1. Introduction
The production of hydrogen without deploying fossil resources is one of the main challenges that have to be overcome for building a future hydrogen economy. By using water as a resource and concentrated solar energy to provide the necessary process heat, hydrogen can be produced basically without CO
2 formation. Because of thermodynamic restrictions, significant yields in the direct thermal splitting of water can only be achieved at temperatures above 2500 K. Although temperatures that high can be reached with solar concentrating systems, they still impose extraordinary demands on materials and reactor design. Thermochemical cycles split water in several steps and enable hydrogen generation at moderate temperatures, which are manageable by today’s technical equipment. Another major advantage is that hydrogen and oxygen are produced in separate steps,
i.e., no separation of hydrogen and oxygen is needed. A number of possible thermochemical cycles exist and have been reviewed over the last years [
1,
2,
3]. Another option would be the electrolysis of water using the electricity generated by a solar power plant. Electrolysis is an already mature technology that is seen as a benchmark for future hydrogen production. Comparative studies that have been published showed that thermochemical cycles have the potential of a higher efficiency than electrolysis and hence the potential to reduce the production costs of hydrogen from water [
4,
5,
6].
Among the most interesting thermochemical cycles are the two step water-splitting cycles using metal oxide redox-systems [
7]. During the first step of this cycle (the regeneration step) the metal oxide is reduced by setting some of its lattice oxygen free according to Reaction (1).
In the next step (the water-splitting step) the reduced and thus activated material is oxidized by water vapor. This means that oxygen ions refill oxygen vacancies of the metal oxide and simultaneously hydrogen is produced (Reaction 2).
Several metal oxide processes have been evaluated, e.g., iron oxide Fe
3O
4/FeO [
8,
9,
10,
11,
12], manganese oxide Mn
3O
4/MnO [
13] and zinc oxide ZnO/Zn [
14]. The high temperatures (2100 K) needed for the reduction of magnetite Fe
3O
4 lead to rapid deactivation of the material. The necessary reduction temperature can be lowered by supporting Fe
3O
4 on yttria-stabilized zirconia (YSZ), for which a new reaction mechanism was proposed [
15,
16]. Mixed-metal ferrites, which incorporate transition metals in the ferrite structure, can be reduced at lower reduction temperatures and are being studied intensively. Such transition metals are manganese (Mn) [
17,
18], cobalt (Co) [
19], nickel (Ni) [
20,
21] and zinc (Zn) [
22,
23,
24]. Several comparative studies have already been published [
25,
26].
Different approaches on solar receiver concepts using mixed ferrites [
27,
28,
29,
30,
31,
32,
33] have been developed. One of those approaches incorporates honeycomb structures that are coated with the active material and placed in a solar receiver-reactor where they act on the one hand as the absorber for the concentrated solar irradiation and on the other hand provide the necessary surface area for the chemical reaction. The advantages of such a concept are its simplicity, as it has no moving parts, and its scalability. Such coated monoliths had shown the capability of water splitting and regeneration at moderate temperatures of 1200 °C. However, a strong deactivation of the material was observed with on-going testing [
34,
35].
First studies with coated SiSiC monoliths were presented in a previous work showing the influence of water splitting temperature and water concentration on the hydrogen production rate [
34]. The work showed that the ferrite coating sintered strongly during the high temperature regeneration step leading to a loss of porosity and surface area. The occurrence of unintended reactions between the ferrite and the SiSiC substrate was suggested but not finally resolved. In the present work, coated SiSiC honeycomb structures with a zinc ferrite redox material were analyzed with different material characterization techniques after they were cycled in a laboratory test set-up in order to resolve any interactions between the substrate and the coating.
2. Experimental Set-Up
As in the previous work the tested honeycomb structures shown in
Figure 1 (outer diameter: 26 mm; length: 50 mm; channel density: 90 channels per square inch) consisted of a substrate of SiSiC coated with a zinc ferrite of the form Zn
xFe
3−xO
4. The SiSiC honeycombs were coated with the zinc ferrite via a washcoating technique employed for the coating of automotive catalysts [
36]. During the washcoating procedure the porous supports were impregnated with a slurry containing the coating powder and aluminum oxide (Al
2O
3) as a binding material. The honeycombs were then dried and fired at 800 °C under an inert gas atmosphere. To prevent any reactions between the redox powder and the support material, a barrier coating layer of zircon dioxide (ZrO
2) was applied on the monolith to offer structural and chemical stabilization. After the impregnation the honeycombs were fired to ensure efficient adhesion of the redox material. With this method the honeycomb structures were coated with a thin layer of the redox material. The coverage was complete but the thickness of the coating varied strongly as will be shown in the following paragraph.
Figure 1.
Siliconized silicon carbide (SiSiC) honeycomb structure used for testing.
Figure 1.
Siliconized silicon carbide (SiSiC) honeycomb structure used for testing.
The laboratory test set-up in which the samples were tested was already described in the previous work [
34]. In the test set-up, a coated honeycomb can be exposed to changing gas atmospheres of either pure inert gas or a mixture of water-vapor and inert gas while it is heated by an electric furnace. In this way the sample undergoes alternately the reduction and the water-splitting step. Hydrogen production was monitored with a mass spectrometer in the off-gas line of the reactor. Excess water was condensed in a gas cooler before the mass spectrometer.
For the present work, the coated honeycomb structures were subjected to different numbers of cycles, one cycle comprising of one water-splitting and one high-temperature regeneration step. For water-splitting, the samples were subjected to a mixture of water vapor and nitrogen for about 20 min. Then the water vapor was switched off and the temperature was increased to 1200 °C in order to regenerate the redox material. During this step and during heating and cooling phases the sample is always flushed with a constant nitrogen stream. After testing, the coated honeycombs were investigated using different methods for material characterization in order to show material transformations and the formation of possible reaction products between the coating and the SiSiC substrate. For the analysis, small pieces were broken off the monoliths after testing. For phase analysis the samples were subjected to X-ray diffractometry (XRD) with CuKα radiation (Siemens, Kristalloflex D5000 diffractometer). Analysis of the X-ray diffraction pattern in order to identify the phases was done with the software tool EVA2 (Bruker) by comparing the diffraction data of the analysed sample against powder diffraction file (pdf) database. The microstructure of the samples was characterized by scanning electron microscopy (Zeiss, Ultra55, cathode FEG) equipped with an energy dispersive X-ray spectroscopy (EDS) unit (Oxford) for microanalysis. For Scanning Electron Microscopy (SEM)-EDS analysis the samples were fixed on small sample holders using conductive silver and/or conductive foil. In order to prevent charging effects, SEM samples were coated with platinum. To gain information on the elemental distribution of a sample, small pieces of the honeycomb or powder samples were embedded in epoxy resin to prepare polished cross-sections, which were then examined by SEM-EDS.
3. Experimental Results
The ferrite loading of all samples tested and the number of cycles they had performed are summarized in
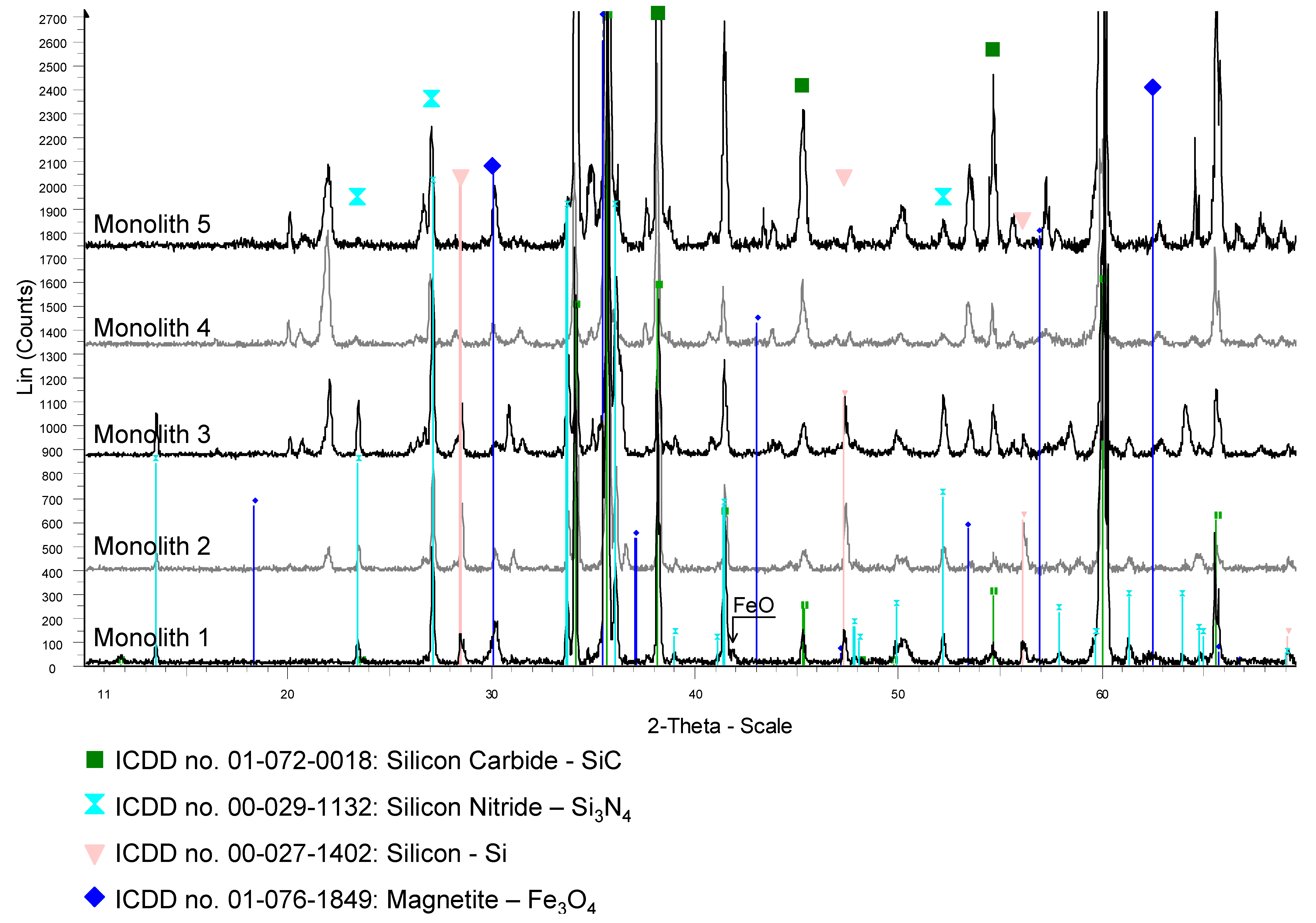
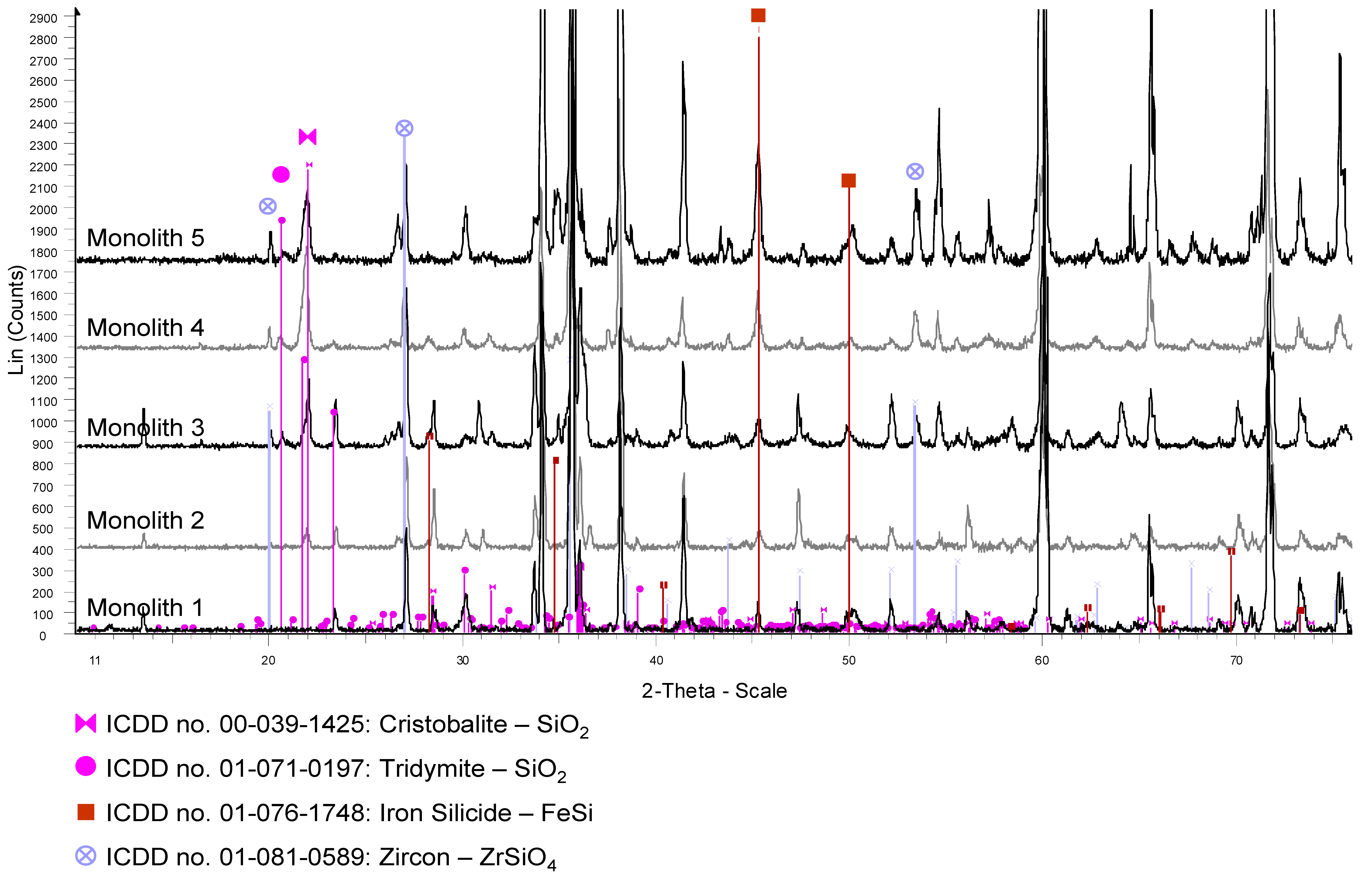
Table 1. Also water splitting and regeneration temperatures are shown. With Monolith 3 and 4 parametric studies concerning the effect of water splitting temperature on the hydrogen production rate were performed. The temperature was kept constant during a water-splitting step but varied from cycle to cycle. Temperatures between 800 and 1200 °C were investigated. With Monolith 5 parametric studies concerning the regeneration temperature were performed varying the temperature between 900 and 1200 °C. After testing, all samples were analyzed via XRD and SEM-EDS analysis as described above. The XRD patterns of the samples are displayed in
Appendix A.
Table 1.
Overview of investigated coated honeycomb structures.
Table 1.
Overview of investigated coated honeycomb structures.
| Sample notation | Ferrite loading per gram of coated monolith | Total ferrite loading | Treatment |
|---|
| Monolith 1 | 0.071 g/gmonolith | 2.30 g | untreated |
| Monolith 2 | 0.070 g/gmonolith | 2.27 g | 1 cycle
water splitting at 900 °C
regeneration at 1200 °C |
| Monolith 3 | 0.104 g/gmonolith | 3.48 g | 25 cycles
water splitting between 800 and 1200 °C
regeneration at 1200 °C |
| Monolith 4 | 0.169 g/gmonolith | 6.12 g | 58 cycles
water splitting between 800 and 1200 °C
regeneration at 1200 °C |
| Monolith 5 | 0.100 g/gmonolith | 3.35 g | 74 cycles
water splitting at 900 °C
regeneration between 900 and 1210 °C |
As shown in the previous work, typically hydrogen production started with a peak at a high level as soon as water vapor was introduced into the reactor but then decreased rapidly within about 10 min to a small but constant level [
34]. No oxygen could be detected in the off-gas of the reactor in all the experiments during the regeneration phase. The release and presence of oxygen in the gas phases would be expected by Reaction (1). It was suggested that the released oxygen reacts with the substrate forming silicon dioxide SiO
2. This will now be verified by comparing the untreated honeycomb (Monolith 1) to the treated honeycombs (Monoliths 2–5).
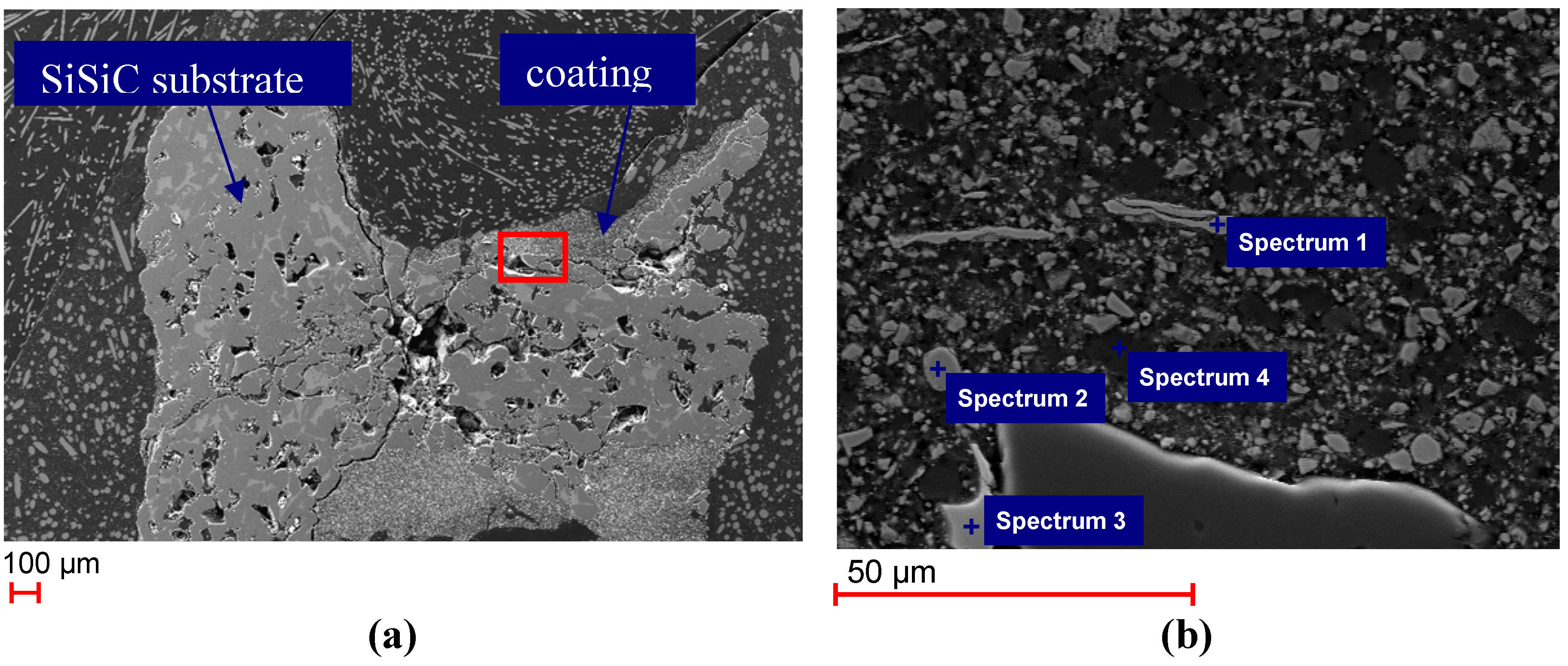
SEM images of the polished cross section of the unused Monolith 1 are shown in
Figure 2. The coating is distributed quite inhomogeneously on the substrate, which becomes clear in
Figure 2a with varying coating thickness between 50 and 300 μm. The encircled area in
Figure 2a is shown as an enlarged cutout in
Figure 2b.
Figure 2.
(
a) Scanning Electron Microscopy (SEM) images of polished cross section of Monolith 1 (untreated); (
b) enlarged cutout of
Figure 2a showing the coating of Monolith 1 and points where energy dispersive X-ray spectroscopy (EDS) were taken.
Figure 2.
(
a) Scanning Electron Microscopy (SEM) images of polished cross section of Monolith 1 (untreated); (
b) enlarged cutout of
Figure 2a showing the coating of Monolith 1 and points where energy dispersive X-ray spectroscopy (EDS) were taken.
EDS analysis taken at different locations on the sample (compare
Figure 2b revealed various elemental compositions (
Table 2). On the one hand, pure iron particles (Spectrum 1) and a zinc and iron containing phase (Spectrum 2) originating from the zinc ferrite did occur. On the other hand, isolated light-colored Zr-bearing crystals were detected (Spectrum 3), which originate from the ZrO
2 barrier coating. The Zn-ferrite, Fe and ZrO
2 grains were embedded in alumina (Spectrum 4), which was used in the coating process as a binding material.
Table 2.
Elements detected in Spectra 1–4 in
Figure 2.
Table 2.
Elements detected in Spectra 1–4 in Figure 2.
| Spectrum | Elements detected |
|---|
| Spectrum 1 | Fe |
| Spectrum 2 | Fe, O, Zn |
| Spectrum 3 | O, Zr |
| Spectrum 4 | Al, O |
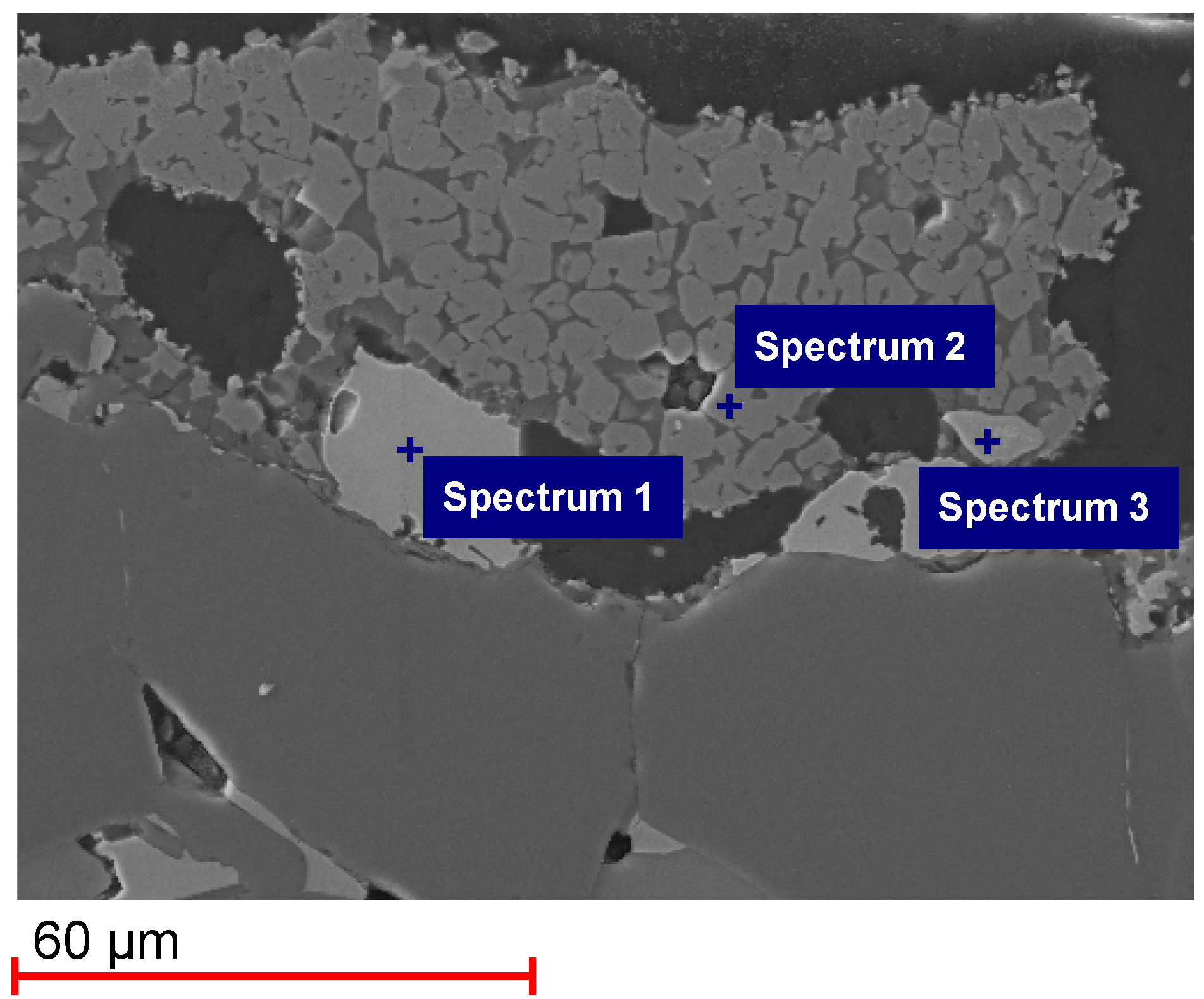
Figure 3 shows an SEM image of Monolith 2, tested for only one cycle. The elements detected in the EDS spectra are shown in
Table 3. The XRD pattern of Monolith 2 is shown in
Appendix A. A Fe-Si phase could be found in the SEM-EDS analysis (Spectrum 1), which was identified by XRD analysis to be FeSi. A Fe-Al-O phase was detected (Spectrum 2), which is probably hercynite FeAl
2O
4. It was not found in the XRD pattern probably because it is below the detection limit of XRD analysis. Finally, a Zr-Si-O phase was found in the SEM-EDS analysis (Spectrum 3), which has been identified in the XRD pattern to be zircon (ZrSiO
4).
Figure 3.
SEM image and EDS spectra of Monolith 2 after 1 cycle.
Figure 3.
SEM image and EDS spectra of Monolith 2 after 1 cycle.
Table 3.
Elements detected in Spectra 1–3 in
Figure 3.
Table 3.
Elements detected in Spectra 1–3 in Figure 3.
| Spectrum | Elements detected |
|---|
| Spectrum 1 | Fe, Si |
| Spectrum 2 | Al, Fe, O |
| Spectrum 3 | O, Si, Zr |
SEM-EDS and XRD analysis of Monoliths 3, 4 and 5 also showed the existence of these new phases; especially FeSi and ZrSiO
4. As well the XRD analysis of all the tested monoliths (Monoliths 2–5) showed clearly the existence of crystalline SiO
2 (cristobalite or tridymite; respectively; see
Appendix A). The presence of these phases shows that multiple reactions have occurred between the different phases present in the coating and the SiSiC substrate. Comparing the peaks heights of the new phases for all monoliths in the XRD patterns (
Appendix A); an increase of peak height with increasing cycle number could be observed; suggesting that the reaction products are formed throughout repeated cycling.
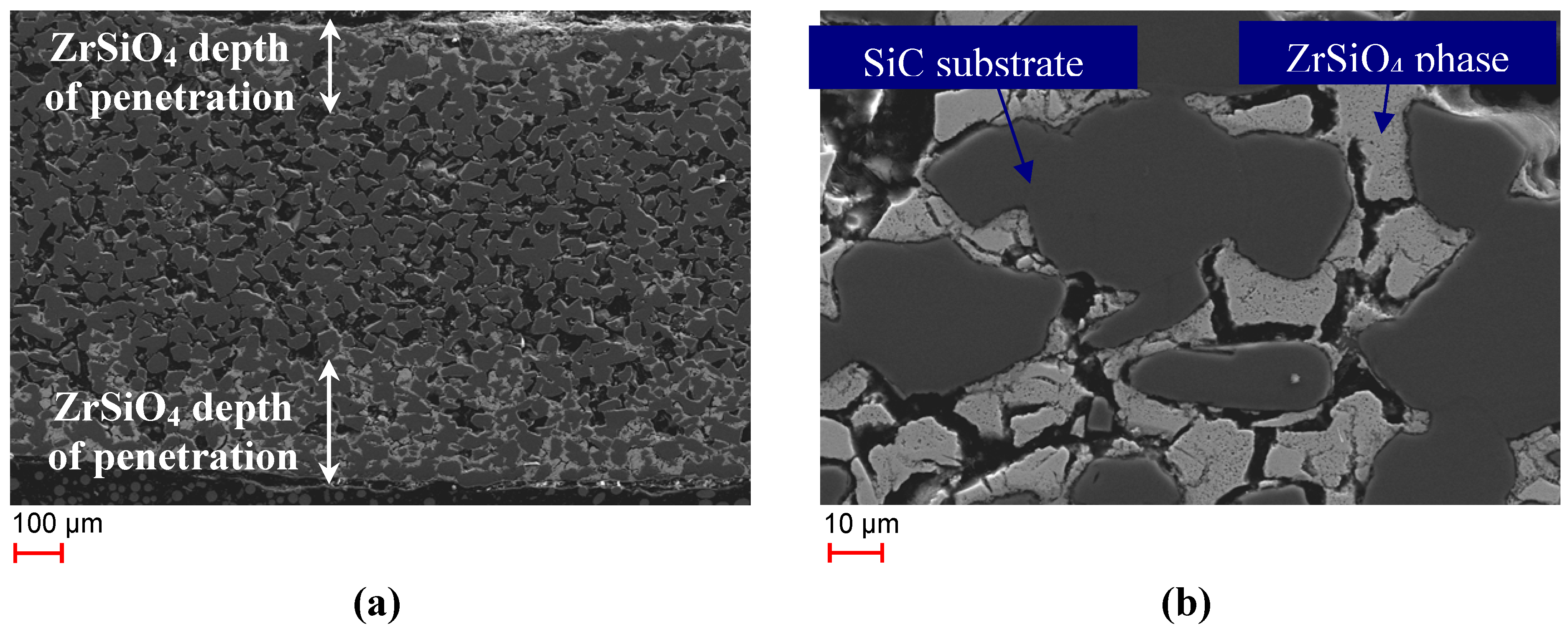
Another interesting observation was made when looking at the cross sections of the SiSiC substrate. The SEM images displayed in
Figure 4 shows a section through a SiSiC bar of Monolith 4 that had been tested for 58 cycles in the laboratory test-rig.
Figure 4.
(
a) SEM image of polished cross-section of Monolith 4 after 58 cycles (
b) enlarged cutout of
Figure 4a.
Figure 4.
(
a) SEM image of polished cross-section of Monolith 4 after 58 cycles (
b) enlarged cutout of
Figure 4a.
In
Figure 4a a light-colored phase that has penetrated about 150–250 µm into the monolith on both sides is visible. SEM-EDS analysis showed that the phase contains Zr, Si and O,
i.e., it is the zircon phase detected in the XRD patterns. The ZrSiO
4 phase encloses the darker SiC grains (
Figure 4b). A thin layer of SiO
2 was detected by SEM-EDS at the contact sites of zircon and the SiC grains. Scattered inclusions of pure ZrO
2 were detected in the ZrSiO
4 phase. The course of formation of the zircon phase inside the substrate is not fully clear though the following scenario appears feasible: First, a silica scale has formed through oxidation of Si and SiC. In the next step adjacent ZrO
2 grains might have been dissolved in the amorphous silica thus reducing its viscosity. Then the viscous zirconia-silicate phase infiltrated the porous SiSiC ceramics and, after approaching a certain composition, ZrSiO
4 has been formed. SEM-EDS analysis of Monoliths 3 and 5 also showed the existence of a ZrSiO
4 phase that had penetrated into the monoliths.
Table 4 shows the thickness of the ZrSiO
4 layer for the different monoliths. The layer of Monolith 4 was thickest which might be due to the combination of high numbers of cycles performed and high temperatures up to 1200 °C.
Table 4.
Thickness of ZrSiO4 phase.
Table 4.
Thickness of ZrSiO4 phase.
| Sample | Thickness of ZrSiO4 phase |
|---|
| Monolith 3 | 30 µm |
| Monolith 4 | 150–250 µm |
| Monolith 5 | 120 µm |
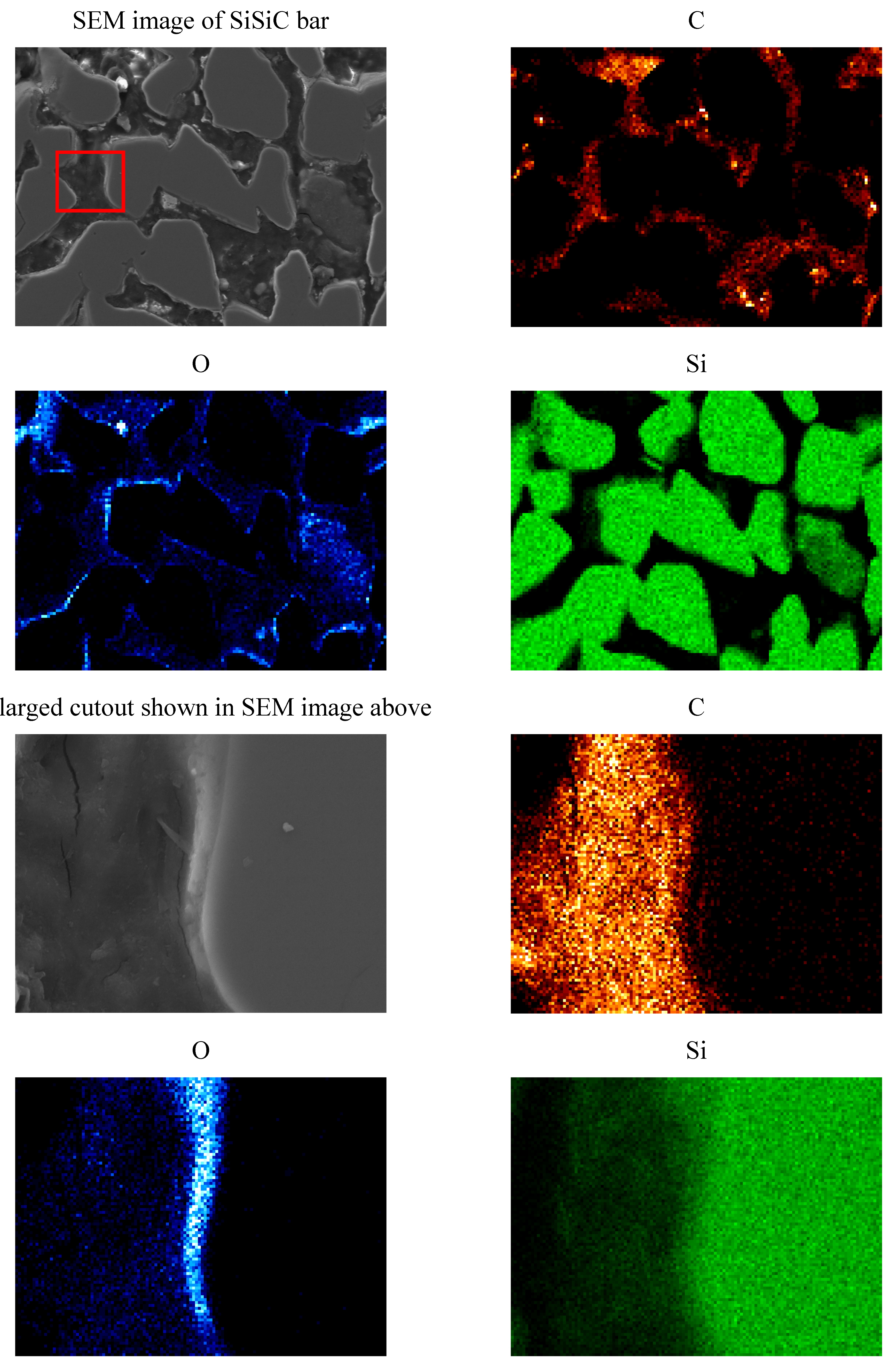
Another finding was that in the inner region of Monolith 4 no free Si is present anymore in contrast to the untreated monoliths where Si is found between the SiC grains. Instead, EDS analyses of the substrate bulk material of Monolith 4 indicated a silica phase that had formed around the inner SiC grains as can be seen in the EDS mapping of Monolith 4 in
Appendix B. The observations suggest that free Si has completely reacted, forming first SiO
2 and then other reaction products. The SiC grains have only oxidized on the edge, forming a protective SiO
2 surface layer. The XRD data of Monolith 4 and 5 confirmed that no Si is present anymore in the sample while in the XRD data of Monoliths 1, 2 and 3 (tested for 0, 1, 25 cycles respectively) a Si peak was still observed. This observation is in correspondence with the findings of Mehan
et al. who investigated the reactions of Si/SiC (polycrystalline SiC crystals immersed in a matrix of silicon, the material consisted of 70% SiC, 25% Si and 5% C) with metals (Ni, Co, Cr) and metal alloys at temperatures up to 1150 °C [
37]. They found that the composite material was depleted of silicon, leaving voids between the converted fiber bundles. Complex mixed silicides had formed in the reacted metal, leading them to conclude that Si had diffused directly into the metallic or alloy phase. Experiments with pure SiC and Si
3N
4 ceramics showed that the reaction was much less pronounced than in presence of silicon-containing materials. They concluded that in the case of SiC and Si
3N
4, much of the surface had been rendered inert by a SiO
2 layer. Their results correspond well to the observations of the present study. The substrate of Monolith 4 was also depleted of Si, suggesting that Si had completely reacted with the coating material.
4. Discussion
The SEM-EDS and the XRD analyses showed clearly that a number of reactions between the SiSiC substrate, the Zn-ferrite coating, the ZrO2 barrier coating and the Al2O3 binder material had occurred during testing of coated SiSiC honeycombs forming new reaction products such as FeSi, ZrSiO4 and SiO2. Especially the fact that no oxygen was detected in the off-gas of the reactor during the regeneration step must be due to the fact that the ferrite is reduced through reaction with Si and SiC, hence forming SiO2.
This leads to the formulation of a new reduction mechanism for the redox material through reaction with free Si and through reaction with SiC:
The formation of CO was not monitored during the tests described above because the mass spectrometer used to monitor the off-gas composition cannot distinguish between CO and N2, used as a carrier gas. Some tests were performed with Argon instead of N2 and in these CO formation was observed in the first couple of reduction steps, meaning that probably both reactions take place in the first cycles. The SiC surface is probably quickly rendered inert by a SiO2 layer forming on the outside of the SiC grains. Thus, we assume that the reduction of the ferrite occurs mainly through reaction with free Si as long as Si is present in the structure. It is suggested that the reduction of the ferrite deteriorates with cycling due to the growing of a SiO2 and ZrSiO4 layer and the disappearance of Si. Thus, reduction might be controlled by permeability and diffusion of silicon or oxygen through the product layer. This is supported when looking at the produced amount of hydrogen over many numbers of cycles.
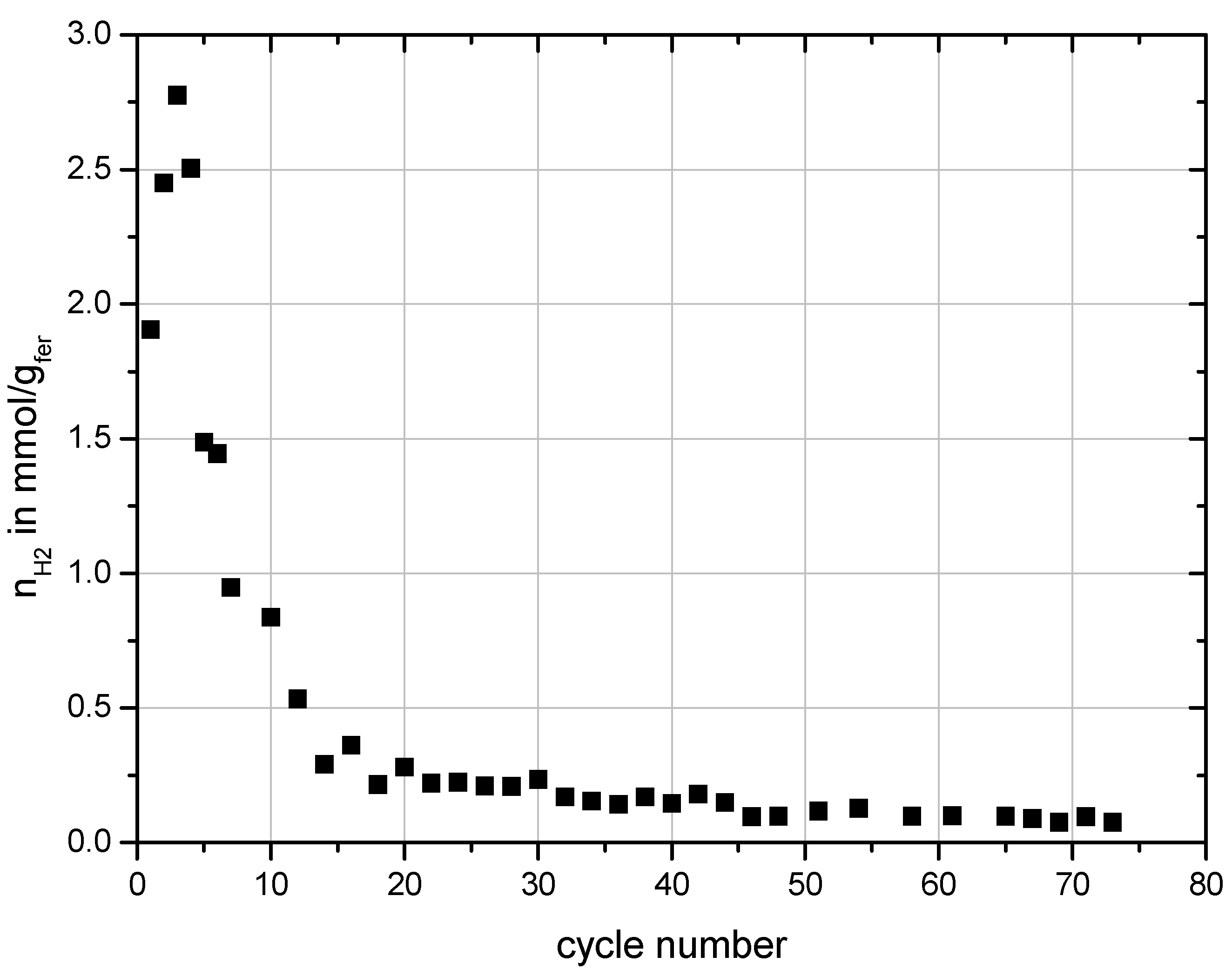
Figure 5 shows the total specific amount of hydrogen produced during the water-splitting steps performed with Monolith 5. In these cycles water-splitting was always performed at 900 °C for 20 min with a constant flow rate of water of 0.59 mmol/s introduced into the reactor. The preceding regeneration step was always performed at 1200 °C. A decline of the hydrogen production especially within the first 20 cycles is observed. Then hydrogen production settled to an almost constant level and only slightly decreased with on-going testing.
Figure 5.
Specific hydrogen production over cycle number performed with Monolith 5.
Figure 5.
Specific hydrogen production over cycle number performed with Monolith 5.
As shown already in the previous work, comparison of SEM images of untreated and treated samples showed that the coating strongly sinters leading to a decrease of initial porosity and thus surface area [
34]. But the SEM images of the monolith after one cycle (Monolith 2) showed that this effect already occurs in the first high temperature reduction step. That means any decrease in hydrogen production due to the loss of surface area should be seen from the first to the second cycle.
The results presented above showed that the substrate is slowly depleted of Si with on-going testing. After about 50 cycles no free Si could be found anymore in the honeycomb and the SiC grains of the substrate had formed a protective layer of SiO
2. It is suggested that this is the reason for the decrease of the hydrogen production rate with on-going testing. As long as free Si is available the reduction proceeds fast but after about 20 cycles the reduction of the ferrite decreases to a very small level when the free Si in the substrate is completely converted to SiO
2 resulting in the very low amounts of hydrogen production after the 20th cycle shown in
Figure 5. At that point, reduction of the ferrite can occur to some extend through thermal reduction and through reaction with SiC by diffusion of oxygen through the SiO
2 layer developed around the SiC grains. In none of the reduction steps any O
2 was monitored in the off-gas of the reactor, meaning that if thermal reduction occurred, the O
2 is either reacted on the monolith or somewhere downstream of the reactor. To what extend these two reactions take place cannot be resolved at this time.