Preparation of Amidoxime Polyacrylonitrile Chelating Nanofibers and Their Application for Adsorption of Metal Ions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentation
2.3. Electrospinning of PAN Nanofibers
2.4. Chemical Modification of PAN Nanofibers
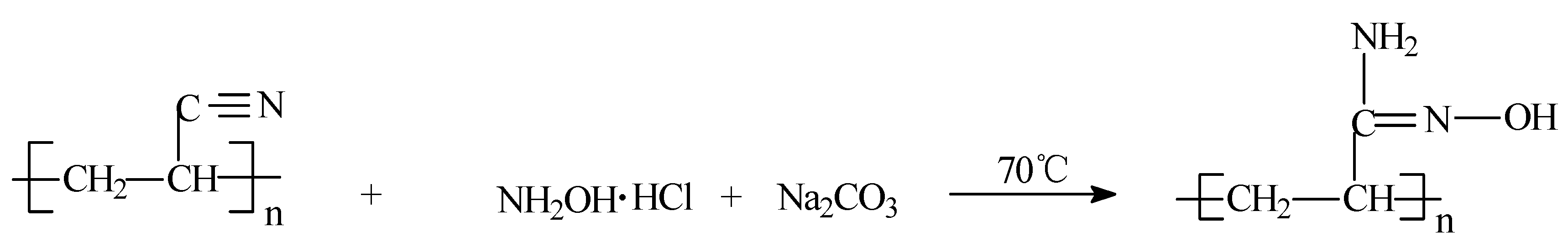
2.5. Adsorption Behaviors
3. Results and Discussion
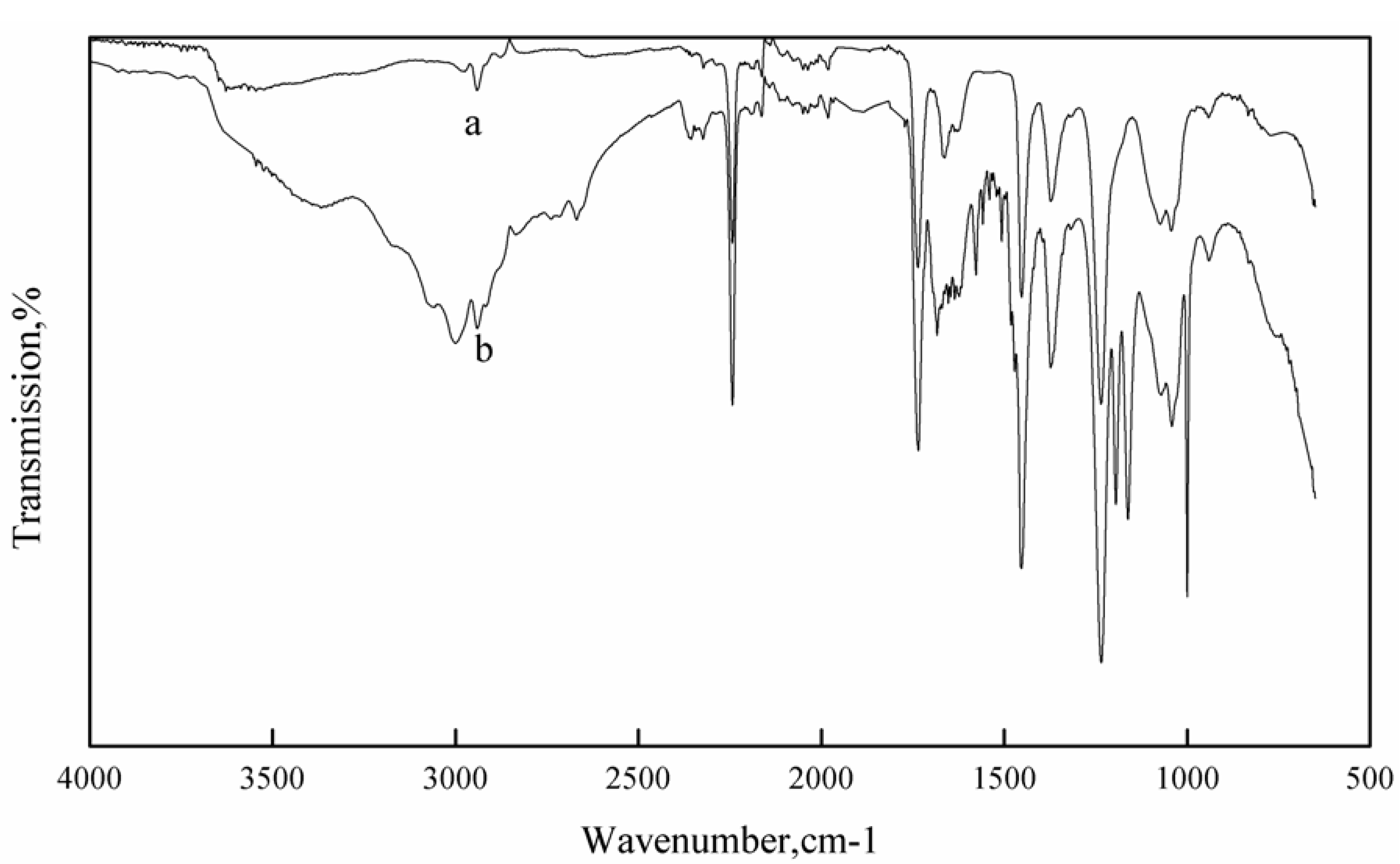
3.1. FT-IR Study
3.2. Conversion of Nitrile Group
| NO. | Time (h) | Temperature (K) | NH2OH content (g/L) | pH value | Conversion (%) | Appearance and properties |
|---|---|---|---|---|---|---|
| 1 | 1 | 313.15 | 40 | 7 | 21.3 | Soft and white |
| 2 | 2 | 313.15 | 40 | 7 | 26.4 | Soft and white |
| 3 | 3 | 313.15 | 40 | 7 | 43.6 | Brittle and white |
| 4 | 4 | 313.15 | 40 | 7 | 51.7 | Brittle and light yellow |
| 5 | 5 | 313.15 | 40 | 7 | 56.2 | Brittle and light yellow |
| 6 | 2 | 333.15 | 40 | 3 | 26.0 | Soft and white |
| 7 | 2 | 333.15 | 40 | 5 | 50.6 | Brittle and light yellow |
| 8 | 2 | 333.15 | 40 | 7 | 57.4 | Brittle and light yellow |
| 9 | 2 | 333.15 | 40 | 9 | 31.4 | Soft and white |
| 10 | 2 | 333.15 | 40 | 11 | 22.2 | Soft and white |
| 11 | 2 | 343.15 | 10 | 7 | 27.8 | Soft and white |
| 12 | 2 | 343.15 | 20 | 7 | 44.1 | Soft and white |
| 13 | 2 | 343.15 | 30 | 7 | 59.3 | Brittle and light yellow |
| 14 | 2 | 343.15 | 40 | 7 | 78.1 | Hard and light yellow |
| 15 | 2 | 343.15 | 50 | 7 | 83.8 | Hard and light yellow |
| 16 | 2 | 313.15 | 40 | 7 | 26.4 | Soft and white |
| 17 | 2 | 323.15 | 40 | 7 | 32.8 | Soft and white |
| 18 | 2 | 333.15 | 40 | 7 | 58.4 | Brittle and light yellow |
| 19 | 2 | 343.15 | 40 | 7 | 78.1 | Hard and light yellow |
| 20 | 2 | 353.15 | 40 | 7 | 86.0 | Hard and light yellow |
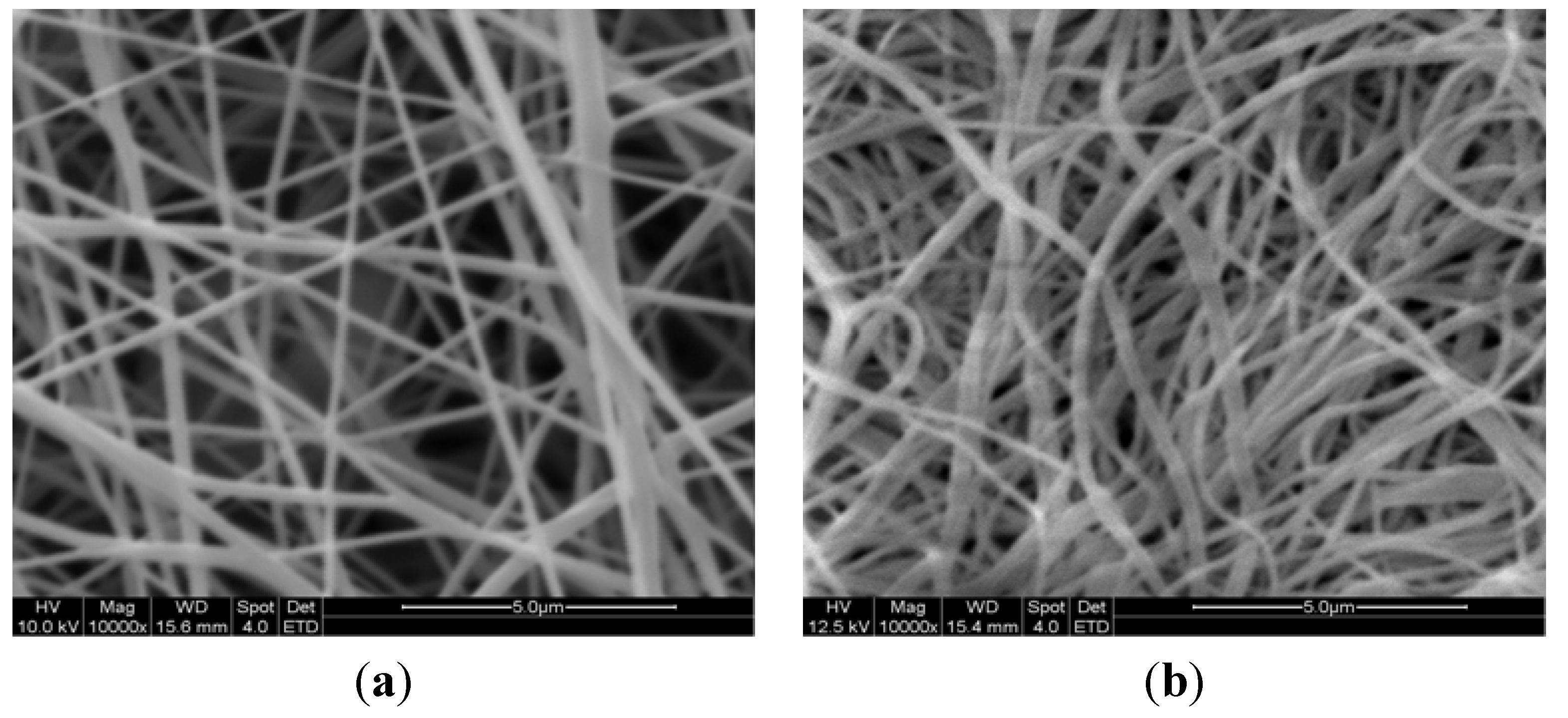
3.3. SEM
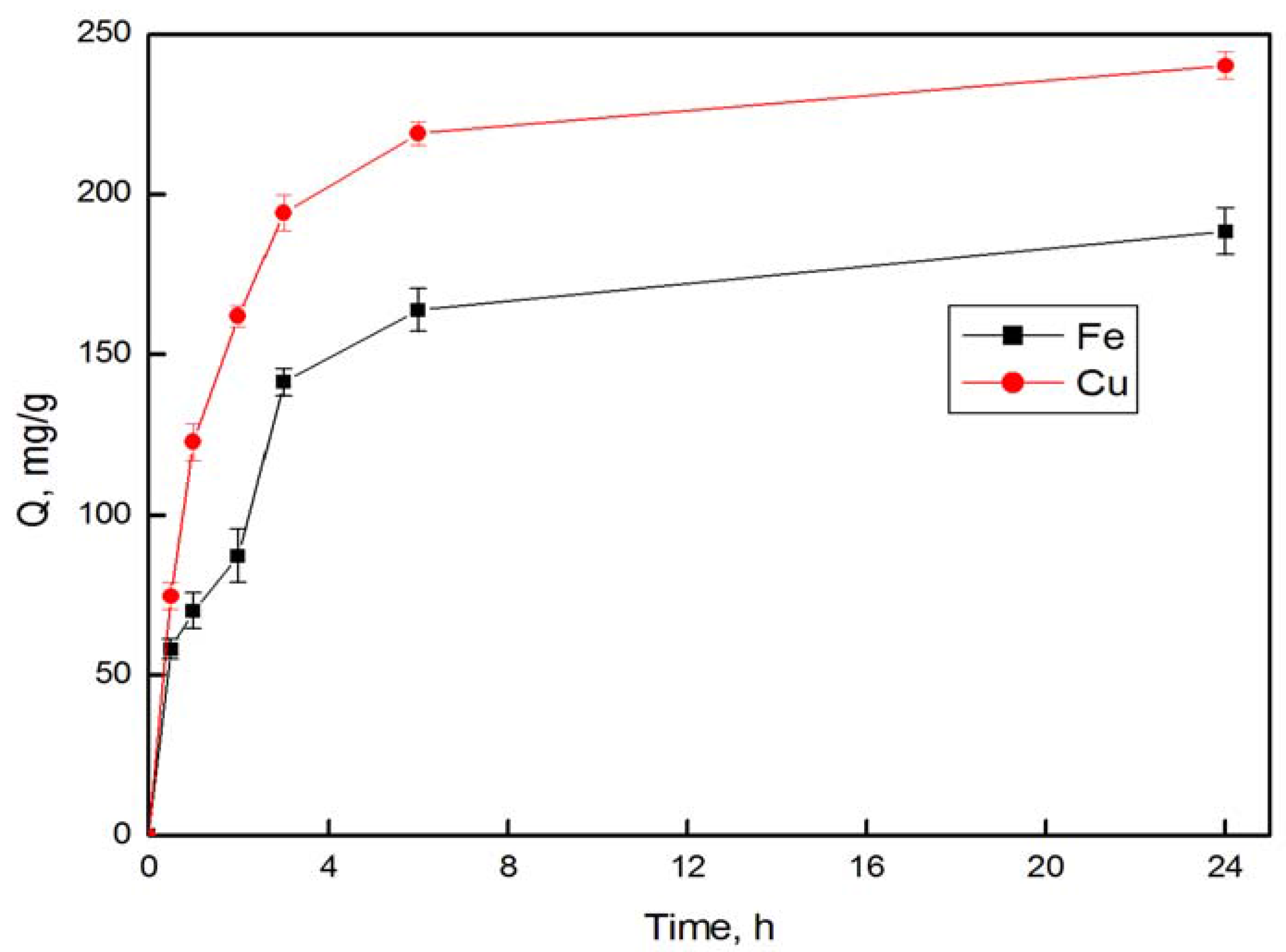
3.4. Adsorption Behaviors

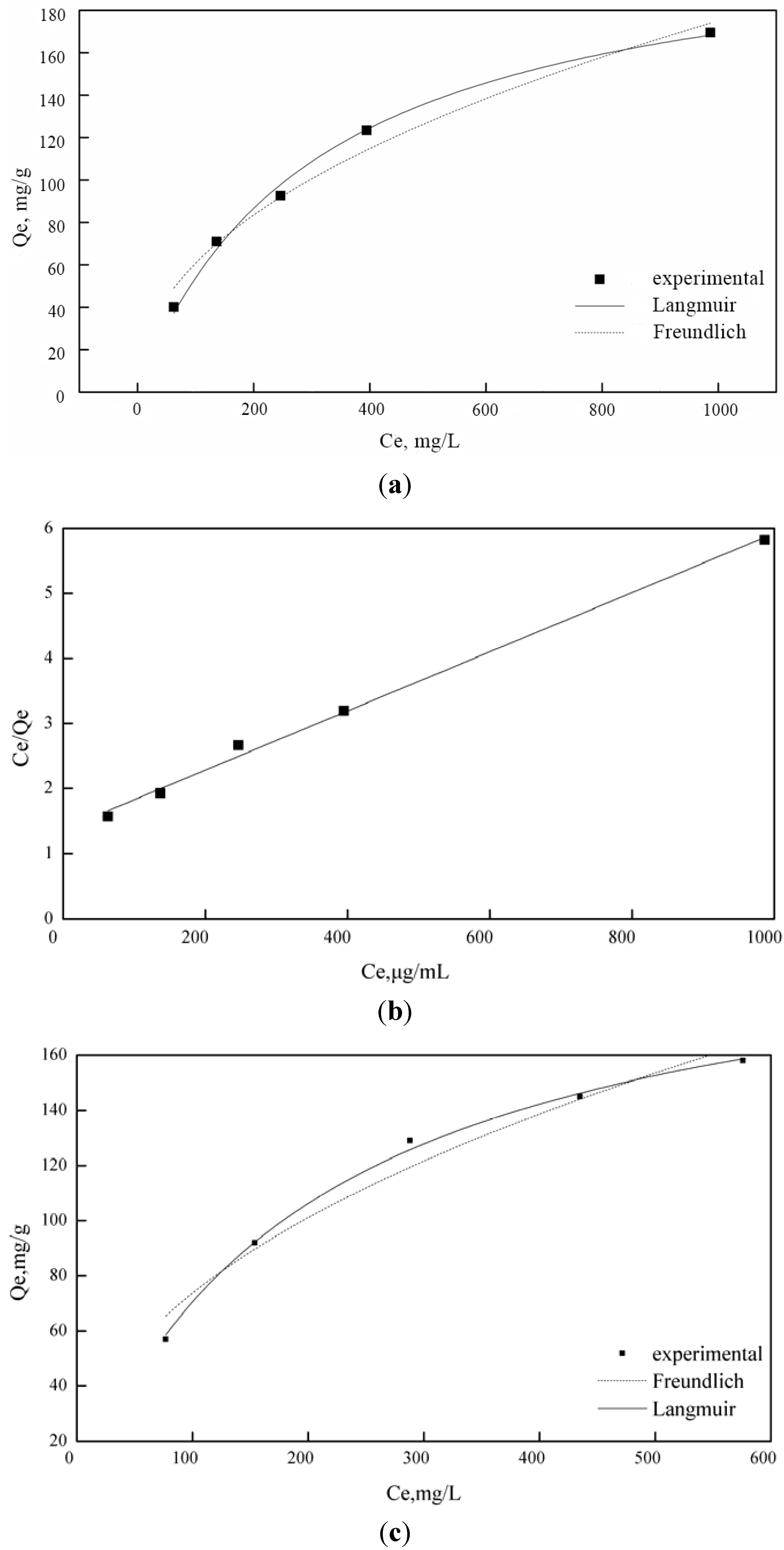
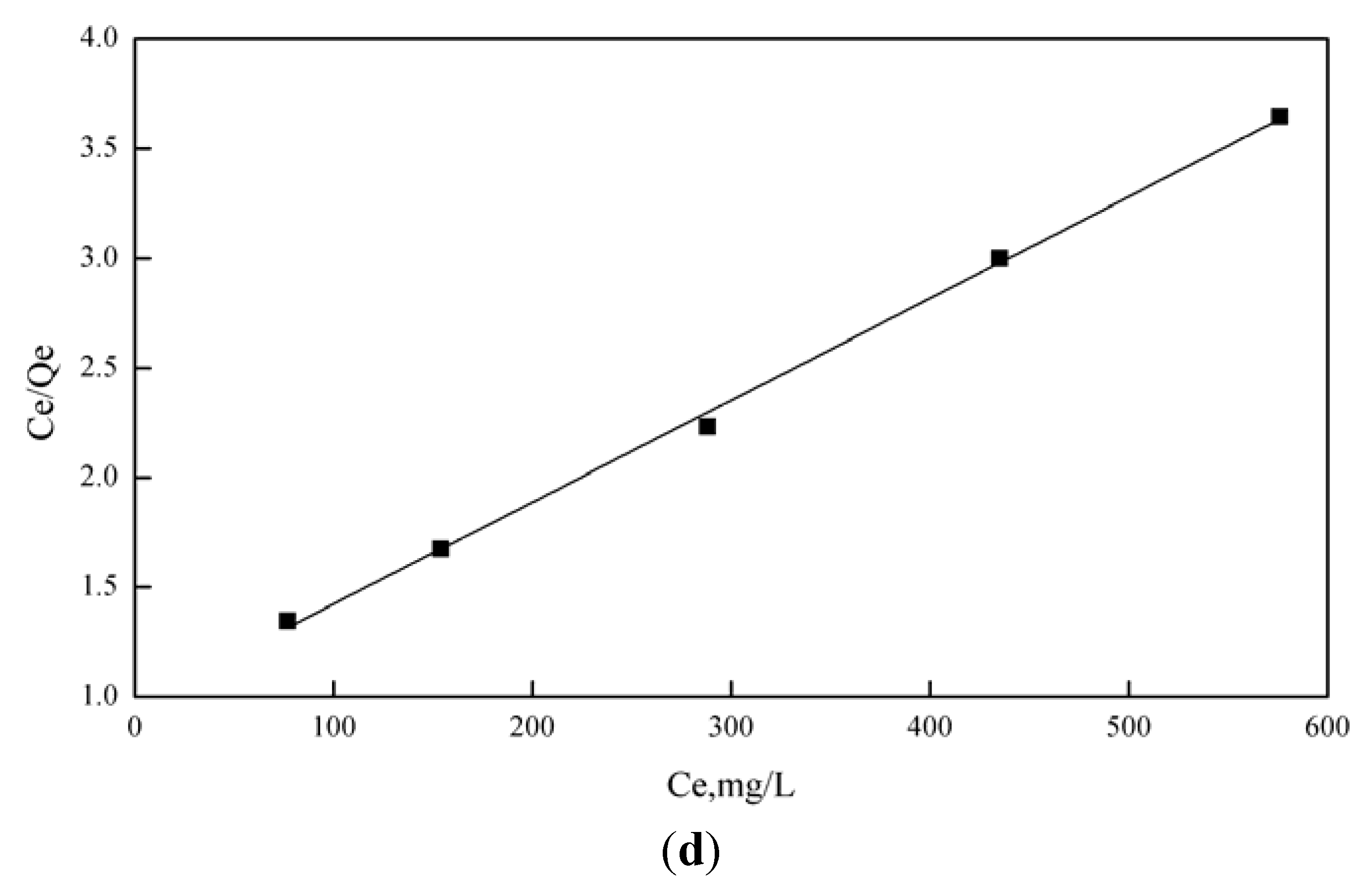
| Metal ions | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | b (L/mg) | r2 | K (mg(1−1/n) L1/n g−1) | n | r2 | |
| Cu2+ | 215.17829 | 0.00488 | 0.99693 | 9.03894 | 0.45573 | 0.96072 |
| Fe3+ | 221.3681 | 0.00321 | 0.99337 | 7.29263 | 0.46002 | 0.97463 |
4. Conclusions
Acknowledgements
References
- Neghlani, P.K.; Rafizadeh, M.; Taromi, F.A. Preparation of aminated-polyacrylonitrile nanofiber membranes for the adsorption of metal ions: Comparison with microfibers. J. Hazard. Mater. 2011, 186, 182–189. [Google Scholar]
- Jain, C.K.; Singhal, D.C.; Sharma, M.K. Adsorption of zinc on bed sediment of River Hindon: Adsorption models and kinetics. J. Hazard. Mater. 2004, 114, 231–239. [Google Scholar]
- Sekar, M.; Sakthi, V.; Rengaraj, S. Kinetics equilibrium adsorption study of lead(II) onto activated carbon prepared from coconut shell. J. Colloid Interface Sci. 2004, 79, 307–313. [Google Scholar]
- Iqbal, M.; Saeed, A.; Zafar, S.I. Hybrid biosorbent: an innovative matrix to enhance the biosorption of Cd(II) from aqueous solution. J. Hazard. Mater. 2007, 148, 47–55. [Google Scholar]
- Miretzky, P.; Saralegui, A.; Cirelli, A.F. Simultaneous heavy metal removal mechanism by dead macrophytes. Chemosphere 2006, 62, 247–254. [Google Scholar]
- Shoushtari, A.M.; Zargaran, M.; Abdouss, M. Preparation and characterization of high efficiency ion-exchange crosslinked acrylic fibers. J. Appl. Polym. Sci. 2006, 101, 2202–2209. [Google Scholar]
- Shin, D.H.; Ko, Y.G.; Choi, U.S.; Kim, W.N. Design of high efficiency chelate fibers with an amine group to remove heavy metal ions and pH-related FT-IR analysis. J. Ind. Eng. Chem. Res. 2004, 43, 2060–2066. [Google Scholar]
- Tahaei, P.; Abdouss, M.; Edrissi, M.; Shoushtari, A.M.; Zargaran, M. Preparation of chelating fibrous polymer by different diamines and study on their physical and chemical properties. Mater. Sci. Eng. Technol. 2008, 39, 839–844. [Google Scholar]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. J. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar]
- Kenawy, E.R.; Bowlin, G.L.; Mansfield, K. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar]
- Liu, H.; Kameoka, J.; Czaplewski, D.A.; Craighead, H.G. Polymeric nanowire chemical sensor. J. Nano. Lett. 2004, 4, 671–675. [Google Scholar]
- Gibson, P.; Schreuder-Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibers. J. Colloid Surf. A Physicochem. Eng. Asp. 2001, 187–188, 469–481. [Google Scholar]
- Sang, Y.; Gu, Q.; Sun, T. Filtration by a novel nanofiber membrane and alumina adsorption to remove copper (II) from groundwater. J. Hazard. Mater. 2008, 153, 860–866. [Google Scholar]
- Sang, Y.; Li, F.; Gu, Q.; Liang, C.Z.; Chen, J.Q. Heavy metal-contaminated groundwater treatment by a novel nanofiber membrane. J. Desalin. 2008, 223, 349–360. [Google Scholar]
- Ma, Z.W.; Masaya, K.; Ramakrishna, S. Immobilization of Cibacron blue F3GA on electrospun polysulphone ultra-fine fiber surfaces towards developing an affinity membrane for albumin adsorption. J. Membr. Sci. 2006, 282, 237–244. [Google Scholar]
- Saeed, K.; Haider, S.; Oh, T.J.; Park, S.Y. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibres and their applications to metal ions adsorption. J. Membr. Sci. 2008, 322, 400–405. [Google Scholar]
- Cai, Y.B.; Gao, D.W.; Wei, Q.F.; Gu, H.L.; Huang, F.L.; Song, L. Effects of ferric chloride on structure, surface morphology and combustion property of electrospun polyacrylonitrile composite nanofibers. Fibers Polym. 2011, 12, 145–150. [Google Scholar]
- Lin, W.P.; Lu, Y.; Zeng, H. Studies of the preparation, structure, and properties of an acrylic chelating fiber containing amidoxime groups. J. Appl. Polym. Sci. 1993, 47, 45–52. [Google Scholar]
- Bilba, N.; Bilba, D.; Moroi, G. Synthesis of a polyacrylamidoxime chelating fiber and its efficiency in the retention of palladium ions. J. Appl. Polym. Sci. 2004, 92, 3730–3735. [Google Scholar]
- Coskun, R.; Soykan, C. Preparation of amidoximated polyester fiber and competitive adsorption of some heavy metal ions from aqueous solution onto this fiber. J. Appl. Polym. Sci. 2009, 112, 1798–1807. [Google Scholar]
- Deng, S.B.; Bai, R.; Chen, J.P. Aminated polyacrylonitrile fibers for lead and copper removal. Langmuir 2003, 19, 5058–5064. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, F.; Xu, Y.; Liao, S.; Yang, D.; Hsieh, Y.-L.; Wei, Q. Preparation of Amidoxime Polyacrylonitrile Chelating Nanofibers and Their Application for Adsorption of Metal Ions. Materials 2013, 6, 969-980. https://doi.org/10.3390/ma6030969
Huang F, Xu Y, Liao S, Yang D, Hsieh Y-L, Wei Q. Preparation of Amidoxime Polyacrylonitrile Chelating Nanofibers and Their Application for Adsorption of Metal Ions. Materials. 2013; 6(3):969-980. https://doi.org/10.3390/ma6030969
Chicago/Turabian StyleHuang, Fenglin, Yunfei Xu, Shiqin Liao, Dawei Yang, You-Lo Hsieh, and Qufu Wei. 2013. "Preparation of Amidoxime Polyacrylonitrile Chelating Nanofibers and Their Application for Adsorption of Metal Ions" Materials 6, no. 3: 969-980. https://doi.org/10.3390/ma6030969
APA StyleHuang, F., Xu, Y., Liao, S., Yang, D., Hsieh, Y.-L., & Wei, Q. (2013). Preparation of Amidoxime Polyacrylonitrile Chelating Nanofibers and Their Application for Adsorption of Metal Ions. Materials, 6(3), 969-980. https://doi.org/10.3390/ma6030969




