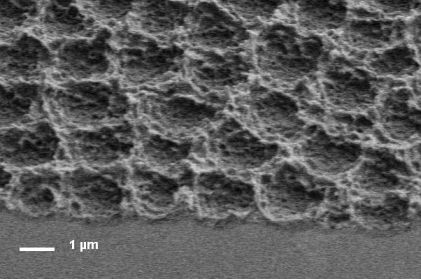
Electrochemically Formed Porous Silica
Abstract
:1. Introduction
2. Anodic Behavior of Silicon in Acidic Fluoride Electrolyte
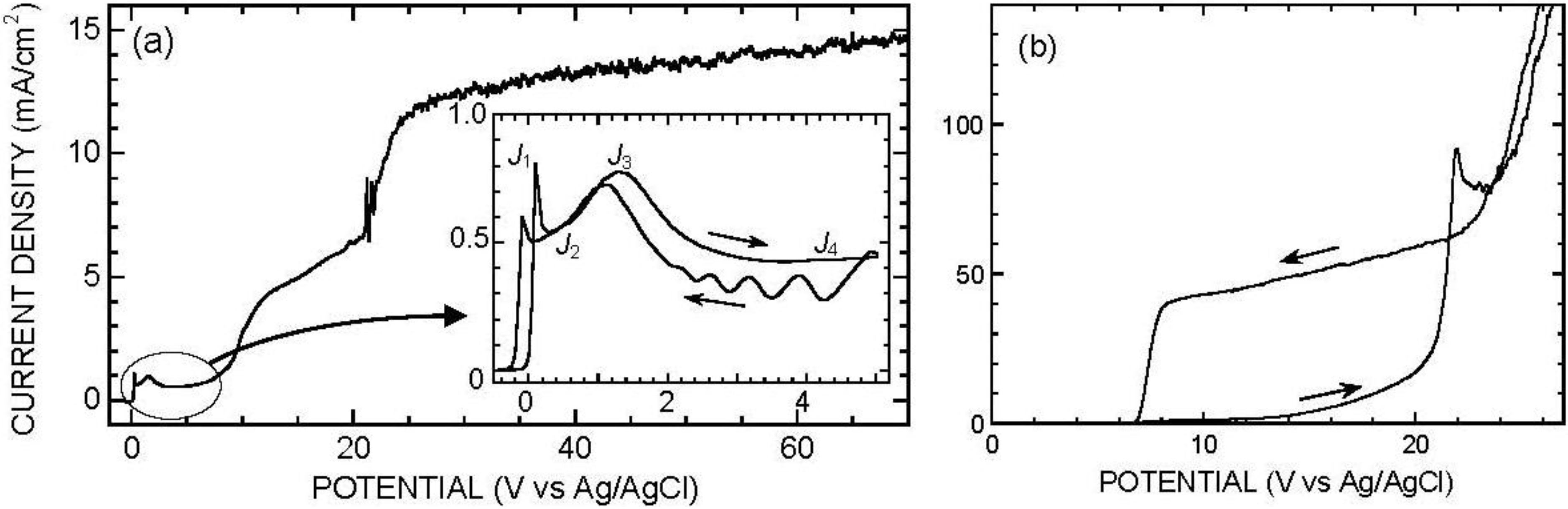
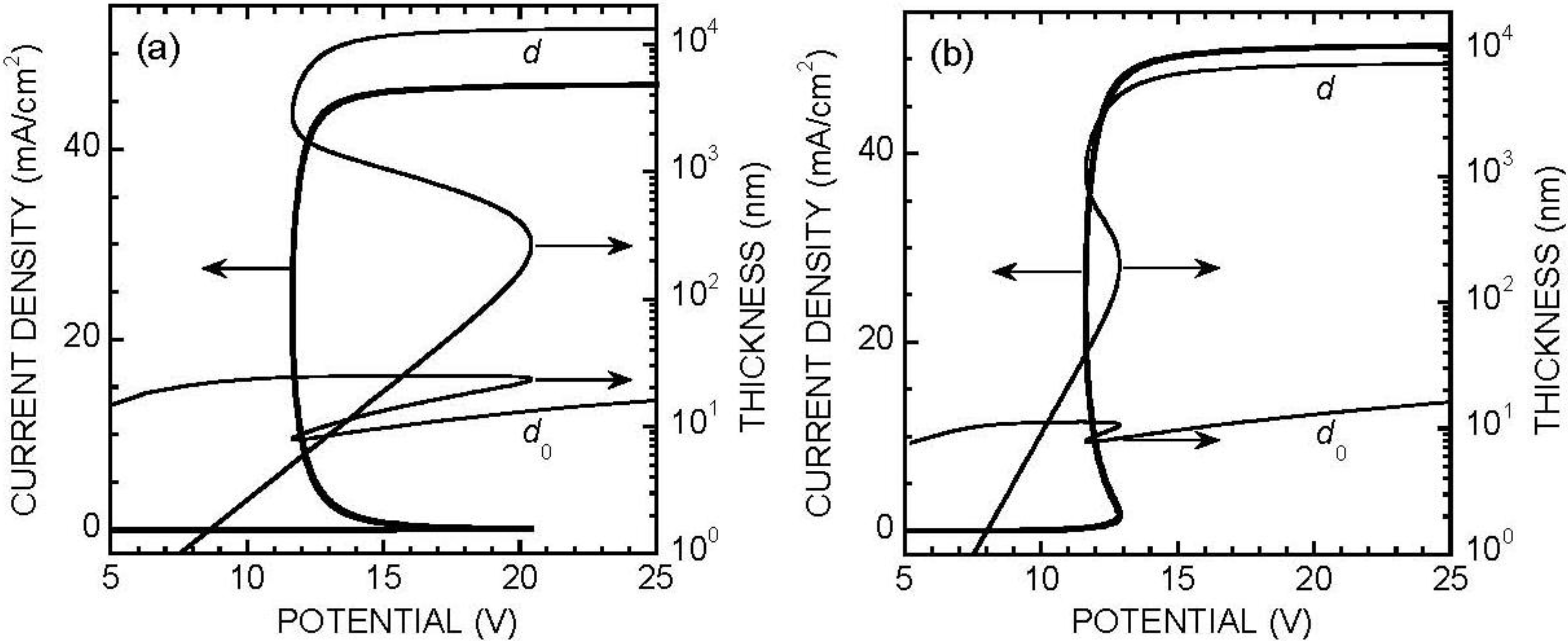
3. Anodic Behavior of Silicon in Neutral Fluoride Electrolyte
4. Porous Silica: Formation Mechanism
4.1. Case of Acidic Fluoride Electrolytes
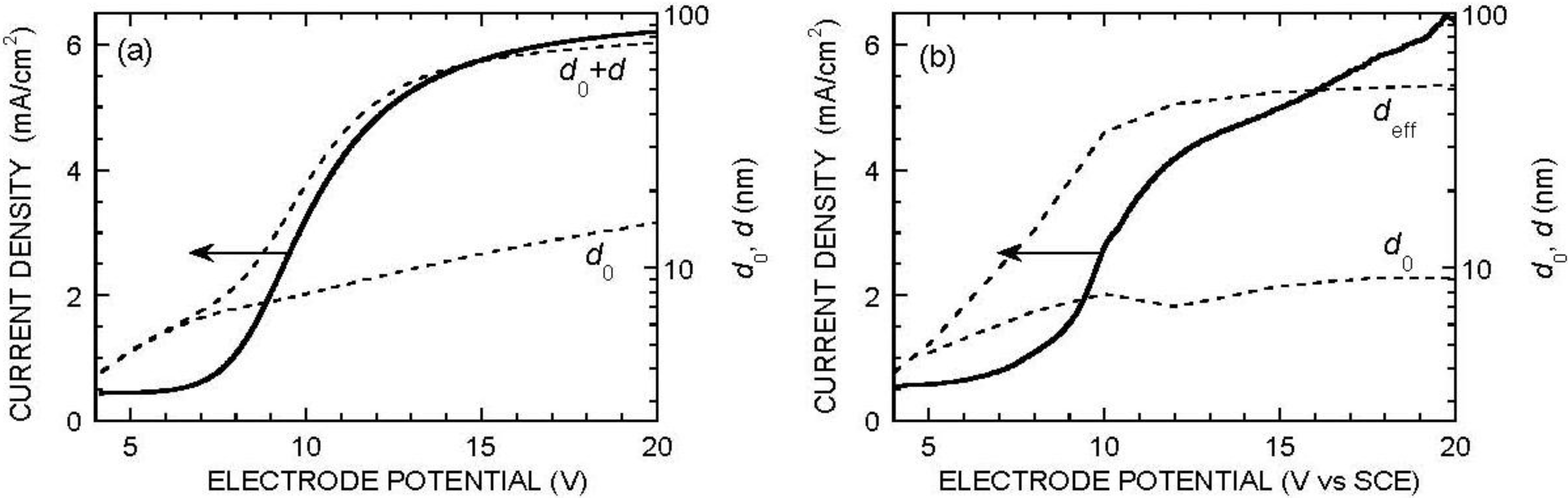
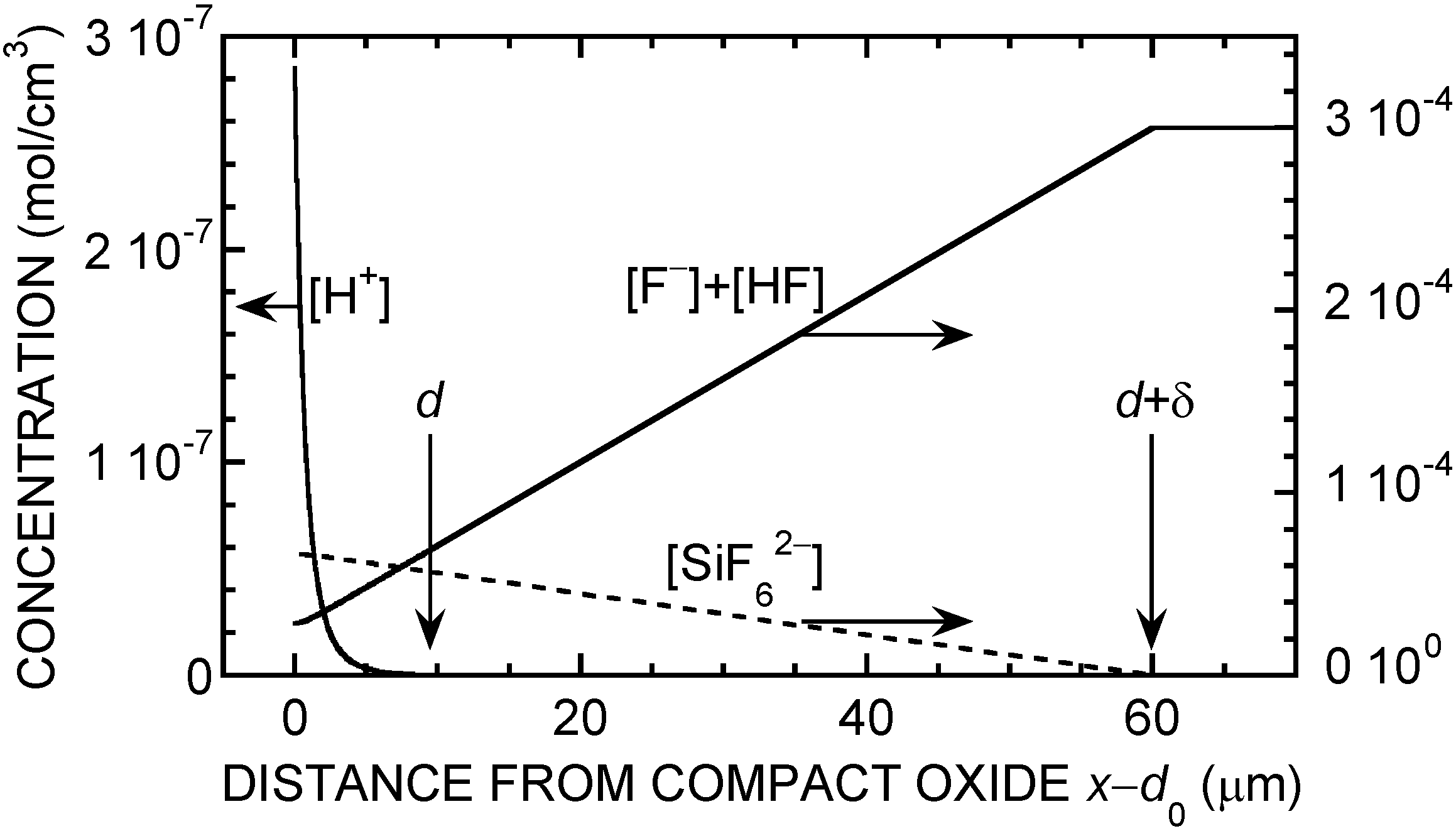
4.2. Case of Neutral Fluoride Electrolytes

5. Porous Silica: Macromorphologies
- -
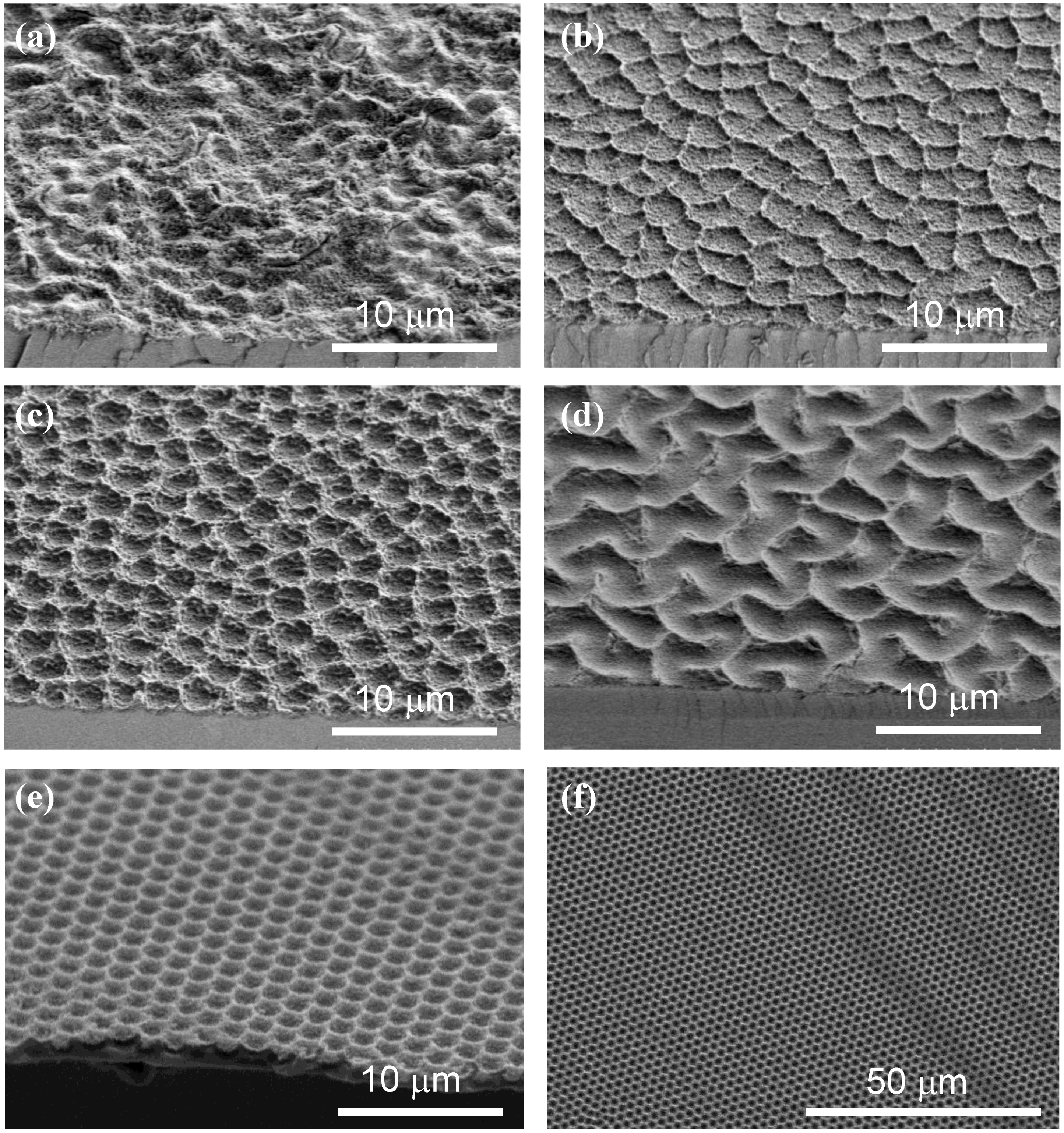
- Disordered (D) (Figure 7a). At low electrode rotation rates and low fluoride concentrations, the top views exhibit a disordered aspect, with bright spots of a few microns in size, with random positions and distributed brightness. The roughness of the films, more quantitatively observed on cleavage planes, has essentially random character.
- -
- Waves (W) (Figure 7b). In a narrow region of potential above the upper boundary of uniform film formation, the films appear decorated by protruding crests of irregular shape. SEM profiles show that the uniform pieces between the crests are slightly bent upwards. This morphology appears as a transition between the uniform morphology and the “bowl” morphology described hereafter.
- -
- Bowls (B) (Figure 7c). For potentials slightly higher than those leading to the wave morphology, the top views exhibit bright circular spots, of similar size and brightness, with a tendency to short range ordering. It has been shown that prepatterning allows one to grow similar structures ordered on the long-range [27] (Figure 7e–f). The SEM profiles indicate that these spots correspond to closely circular depressions (bowls) in the surface of the film, which may look like macropores in the most extreme cases. The Si/SiO2 interface also exhibits bowl shapes.
- -
- Labyrinths (L) (Figure 7d). For still higher potentials, the bowls tend to coalesce and to form meandering stripes. The collection of parallel stripes then exhibits a labyrinth shape.
- -
- Finally, a fifth morphology (B’) appears at very high potentials, which has been regarded as a variant of B. However, it has a more random character and must probably be regarded as a distinct morphology, intermediate between B and D.

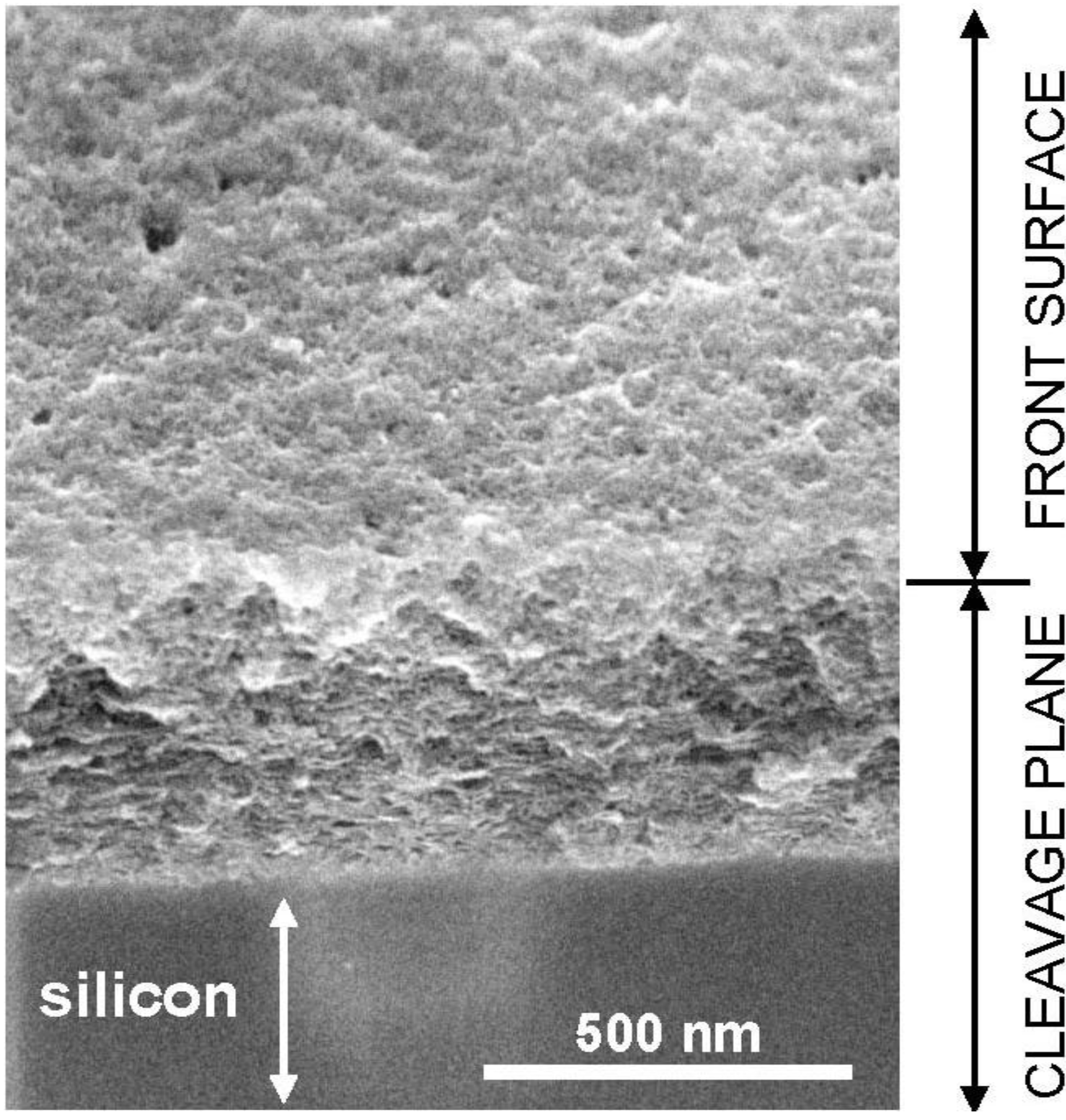
6. Porous Silica on the Nanometric Scale: The Stratified Structure
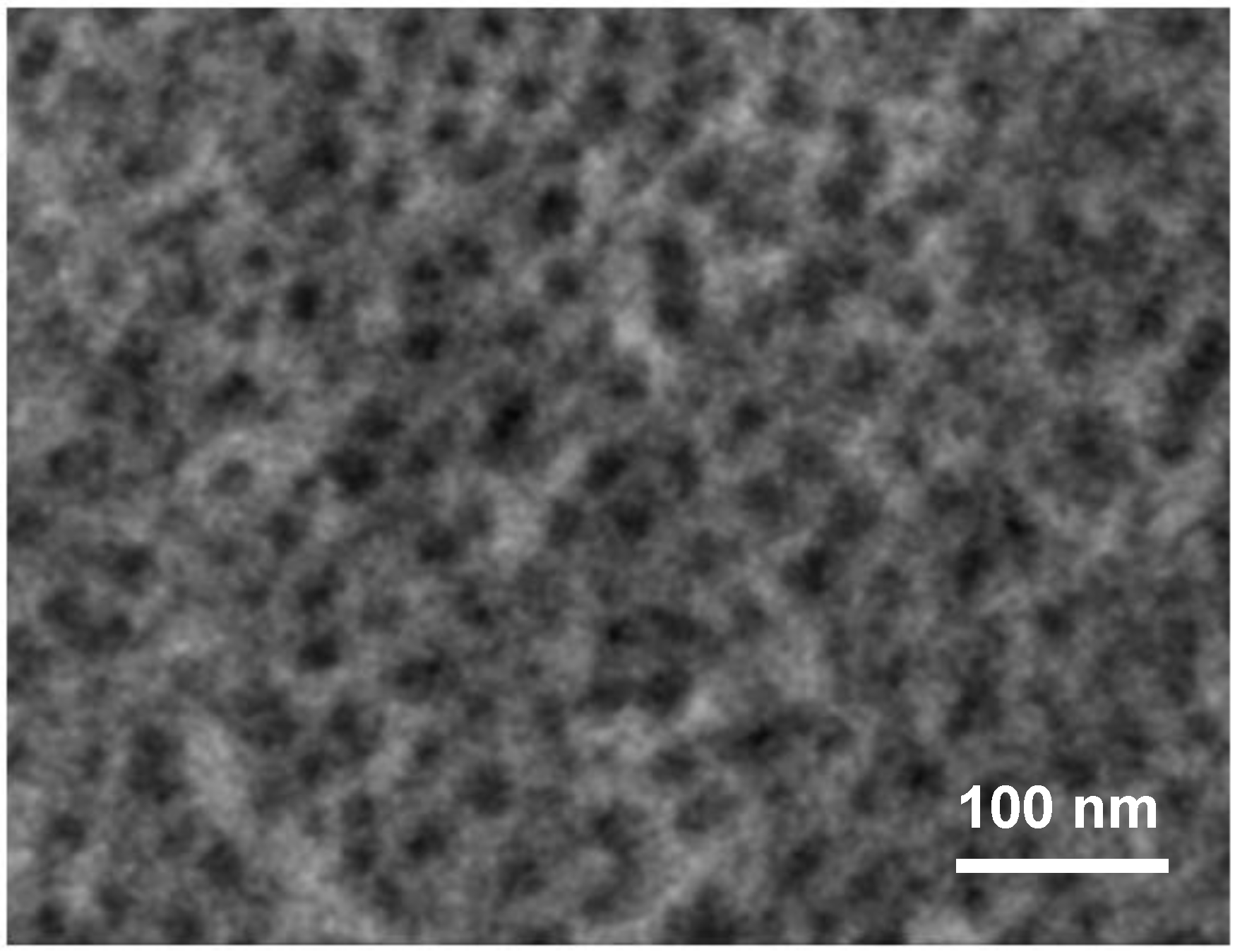
6.1. Observation of the Stratified Structure

6.2. Origin of the Stratified Structure
- -
- -
- The stacking period in acidic medium, obtained from the oxide profile as a function of time. This profile was deduced from a quantitative analysis of infrared absorption data during oxide dissolution triggered at various times of the oscillation period, giving evidence for successive layers of a slowly-dissolving oxide and a fast-dissolving one [35].
- -

6.3. Why a Stratified Structure rather than Nanopores or Nanotubes?
7. Conclusion and Perspectives
Acknowledgments
References
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Jessensky, O.; Müller, F.; Gösele, U. Self-organized formation of hexagonal pore structures in anodic alumina. J. Electrochem. Soc. 1998, 145, 3735–3740. [Google Scholar] [CrossRef]
- Beranek, R.; Hildebrand, H.; Schmuki, P. Self-organized porous titanium oxide prepared in H2SO4/HF electrolytes. Electrochem. Solid State Lett. 2003, 6, B12–B14. [Google Scholar]
- Macak, J.M.; Schmuki, P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim. Acta 2006, 52, 1258–1264. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Schmuki, P. Self-organized high aspect ratio porous hafnium oxide prepared by electrochemical anodization. Electrochem. Commun. 2005, 7, 49–52. [Google Scholar]
- Lee, W.-J.; Smyrl, W.H. Zirconium oxide nanotubes synthesized via direct electrochemical anodization. Electrochem. Solid State Lett. 2005, 8, B7–B9. [Google Scholar]
- Sieber, I.V.; Schmuki, P. Porous tantalum oxide prepared by electrochemical anodic oxidation. J. Electrochem. Soc. 2005, 152, C639–C644. [Google Scholar] [CrossRef]
- Choi, J.; Lim, J.H.; Lee, S.C.; Chang, J.H.; Kim, K.J.; Cho, M.A. Porous niobium oxide films prepared by anodization in HF/H3PO4. Electrochim. Acta 2006, 51, 5502–5507. [Google Scholar]
- Yang, M.; Shrestha, N.K.; Schmuki, P. Thick porous tungsten trioxide films by anodization of tungsten in fluoride containing phosphoric acid electrolyte. Electrochem. Commun. 2009, 11, 1908–1911. [Google Scholar] [CrossRef]
- Miyata, H.; Suzuki, T.; Fukuoka, A.; Sawada, T.; Watanabe, M.; Noma, T.; Takada, K.; Mukaide, T.; Kuroda, K. Silica films with a single-crystalline mesoporous structure. Nature Mater. 2004, 3, 651–656. [Google Scholar] [CrossRef]
- Revesz, A.G. Anodic oxidation of silicon in KNO3–N–methylacetamide solution: Interface properties. J. Electrochem. Soc. 1967, 114, 629–632. [Google Scholar] [CrossRef]
- Gaspard, F.; Halimaoui, A.; Sarrabayrouse, G. Electrical properties of thin anodic silicon dioxide layers grown in pure water. Rev. Phys. Appl. 1987, 22, 65–69. [Google Scholar]
- Properties of Porous Silicon; Canham, L.T. (Ed.) INSPEC: London, UK, 1997.
- Memming, R.; Schwandt, G. Anodic dissolution of silicon in hydrofluoric acid solutions. Surf. Sci. 1966, 4, 109–124. [Google Scholar]
- Memming, R.; Schwandt, G. Potential distribution and formation of surface states at the silicon-electrolyte interface. Surf. Sci. 1966, 5, 97–110. [Google Scholar] [CrossRef]
- Gerischer, H.; Lübke, M. Electrolytic growth and dissolution of oxide layers on silicon in aqueous solutions of fluorides. Ber. Bunsenges. Phys. Chem. 1988, 92, 573–577. [Google Scholar] [CrossRef]
- Gershinskii, A.E.; Mironova, L.V. Behavior of silicon in aqueous HF solutions. Soviet Electrochem. 1989, 25, 1224–1230. [Google Scholar]
- Eddowes, M.J. Anodic dissolution of p- and n-type silicon: Kinetic study of the chemical mechanism. J. Electroanal. Chem. 1990, 280, 297–311. [Google Scholar] [CrossRef]
- Chazalviel, J.-N.; Etman, M.; Ozanam, F. A voltammetric study of the anodic dissolution of p-Si in fluoride electrolytes. J. Electroanal. Chem. 1991, 197, 533–540. [Google Scholar] [CrossRef]
- Zhang, X.G. Electrochemistry of Silicon and Its Oxide; Kluwer/Plenum: New York, NY, USA, 2001. [Google Scholar]
- Lehmann, V. Electrochemistry of Silicon; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- da Fonseca, C.; Ozanam, F.; Chazalviel, J.-N. In-situ infrared characterisation of the interfacial oxide during the anodic dissolution of a silicon electrode in a fluoride electrolyte. Surf. Sci. 1996, 365, 1–14. [Google Scholar] [CrossRef]
- Lharch, M.; Chazalviel, J.-N.; Ozanam, F.; Aggour, M.; Wehrspohn, R.B. In situ investigation of porous anodic films of silica. Phys. Status Solidi (a) 2003, 197, 39–45. [Google Scholar] [CrossRef]
- Frey, S.; Grésillon, B.; Ozanam, F.; Chazalviel, J.-N.; Carstensen, J.; Föll, H.; Wehrspohn, R.B. Self-organized macrostructures in anodically formed mesoporous silica. Electrochem. Solid State Lett. 2005, 8, B25–B29. [Google Scholar]
- Venkateswara-Rao, A.; Ozanam, F.; Chazalviel, J.-N. In-situ Fourier-transform electromodulated infrared study of porous silicon formation: Evidence for solvent effects on the vibrational linewidths. J. Electrochem. Soc. 1991, 138, 153–159. [Google Scholar] [CrossRef]
- Belaïdi, A.; Safi, M.; Ozanam, F.; Chazalviel, J.-N.; Gorochov, O. Surface chemistry during porous silicon formation in dilute fluoride electrolytes. J. Electrochem. Soc. 1999, 146, 2659–2664. [Google Scholar] [CrossRef]
- Föll, H. Properties of silicon-electrolyte junctions and their application to silicon characterization. Appl. Phys. A 1991, 53, 8–19. [Google Scholar] [CrossRef]
- Chazalviel, J.-N.; Ozanam, F.; Etman, M.; Paolucci, F.; Peter, L.M.; Stumper, J. The p-Si/fluoride interface in the anodic region: Damped and/or sustained oscillations. J. Electroanal. Chem. 1992, 327, 343–349. [Google Scholar] [CrossRef]
- Ozanam, F.; Chazalviel, J.-N. Resonant and Nonresonant behavior of the anodic dissolution of silicon in fluoride media: An impedance study. J. Electrochem. Soc. 1992, 139, 2491–2501. [Google Scholar] [CrossRef]
- Lewerenz, H.J.; Aggour, M. On the origin of photocurrent oscillation at Si electrodes. J. Electroanal. Chem. 1993, 351, 159–168. [Google Scholar] [CrossRef]
- Aggour, M.; Giersig, M.; Lewerenz, H.J. Interface condition of n-Si(111) during photocurrent oscillations in NH4F solutions. J. Electroanal. Chem. 1995, 383, 67–74. [Google Scholar] [CrossRef]
- Chazalviel, J.-N.; da Fonseca, C.; Ozanam, F. In-situ infrared study of the oscillating anodic dissolution of silicon in fluoride electrolytes. J. Electrochem. Soc. 1998, 145, 964–973. [Google Scholar] [CrossRef]
- Carstensen, J.; Prange, R.; Föll, H. A model for current-voltage oscillations at the silicon electrode and comparison with experimental results. J. Electrochem. Soc. 1999, 146, 1134–1140. [Google Scholar]
- Grzanna, J.; Jungblut, H.; Lewerenz, H.J. A model for electrochemical oscillations at the Si/electrolyte contact: Part I. Theoretical development. J. Electroanal. Chem. 2000, 486, 181–189. [Google Scholar] [CrossRef]
- Grzanna, J.; Jungblut, H.; Lewerenz, H.J. A model for electrochemical oscillations at the Si/electrolyte contact: Part II. Simulations and experimental results. J. Electroanal. Chem. 2000, 486, 190–203. [Google Scholar] [CrossRef]
- Foca, E.; Carstensen, J.; Föll, H. Modelling electrochemical current and potential oscillations at the Si electrode. J. Electroanal. Chem. 2007, 603, 175–202. [Google Scholar] [CrossRef]
- Grzanna, J.; Jungblut, H.; Lewerenz, H.J. Nano- and macropores in the model for current oscillations at the Si/electrolyte contact. Phys. Status Solidi (a) 2007, 204, 1245–1249. [Google Scholar] [CrossRef]
- Hassan, H.H.; Sculfort, J.L.; Etman, M.; Ozanam, F.; Chazalviel, J.-N. Kinetic and diffusional limitations to the anodic dissolution of p-Si in fluoride media. J. Electroanal. Chem. 1995, 380, 55–61. [Google Scholar] [CrossRef]
- Cattarin, S.; Frateur, I.; Musiani, M.; Tribollet, B. Electrodissolution of p-Si in acidic fluoride media-modeling of the steady state. J. Electrochem. Soc. 2000, 147, 3277–3282. [Google Scholar] [CrossRef]
- Lharch, M.; Aggour, M.; Chazalviel, J.-N.; Ozanam, F. Anodic dissolution and electroluminescence of p-Si at high potentials in fluoride media. J. Electrochem. Soc. 2002, 149, C250–C255. [Google Scholar] [CrossRef]
- Chazalviel, J.-N.; Ozanam, F.; Lharch, M.; Choi, J.; Wehrspohn, R.B. Controlled formation of thick anodic films of mesoporous silica. In Proceedings of 203rd ECS Meeting, Paris, France, 27 April–2 May 2003.
- Frey, S.; Keipert, S.; Chazalviel, J.-N.; Ozanam, F.; Carstensen, J.; Föll, H. Electrochemical formation of porous silica: Toward an understanding of the mechanisms. Phys. Status Solidi (a) 2007, 204, 1250–1254. [Google Scholar] [CrossRef]
- Chazalviel, J.-N. Ionic processes through the interfacial oxide in the anodic dissolution of silicon. Electrochim. Acta 1992, 37, 865–875. [Google Scholar] [CrossRef]
- Mende, G. Anodic oxidation of Silicon as a low-temperature passivation technique. In Semiconductor Micromachining; Campbell, S.A., Lewerenz, H.J., Eds.; Wiley: New York, NY, USA, 1998; Volume 2, pp. 263–312. [Google Scholar]
- Hoar, T.P.; Mott, N.F. A mechanism for the formation of porous anodic oxide films on aluminium. J. Phys. Chem. Solids 1959, 9, 97–99. [Google Scholar] [CrossRef]
- Garcia-Vergara, S.J.; Iglesias-Rubianes, L.; Blanco-Pinzon, C.E.; Skeldon, P.; Thompson, G.E.; Campestrini, P. Mechanical instability and pore generation in anodic alumina. Proc. Roy. Soc. A 2006, 462, 2345–2358. [Google Scholar] [CrossRef]
- Su, Z.X.; Zhou, W.Z. Formation mechanism of porous anodic aluminium and titanium oxides. Adv. Mater. 2008, 20, 3663–3667. [Google Scholar]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Amin, M.A.; Frey, S.; Ozanam, F.; Chazalviel, J.-N. Macromorphologies in electrochemically formed porous silica. Electrochim. Acta 2008, 53, 4485–4494. [Google Scholar] [CrossRef]
- Chazalviel, J.-N.; Cortès, R.; Maroun, F.; Ozanam, F. Stratified structure of anodically formed mesoporous silica. Phys. Status Solidi (a) 2009, 206, 1229–1234. [Google Scholar] [CrossRef]
- Chazalviel, J.-N.; Ozanam, F. Electrochemically formed porous silica as a template for metal electrodeposition. ECS Trans. 2010, 25(27), 131–137. [Google Scholar]
- Lehmann, V. On the origin of electrochemical oscillations at silicon electrodes. J. Electrochem. Soc. 1995, 143, 1313–1318. [Google Scholar] [CrossRef]
- Parkhutik, V.; Matveeva, E. Observation of new oscillatory phenomena during the electrochemical anodization of silicon. Electrochem. Solid State Lett. 1999, 2, 371–374. [Google Scholar] [CrossRef]
- Parkhutik, V.; Matveeva, E.; Perez, R.; Alamo, J.; Beltrán, D. Mechanism of large oscillations of anodic potential during anodization of silicon in H3PO4/HF solutions. Mater. Sci. Engin. B 2000, 69, 553–558. [Google Scholar] [CrossRef]
- Schefold, J.; Lincot, D.; Ambard, A.; Kerrec, O. The cyclic nature of corrosion of Zr and Zr–Sn in high-temperatuure water (633 K). A long-term in situ impedance spectroscopic study. J. Electrochem. Soc. 2003, 150, B451–B461. [Google Scholar] [CrossRef]
- Yang, M.; Shrestha, N.K.; Schmuki, P. Toward self-ordered silica nanotubes by electrochemical anodization of Si(100). Electrochem. Solid State Lett. 2010, 13, C25–C28. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/)
Share and Cite
Chazalviel, J.-N.; Ozanam, F. Electrochemically Formed Porous Silica. Materials 2011, 4, 825-844. https://doi.org/10.3390/ma4050825
Chazalviel J-N, Ozanam F. Electrochemically Formed Porous Silica. Materials. 2011; 4(5):825-844. https://doi.org/10.3390/ma4050825
Chicago/Turabian StyleChazalviel, Jean-Noël, and François Ozanam. 2011. "Electrochemically Formed Porous Silica" Materials 4, no. 5: 825-844. https://doi.org/10.3390/ma4050825
APA StyleChazalviel, J.-N., & Ozanam, F. (2011). Electrochemically Formed Porous Silica. Materials, 4(5), 825-844. https://doi.org/10.3390/ma4050825