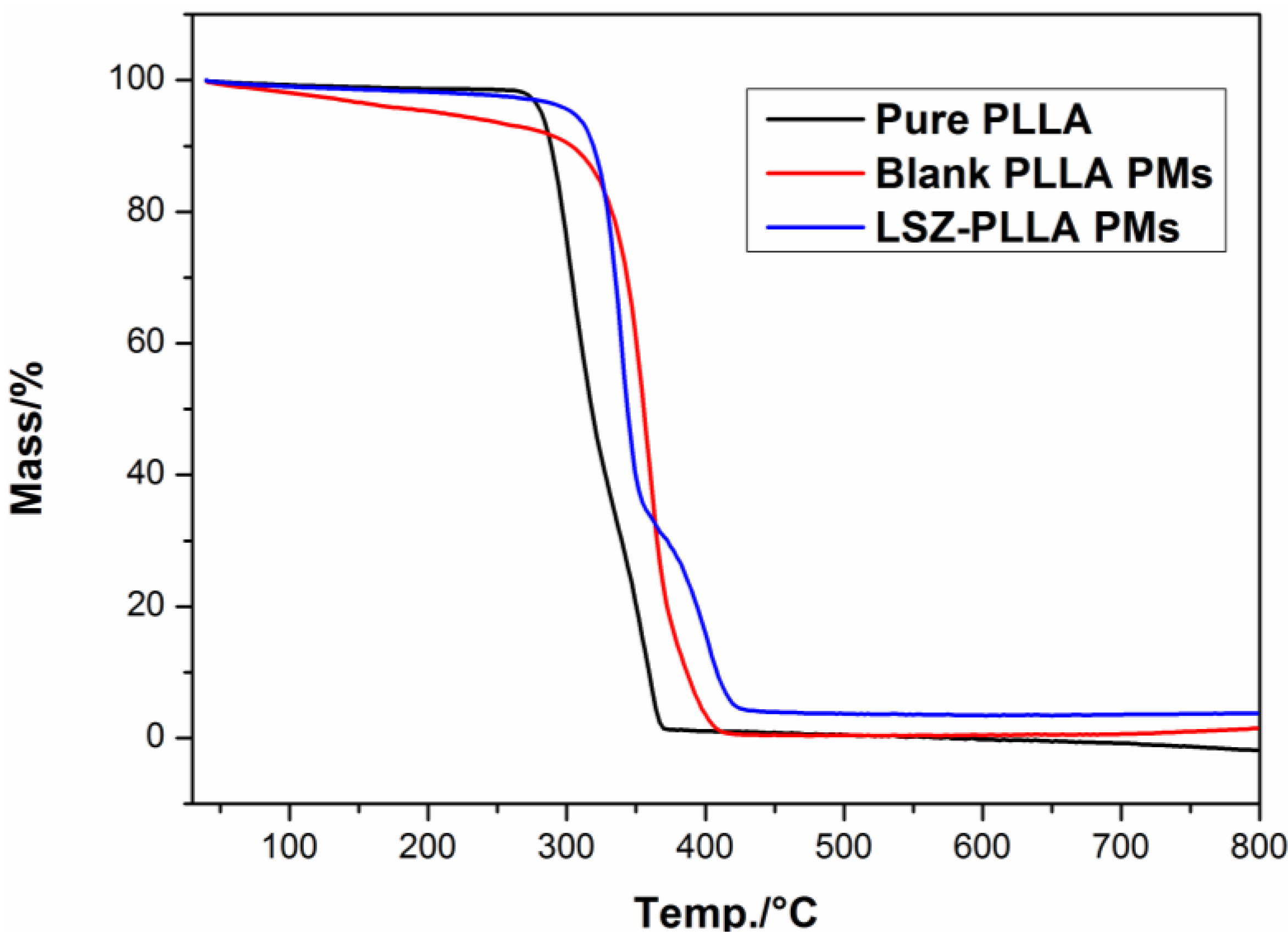
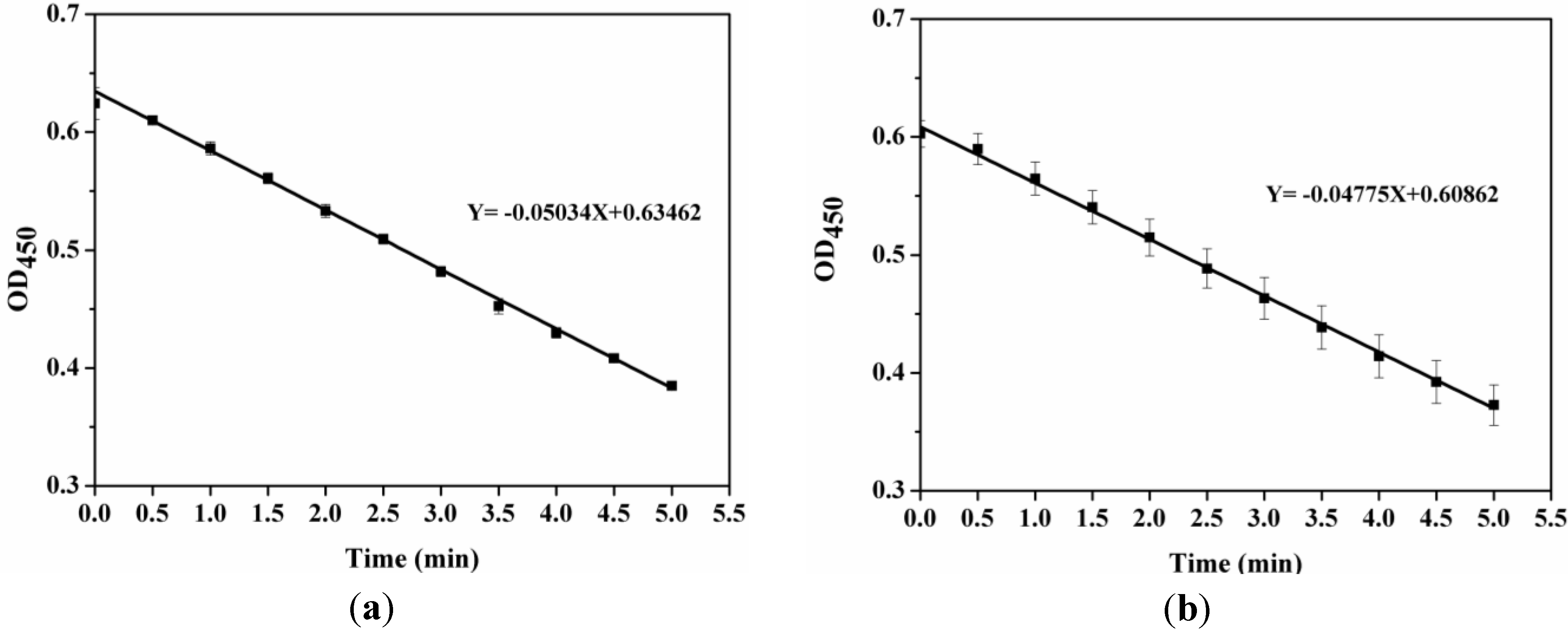
2.4. Activity Assay of LSZ before and after Precipitation Process
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Preparation of LSZ-PLLA PMs
3.2.2. Characterization of Surface Morphology
3.2.3. Investigation of Particle Size, Porosity and Tapped Density
3.2.4. Measurement of DL and EE
3.2.5. Study of Drug Release Profiles
3.2.6. Characterization of Physicochemical Properties of LSZ-PLLA PMs
3.2.7. Determination of Enzyme Activity Retention Rate of LSZ
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yoon, S.S.; Eto, H.; Lin, C.M.; Nakamura, H.; Pawlik, T.M.; Song, S.U.; Tanabe, K.K. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999, 59, 6251–6256. [Google Scholar] [PubMed]
- Aitken, M.L.; Moss, R.B.; Waltz, D.A.; Dovey, M.E.; Tonelli, M.R.; McNamara, S.C.; Gibson, R.L.; Ramsey, B.W.; Carter, B.J.; Reynolds, T.C. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 2001, 12, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Lillard, J.W. Past, present, and future technologies for oral delivery of therapeutic proteins. J. Pharm. Sci. 2008, 97, 2497–2523. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.C. Oral peptide and protein delivery: Unfulfilled promises? Drug Discov. Today 2003, 8, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, X.; Wei, J.; Yan, L.; Jing, X. Application of the biodegradable diblock copolymer poly (L-lactide)-block-poly (L-cysteine): Drug delivery and protein conjugation. J. Appl. Polym. Sci. 2010, 118, 1738–1742. [Google Scholar]
- Ghassemi, A.; Van Steenbergen, M.; Talsma, H.; Van Nostrum, C.; Jiskoot, W.; Crommelin, D.; Hennink, W. Microspheres of hydrophilic PLGA highly attractive for protein delivery. J. Control. Release 2010, 148. [Google Scholar] [CrossRef]
- Wanakule, P.; Liu, G.W.; Fleury, A.T.; Roy, K. Nano-inside-micro: Disease-responsive microgels with encapsulated nanoparticles for intracellular drug delivery to the deep lung. J. Control. Release 2012, 162, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S. Pulmonary delivery of drugs for bone disorders. Adv. Drug Deliver. Rev. 2000, 42, 239–248. [Google Scholar] [CrossRef]
- Vujanic, A.; Sutton, P.; Snibson, K.J.; Yen, H.H.; Scheerlinck, J.P. Mucosal vaccination: Lung versus nose. Vet. Immunol. Immunopathol. 2012, 148, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Z.; Wang, G.Y.; Wang, S.B.; Feng, J.G.; Liu, Y.G.; Kang, Y.Q. Preparation of poly-(methyl vinyl ether-co-maleic anhydride) nanoparticles by solution-enhanced dispersion by supercritical CO2. Materials 2012, 5, 1841–1852. [Google Scholar] [CrossRef]
- Alnaief, M.; Alzaitoun, M.A.; Garcia-Gonzalez, C.A.; Smirnova, I. Preparation of biodegradable nanoporous microspherical aerogel based on alginate. Carbohyd. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohyd. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Kawagishi, K.; Saito, H.; Furukawa, H.; Horie, K. Superior nanoporous polyimides via supercritical CO2 drying of jungle-gym-type polyimide gels. Macromol. Rapid Commun. 2007, 28, 96–100. [Google Scholar] [CrossRef]
- Ji, C.; Annabi, N.; Khademhosseini, A.; Dehghani, F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011, 7, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Rinki, K.; Tripathi, S.; Dutta, P.K.; Dutta, J.; Hunt, A.J.; Macquarrie, D.J.; Clark, J.H. Direct chitosan scaffold formation via chitin whiskers by a supercritical carbon dioxide method: A green approach. J. Mater. Chem. 2009, 19, 8651–8655. [Google Scholar] [CrossRef]
- White, L.J.; Hutter, V.; Tai, H.; Howdle, S.M.; Shakesheff, K.M. The effect of processing variables on morphological and mechanical properties of supercritical CO2 foamed scaffolds for tissue engineering. Acta Biomater. 2012, 8, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hedin, N. Synthesis of microporous organic polymers with high CO2-over-N2 selectivity and CO2 adsorption. J. Mater. Chem. A 2013, 1, 3406–3414. [Google Scholar] [CrossRef]
- Yoganathan, R.B.; Mammucari, R.; Foster, N.R. A Green Method for Processing Polymers using Dense Gas Technology. Materials 2010, 3, 3188–3203. [Google Scholar] [CrossRef]
- Siegmann, R.; Moller, E.; Beuermann, S. Propagation Rate Coefficients for Homogeneous Phase VDF–HFP Copolymerization in Supercritical CO2. Macromol. Rapid Commun. 2012, 33, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Kamrupi, I.; Pokhrel, B.; Kalita, A.; Boruah, M.; Dolui, S.; Boruah, R. Synthesis of macroporous polymer particles by suspension polymerization using supercritical carbon dioxide as a pressure–adjustable porogen. Adv. Polym. Technol. 2012, 31, 154–162. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I.; Adami, R.; Caputo, G. Expanded micro-particles by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluid. 2008, 44, 98–108. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Mechanisms controlling supercritical antisolvent precipitate morphology. Chem. Eng. J. 2011, 169, 358–370. [Google Scholar] [CrossRef]
- De Diego, Y.P.; Pellikaan, H.C.; Wubbolts, F.E.; Witkamp, G.J.; Jansens, P.J. Operating regimes and mechanism of particle formation during the precipitation of polymers using the PCA process. J. Supercrit. Fluid. 2005, 35, 147–156. [Google Scholar]
- Kim, Y.H.; Shing, K.S. Supercritical fluid-micronized ipratropium bromide for pulmonary drug delivery. Powder Technol. 2008, 182, 25–32. [Google Scholar] [CrossRef]
- Chen, A.Z.; Zhao, C.; Wang, S.B.; Liu, Y.G.; Lin, D.L. Generation of porous poly-L-lactide microspheres by emulsion-combined precipitation with a compressed CO2 antisolvent process. J. Mater. Chem. B 2013, 1, 2967. [Google Scholar] [CrossRef]
- Chen, A.Z.; Wang, G.Y.; Wang, S.B.; Li, L.; Liu, Y.G.; Zhao, C. Formation of methotrexate-PLLA-PEG-PLLA composite microspheres by microencapsulation through a process of suspension-enhanced dispersion by supercritical CO2. Int. J. Nanomed. 2012, 7, 3013–3022. [Google Scholar] [CrossRef]
- Winters, M.A.; Knutson, B.L.; Debenedetti, P.G.; Sparks, H.G.; Przybycien, T.M.; Stevenson, C.L.; Prestrelski, S.J. Precipitation of proteins in supercritical carbon dioxide. J. Pharm. Sci. 1996, 85, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.F.; Wu, L.; Zhang, M.; Zhao, Y.L. Viscoelasticity and thermal stability of polylactide composites with various functionalized carbon nanotubes. Polym. Degrad. Stabil. 2008, 93, 1577–1584. [Google Scholar] [CrossRef]
- Chen, A.Z.; Pu, X.M.; Yin, G.F.; Zhao, C.; Wang, S.B.; Liu, Y.G.; Wang, G.Y.; Kang, Y.Q. Study of lysozyme-polymer composite microparticles in supercritical CO2. J. Appl. Polym. Sci. 2012, 125, 3175–3183. [Google Scholar] [CrossRef]
- Van de Weert, M.; Hoechstetter, J.; Hennink, W.E.; Crommelin, D.J.A. The effect of a water/organic solvent interface on the structural stability of lysozyme. J. Control. Release 2000, 68, 351–359. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kang, Y.-Q.; Zhao, C.; Chen, A.-Z.; Wang, S.-B.; Liu, Y.-G.; Wu, W.-G.; Su, X.-Q. Study of Lysozyme-Loaded Poly-L-Lactide (PLLA) Porous Microparticles in a Compressed CO2 Antisolvent Process. Materials 2013, 6, 3571-3583. https://doi.org/10.3390/ma6083571
Kang Y-Q, Zhao C, Chen A-Z, Wang S-B, Liu Y-G, Wu W-G, Su X-Q. Study of Lysozyme-Loaded Poly-L-Lactide (PLLA) Porous Microparticles in a Compressed CO2 Antisolvent Process. Materials. 2013; 6(8):3571-3583. https://doi.org/10.3390/ma6083571
Chicago/Turabian StyleKang, Yong-Qiang, Chen Zhao, Ai-Zheng Chen, Shi-Bin Wang, Yuan-Gang Liu, Wen-Guo Wu, and Xiao-Qian Su. 2013. "Study of Lysozyme-Loaded Poly-L-Lactide (PLLA) Porous Microparticles in a Compressed CO2 Antisolvent Process" Materials 6, no. 8: 3571-3583. https://doi.org/10.3390/ma6083571





