Metal-Exchanged β Zeolites as Catalysts for the Conversion of Acetone to Hydrocarbons
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
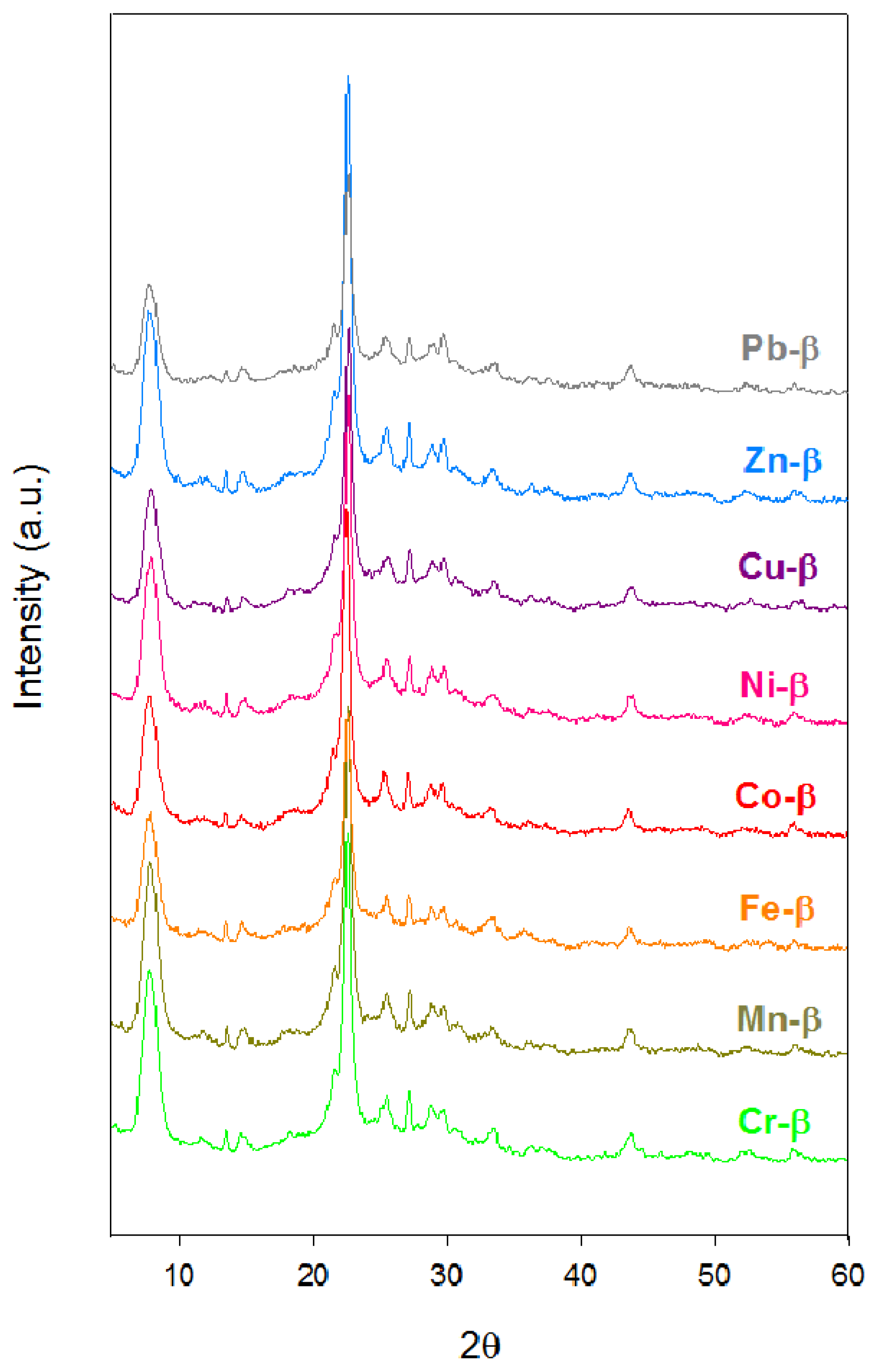
| Catalyst | Si/Al ratio | Metal content (%) | Me/Al ratio | Exchange degree (%) | Crystallinity (%) |
|---|---|---|---|---|---|
| H-β | 12.5 | - | - | - | 100 |
| Cr-β | 27.9 | 1.00 | 0.44 | - | 94 |
| Mn-β | 15.3 | 0.89 | 0.23 | 46 | 83 |
| Fe-β | 49.9 | 4.80 | 3.77 | - | 64 |
| Co-β | 13.7 | 1.44 | 0.35 | 70 | 90 |
| Ni-β | 15.5 | 0.80 | 0.21 | 43 | 81 |
| Cu-β | 13.7 | 2.06 | 0.48 | 98 | 84 |
| Zn-β | 16.2 | 1.22 | 0.32 | 64 | 99 |
| Al-β | 14.5 | - | - | - | 86 |
| Pb-β | 16.7 | 1.50 | 0.42 | 84 | 65 |

| Catalyst | SBET (m2 g−1) | Mesopore volume (cm3 g−1) | Micropore volume (cm3 g−1) |
|---|---|---|---|
| H-β | 582 | 0.89 | 0.22 |
| Cr-β | 540 | 0.88 | 0.19 |
| Mn-β | 531 | 0.90 | 0.19 |
| Fe-β | 513 | 0.89 | 0.18 |
| Co-β | 513 | 0.79 | 0.19 |
| Ni-β | 505 | 0.84 | 0.17 |
| Cu-β | 499 | 0.84 | 0.18 |
| Zn-β | 525 | 0.89 | 0.19 |
| Al-β | 522 | 0.85 | 0.19 |
| Pb-β | 545 | 0.85 | 0.18 |


2.2. Catalytic Activity
| Catalyst | Conversion (%) | Relative yields (wt %) | |||
|---|---|---|---|---|---|
| CO2 | Aliphatics 1 | Aromatics | |||
| Benzene derivatives 2 | Naphthalene derivatives 3 | ||||
| H-β | 96.8 | 6.7 | 37.6 | 40.4 | 12.1 |
| Cr-β | 81.5 | 5.6 | 32.5 | 36.4 | 7.0 |
| Mn-β | 81.9 | 6.0 | 35.7 | 40.2 | - |
| Fe-β | 37.5 | 2.6 | 17.1 | 17.8 | - |
| Co-β | 39.6 | 3.3 | 28.3 | 8.0 | - |
| Ni-β | 42.0 | 3.7 | 29.6 | 8.7 | - |
| Cu-β | 58.5 | 16.6 | 41.9 | - | - |
| Zn-β | 79.5 | 4.3 | 29.0 | 42.7 | 3.5 |
| Al-β | 91.6 | 2.8 | 24.2 | 50.0 | 14.5 |
| Pb-β | 45.1 | 6.0 | 25.3 | 13.9 | - |
| Catalyst | C3-= | iC4 | iC4= | nC4 | nC4= | C5-C6 | C5-C6= |
|---|---|---|---|---|---|---|---|
| H-β | 6.9 | 11.3 | 7.5 | 1.0 | 4.3 | 3.6 | 3.0 |
| Cr-β | 5.6 | 6.9 | 17.0 | - | - | - | 3.0 |
| Mn-β | 5.0 | 6.9 | 14.3 | - | 5.7 | - | 3.8 |
| Fe-β | 2.6 | 3.3 | 11.2 | - | - | - | - |
| Co-β | 1.5 | 1.2 | 23.2 | - | 1.3 | - | 1.1 |
| Ni-β | 6.8 | 5.1 | 16.0 | - | 1.7 | - | - |
| Cu-β | 7.5 | 2.1 | 31.3 | - | - | - | 1.0 |
| Zn-β | 4.2 | 4.7 | 14.6 | - | 1.7 | - | 3.8 |
| Al-β | 2.7 | 7.2 | 6.9 | 0.5 | 3.1 | 1.8 | 1.9 |
| Pb-β | 3.3 | 3.0 | 13.8 | - | 3.4 | - | 1.8 |
| Catalyst | B | T | A8 | TMB | A9 | TeMB | A10–A11 |
|---|---|---|---|---|---|---|---|
| H-β | 1.4 | 8.7 | 13.9 | 8.8 | 4.2 | 1.2 | 2.1 |
| Cr-β | 0.9 | 5.9 | 10.1 | 10.7 | 3.0 | 3.7 | 3.0 |
| Mn-β | 0.7 | 4.6 | 7.4 | 9.6 | 2.5 | 5.4 | 10.0 |
| Fe-β | - | 2.7 | 2.9 | 6.0 | - | 3.4 | 2.8 |
| Co-β | - | 1.1 | - | 3.1 | - | - | 3.8 |
| Ni-β | - | 4.3 | 2.6 | 1.8 | - | - | - |
| Cu-β | - | - | - | - | - | - | - |
| Zn-β | 1.3 | 7.0 | 11.8 | 11.4 | 3.6 | 2.9 | 4.7 |
| Al-β | 1.2 | 7.9 | 14.9 | 14.9 | 3.8 | 2.6 | 4.6 |
| Pb-β | - | 3.4 | 3.9 | 6.6 | - | - | - |



3. Experimental Section
3.1. Ion-Exchange Procedure
3.2. Characterization of Catalysts
3.3. Catalytic Activity
4. Conclusions
Acknowledgments
References
- Chang, C.D.; Silvestri, A.J. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J. Catal. 1977, 47, 249–259. [Google Scholar] [CrossRef]
- Mentzel, U.V.; Holm, M.S. Utilization of biomass: Conversion of model compounds to hydrocarbons over zeolite H-ZSM-5. Appl. Catal. A 2011, 396, 59–67. [Google Scholar] [CrossRef]
- Knifton, J.F.; Dai, P.S.E. Diisopropyl ether syntheses from crude acetone. Catal. Lett. 1999, 57, 193–197. [Google Scholar] [CrossRef]
- Jesse, T.W.; Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of butanol from starch-based waste packing peanuts and agricultural waste. J. Ind. Microbiol. Biotechnol. 2002, 29, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Claassen, P.A.M.; van Lier, J.B.; Lopez Contreras, A.M.; van Niel, E.W.J.; Sijtsma, L.; Stams, A.J.M.; de Vries, S.S.; Weusthuis, R.A. Utilisation of biomass for the supply of energy carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Matsumoto, T.; Kanai, H.; Utani, K.; Matsumura, Y.; Shen, W.-J.; Imamura, S. Catalytic steam reforming of ethanol to produce hydrogen and acetone. Appl. Catal. A 2005, 279, 273–277. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Aguayo, A.T.; Atutxa, A.; Aguado, R.; Olazar, M.; Bilbao, J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. II. Aldehydes, ketones, and acids. Ind. Eng. Chem. Res. 2004, 43, 2619–2626. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Lappas, A.A.; Pilavachi, P.A. In-situ upgrading of biomass pyrolysis vapors: Catalyst screening on a fixed bed reactor. Bioresour. Technol. 2011, 102, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, E.; Mizutani, Y.; Tago, T.; Masuda, T. Production of ketones from sewage sludge over zirconia-supporting iron oxide catalysts in a steam atmosphere. Appl. Catal. B 2006, 68, 154–159. [Google Scholar] [CrossRef]
- Funai, S.; Satoh, Y.; Satoh, Y.; Tajima, K.; Tago, T.; Masuda, T. Development of a new conversion process consisting of hydrothermal treatment and catalytic reaction using ZrO2–FeOX catalyst to convert fermentation residue into useful chemicals. Top. Catal. 2010, 53, 654–658. [Google Scholar] [CrossRef]
- Funai, S.; Tago, T.; Masuda, T. Selective production of ketones from biomass waste containing a large amount of water using an iron oxide catalyst. Catal. Today 2011, 164, 443–446. [Google Scholar] [CrossRef]
- Baigrie, L.M.; Cox, R.A.; Slebocka-Tilk, H.; Tencer, M.; Tidwell, T.T. Acid-catalyzed enolization and aldol condensation of acetaldehyde. J. Am. Chem. Soc. 1985, 107, 3640–3645. [Google Scholar] [CrossRef]
- Novakova, J.; Kubelkova, L.; Dolejsek, Z. TPD/MS studies of the interaction of simple ketones with ZSM-5 zeolites. J. Mol. Catal. 1987, 39, 195–202. [Google Scholar] [CrossRef]
- Salvapati, G.S.; Ramanamurty, K.V.; Janardanarao, M. Selective catalytic self-condensation of acetone. J. Mol. Catal. 1989, 54, 9–30. [Google Scholar] [CrossRef]
- Panov, A.G.; Fripiat, J.J. Acetone condensation reaction on acid catalysts. J. Catal. 1998, 178, 188–197. [Google Scholar] [CrossRef]
- Veloso, C.O.; Monteiro, J.L.F.; Sousa-Aguiar, E.F. Aldol condensation of acetone over alkali cation exchanged zeolites. Stud. Surf. Sci. Catal. 1994, 84, 1913–1920. [Google Scholar]
- Hutchings, G.J.; Johnston, P.; Lee, D.F.; Williams, C.D. Acetone conversion to isobutene in high selectivity using zeolite β catalyst. Catal. Lett. 1993, 21, 49–53. [Google Scholar] [CrossRef]
- Tago, T.; Konno, H.; Ikeda, S.; Yamazaki, S.; Ninomiya, W.; Nakasaka, Y.; Masuda, T. Selective production of isobutylene from acetone over alkali metal ion-exchanged BEA zeolites. Catal. Today 2011, 164, 158–162. [Google Scholar] [CrossRef]
- Esquivel, D.; Cruz-Cabeza, A.J.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Local environment and acidity in alkaline and alkaline-earth exchanged β zeolite: Structural analysis and catalytic properties. Micropor. Mesopor. Mat. 2011, 142, 672–679. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ionics 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Armor, J.N. Metal-exchanged zeolites as catalysts. Micropor. Mesopor. Mat. 1998, 22, 451–456. [Google Scholar] [CrossRef]
- Esquivel, D.; Cruz-Cabeza, A.J.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Enhanced concentration of medium strength brönsted acid sites in aluminium-modified β zeolite. Catal. Lett. 2012. [Google Scholar] [CrossRef]
- Mauvezin, M.; Delahay, G.; Coq, B.; Kieger, S.; Jumas, J.C.; Olivier-Fourcade, J. Identification of iron species in Fe-BEA: Influence of the exchange level. J. Phys. Chem. B 2001, 105, 928–935. [Google Scholar] [CrossRef]
- Penzien, J.; Muller, T.E.; Lercher, J.A. Hydroamination of 6-aminohex-1-yne over zinc based homogeneous and zeolite catalysts. Micropor. Mesopor. Mat. 2001, 48, 285–291. [Google Scholar] [CrossRef]
- Pelmenschikov, A.G.; van Santen, R.A.; Jänchen, J.; Meijer, E. CD3CN as a probe of lewis and bronsted acidity of zeolites. J. Phys. Chem. 1993, 97, 11071–11074. [Google Scholar] [CrossRef]
- Flego, C.; Perego, C. Acetone condensation as a model reaction for the catalytic behavior of acidic molecular sieves: A UV-Vis study. Appl. Catal. A 2000, 192, 317–329. [Google Scholar] [CrossRef]
- Parker, L.M.; Bibby, D.M.; Miller, I.J. Formation of ketenes by reaction of carboxylic acids over alkali metal-exchanged zeolites. J. Catal. 1991, 129, 438–446. [Google Scholar] [CrossRef]
- Olah, G.A.; Ip, W.M. Catalysis of solid superacids. 23. Selective condensation of acetone to mesitylene over superacidic nafion-H and comparison with acidic-basic tungsten(VI) oxide/alumina or zirconium(IV) oxide/alumina catalysts. New J. Chem. 1988, 12, 299–301. [Google Scholar]
- Roldán, R.; Romero, F.J.; Jiménez, C.; Borau, V.; Marinas, J.M. transformation of mixtures of benzene and xylenes into toluene by transalkylation on zeolites. Appl. Catal. A 2004, 266, 203–210. [Google Scholar] [CrossRef]
- Tago, T.; Konno, H.; Sakamoto, M.; Nakasaka, Y.; Masuda, T. Selective synthesis for light olefins from acetone over ZSM-5 zeolites with nano- and macro-crystal sizes. Appl. Catal. A 2011, 403, 183–191. [Google Scholar] [CrossRef]
- Mikkelsen, O.; Kolboe, S. The conversion of methanol to hydrocarbons over zeolite H-beta. Micropor. Mesopor. Mat. 1999, 29, 173–184. [Google Scholar] [CrossRef]
- Corma, A.; Martinez, A.; Arroyo, P.A.; Monteiro, J.L.F.; Sousa-Aguiar, E.F. Isobutane/2-butene alkylation on zeolite beta: Influence of post-synthesis treatments. Appl. Catal. A 1996, 142, 139–150. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Lee, D.F.; Lynch, M. Methanol conversion to hydrocarbons over zeolite H-ZSM-5: Comments on the formation of C4 hydrocarbons at low reaction temperatures. Appl. Catal. A 1993, 106, 115–123. [Google Scholar] [CrossRef]
- Itoh, H.; Hattori, T.; Murakami, Y. Product distribution in the conversion of methanol on partially ion-exchanged mordenites. Appl. Catal. 1982, 2, 19–37. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cruz-Cabeza, A.J.; Esquivel, D.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Metal-Exchanged β Zeolites as Catalysts for the Conversion of Acetone to Hydrocarbons. Materials 2012, 5, 121-134. https://doi.org/10.3390/ma5010121
Cruz-Cabeza AJ, Esquivel D, Jiménez-Sanchidrián C, Romero-Salguero FJ. Metal-Exchanged β Zeolites as Catalysts for the Conversion of Acetone to Hydrocarbons. Materials. 2012; 5(1):121-134. https://doi.org/10.3390/ma5010121
Chicago/Turabian StyleCruz-Cabeza, Aurora J., Dolores Esquivel, César Jiménez-Sanchidrián, and Francisco J. Romero-Salguero. 2012. "Metal-Exchanged β Zeolites as Catalysts for the Conversion of Acetone to Hydrocarbons" Materials 5, no. 1: 121-134. https://doi.org/10.3390/ma5010121





