Uniform and Conformal Carbon Nanofilms Produced Based on Molecular Layer Deposition
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of CNFs
2.2. Preparation of Au Nanoparticles Coated with CNFs and Tubular Carbon Nanofibers
2.3. Characterization of CNFs
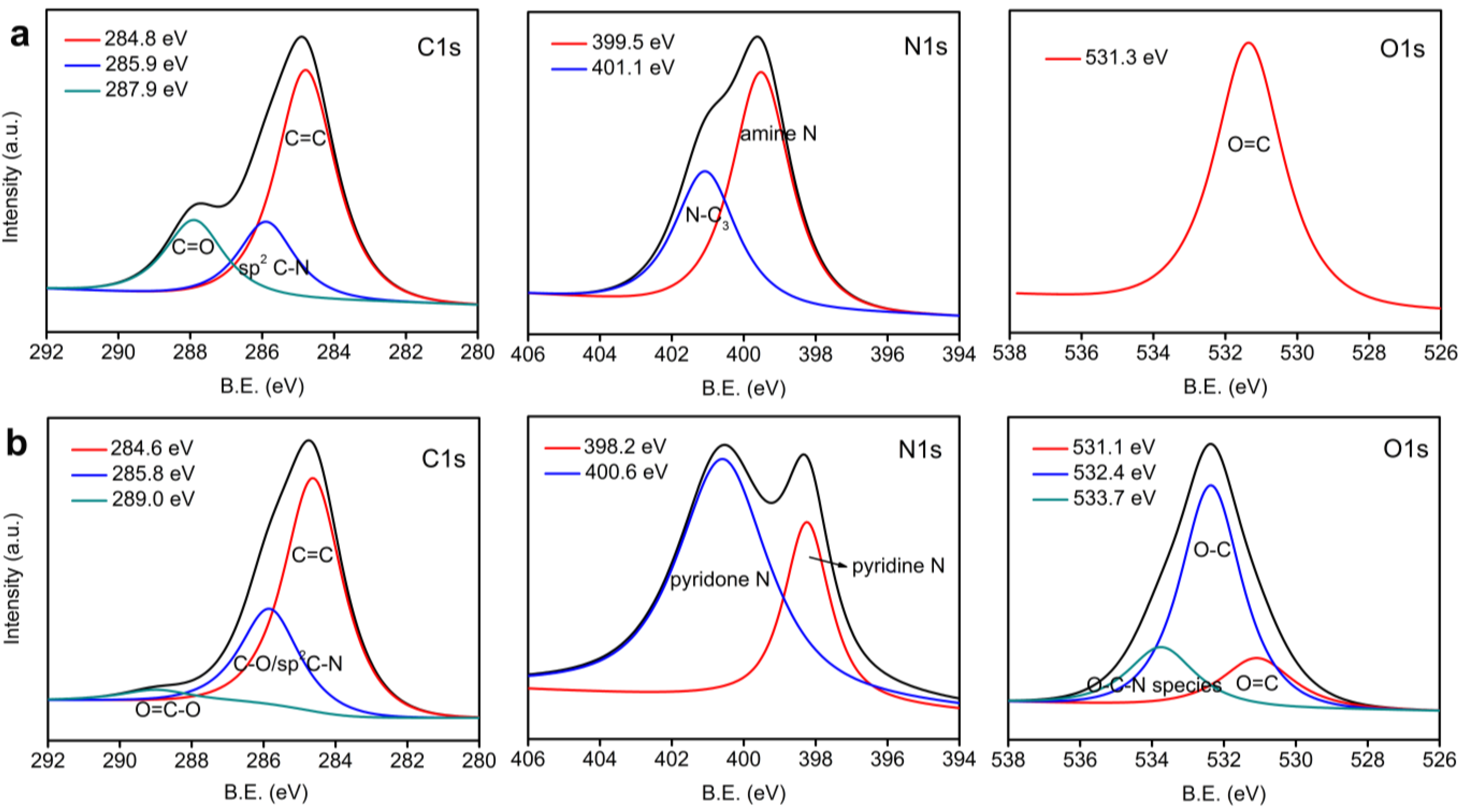
3. Results and Discussion
3.1. Structure Analysis and Properties of CNFs



| Sample | C | N | O |
|---|---|---|---|
| Polyimide film | 66.66% | 12.44% | 20.90% |
| CNF | 81.49% | 4.72% | 13.79% |
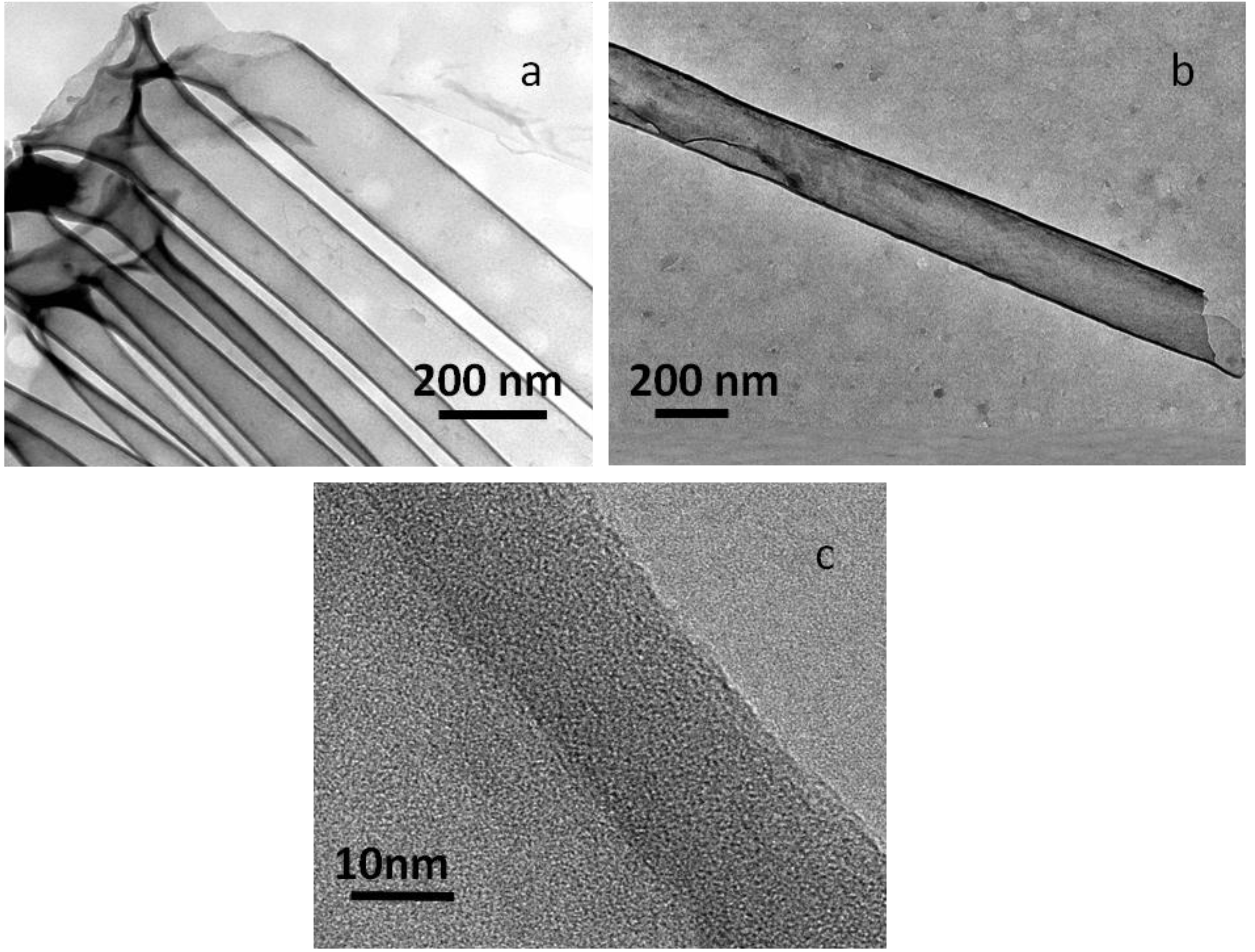
3.2. Au/C Nanoparticles and C-NTs

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bin, Y.Z.; Chen, Q.Y.; Nakamura, Y.; Tsuda, K.; Matsuo, M. Preparation and characterization of carbon films prepared from poly(vinyl alcohol) containing metal oxide and nano fibers with iodine pretreatment. Carbon 2007, 45, 1330–1339. [Google Scholar]
- Yang, D.; Xia, L.; Zhao, H.; Hu, X.; Liu, Y.; Li, J.; Wan, X. Preparation and characterization of an ultrathin carbon shell coating a silver core for shell-isolated nanoparticle-enhanced Raman spectroscopy. Chem. Commun. 2011, 47, 5873–5875. [Google Scholar] [CrossRef]
- Foley, H.C. Carbogenic molecular sieves: Synthesis, properties and applications. Microporous Mater. 1995, 4, 407–433. [Google Scholar] [CrossRef]
- Sheem, K.Y.; Song, E.H.; Lee, Y.H. High-rate charging performance using high-capacity carbon nanofilms coated on alumina nanoparticles for lithium ion battery anode. Electrochim. Acta 2012, 78, 223–228. [Google Scholar] [CrossRef]
- Tsang, S.C.; Caps, V.; Paraskevas, I.; Chadwick, D.; Thompsett, D. Magnetically separable, carbon-supported nanocatalysts for the manufacture of fine chemicals. Angew. Chem. Int. Ed. 2004, 43, 5645–5649. [Google Scholar] [CrossRef]
- Lahiri, I.; Choi, W. Carbon nanostructures in lithium ion batteries: Past, present, and future. Crit. Rev. Solid State Mater. Sci. 2013, 38, 128–166. [Google Scholar] [CrossRef]
- Amaratunga, G.A.J.; Chhowalla, M.; Kiely, C.J.; Alexandrou, I.; Aharonov, R.; Devenish, R.M. Hard elastic carbon thin films from linking of carbon nanoparticles. Nature 1996, 383, 321–323. [Google Scholar] [CrossRef]
- Zhang, Z.; Dua, R.; Zhang, L.; Zhu, H.; Zhang, H.; Wang, P. Carbon-layer-protected cuprous oxide nanowire arrays for efficient water reduction. ACS Nano 2013, 7, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Noborisaka, M.; Hirako, T.; Shirakura, A.; Suzuki, T. Hardness and Surface Roughness of Hydrogenated Amorphous Carbon Films Synthesized by Atmospheric Pressure Plasma Enhanced CVD Method with Various Pulse Frequencies. Surf. Coat. Technol. 2013, 215, 460–464. [Google Scholar]
- Lifshitz, Y. Diamond-like carbon—Present status. Diam. Relat. Mater. 1999, 8, 1659–1676. [Google Scholar] [CrossRef]
- Panchal, V.; Neergat, M.; Bhandarkar, U. Synthesis and characterization of carbon coated nanoparticles produced by a continuous low-pressure plasma process. J. Nanopart. Res. 2011, 13, 3825–3833. [Google Scholar] [CrossRef]
- Niakan, H.; Yang, Q.; Szpunar, J.A. Structure and properties of diamond-like carbon thin films synthesized by biased target ion beam deposition. Surf. Coat. Technol. 2013, 223, 11–16. [Google Scholar] [CrossRef]
- Ishpal; Panwar, O.S.; Kumar, M.; Kumar, S. Effect of ambient gaseous environment on the properties of amorphous carbon thin films. Mater. Chem. Phys. 2011, 125, 558–567. [Google Scholar]
- Caiulo, N.; Yu, C.H.; Yu, K.M.K.; Lo, C.C.H.; Oduro, W.; Thiebaut, B.; Bishop, P.; Tsang, S.C. Carbon-decorated FePt nanoparticles. Adv. Funct. Mater. 2007, 17, 1392–1396. [Google Scholar] [CrossRef]
- Zhu, G.X.; Wei, X.W.; Xia, C.J.; Ye, Y. Solution route to single crystalline dendritic cobalt nanostructures coated with carbon shells. Carbon 2007, 45, 1160–1166. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Adelhelm, C.; Balden, M.; Rinke, M.; Stueber, M. Influence of doping (Ti, V, Zr, W) and annealing on the sp2 carbon structure of amorphous carbon films. J. Appl. Phys. 2009, 105, 033522:1–033522:9. [Google Scholar] [CrossRef]
- Kumari, L.; Subramanyam, S.V. Structural, optical and electrical properties of sulfur-incorporated amorphous carbon films. Appl. Phys. 2009, 95, 343–349. [Google Scholar]
- Chen, Y.D.; Yang, R.T. Preparation of carbon molecular-sieve membrane and diffusion of binary-mixtures in the membrane. Ind. Eng. Chem. Res. 1994, 33, 3146–3153. [Google Scholar] [CrossRef]
- Centeno, T.A.; Fuertes, A.B. Supported carbon molecular sieve membranes based on a phenolic resin. J. Membr. Sci. 1999, 160, 201–211. [Google Scholar] [CrossRef]
- Hatori, H.; Yamada, Y.; Shiraishi, M. Preparation of macroporous carbon-films from polyimide by phase inversion method. Carbon 1992, 30, 303–304. [Google Scholar] [CrossRef]
- Takeichi, T.; Eguchi, Y.; Kaburagi, Y.; Hishiyama, Y.; Inagaki, M. Carbonization and graphitization of kapton-type polyimide films prepared from polyamide alkyl ester. Carbon 1998, 36, 117–122. [Google Scholar] [CrossRef]
- Smirnova, V.E.; Gofman, I.V.; Maritcheva, T.A.; Yudin, V.E.; Eto, K.; Takeichi, T.; Kaburagi, Y.; Hishiyama, Y. The effect of different orientations in rigid rod polyimide films on the graphitized products. Carbon 2007, 45, 839–846. [Google Scholar] [CrossRef]
- Hishiyama, Y.; Yoshida, A.; Inagaki, M. Structure and microtexture of graphitized carbon film derived from aromatic polyimide film Upilex. Carbon 1998, 36, 1113–1117. [Google Scholar] [CrossRef]
- Putkonen, M.; Harjuoja, J.; Sajavaara, T.; Niinisto, L. Atomic layer deposition of polyimide thin films. J. Mater. Chem. 2007, 17, 664–669. [Google Scholar] [CrossRef]
- Ott, A.W.; Klaus, J.W.; Johnson, J.M.; George, S.M. Al2O3 thin film growth on Si(100) using binary reaction sequence chemistry. Thin Solid Films 1997, 292, 135–144. [Google Scholar] [CrossRef]
- Das, A.; Pisana, S.; Chakraborty, B.; Piscanec, S.; Saha, S.K.; Waghmare, U.V.; Novoselov, K.S.; Krishnamurthy, H.R.; Geim, A.K.; Ferrari, A.C.; et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 2008, 3, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiang, X.; Li, F. Facile synthesis and novel electrocatalytic performance of nanostructured Ni-Al layered double hydroxide/carbon nanotube composites. J. Mater. Chem. 2010, 20, 3944–3952. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yan, X.P. A dehydration and stabilizer-free approach to production of stable water dispersions of graphene nanosheets. J. Mater. Chem. 2010, 20, 4328–4332. [Google Scholar] [CrossRef]
- Pastine, S.J.; Okawa, D.; Kessler, B.; Rolandi, M.; Llorente, M.; Zettl, A.; Frechet, J.M.J. A facile and patternable method for the surface modification of carbon nanotube forests using perfluoroarylazides. J. Am. Chem. Soc. 2008, 130, 4238–4239. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Raymundo Pinero, E.; Cazorla Amoros, D.; Linares Solano, A.; Find, J.; Wild, U.; Schlogl, R. Structural characterization of N-containing activated carbon fibers prepared from a low softening point petroleum pitch and a melamine resin. Carbon 2002, 40, 597–608. [Google Scholar]
- Wojtowicz, M.A.; Pels, J.R.; Moulijn, J.A. The fate of nitrogen functionalities in coal during pyrolysis and combustion. Fuel 1995, 74, 507–516. [Google Scholar] [CrossRef]
- Jaouen, F.; Marcotte, S.; Dodelet, J.-P.; Lindbergh, G. Oxygen reduction catalysts for polymer electrolyte fuel cells from the pyrolysis of iron acetate adsorbed on various carbon supports. J. Phys. Chem. 2003, 107, 1376–1386. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, P.; Wang, G.; Gao, Z.; Chen, H.; Wang, Y.; Qin, Y. Uniform and Conformal Carbon Nanofilms Produced Based on Molecular Layer Deposition. Materials 2013, 6, 5602-5612. https://doi.org/10.3390/ma6125602
Yang P, Wang G, Gao Z, Chen H, Wang Y, Qin Y. Uniform and Conformal Carbon Nanofilms Produced Based on Molecular Layer Deposition. Materials. 2013; 6(12):5602-5612. https://doi.org/10.3390/ma6125602
Chicago/Turabian StyleYang, Peng, Guizhen Wang, Zhe Gao, He Chen, Yong Wang, and Yong Qin. 2013. "Uniform and Conformal Carbon Nanofilms Produced Based on Molecular Layer Deposition" Materials 6, no. 12: 5602-5612. https://doi.org/10.3390/ma6125602





