Effect of Molecular Flexibility on the Nematic-to-Isotropic Phase Transition for Highly Biaxial Molecular Non-Symmetric Liquid Crystal Dimers
Abstract
:1. Introduction
2. Experimental Section
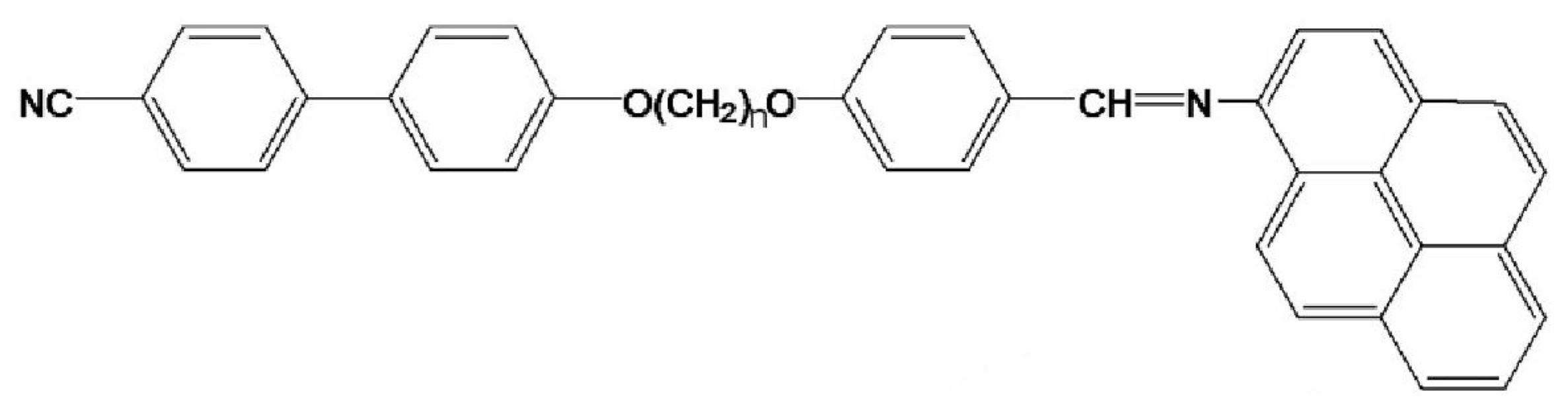
2.1. Liquid Crystal Materials
2.2. Specific Heat Measurements
2.3. Dielectric Measurements
3. Results and Discussion
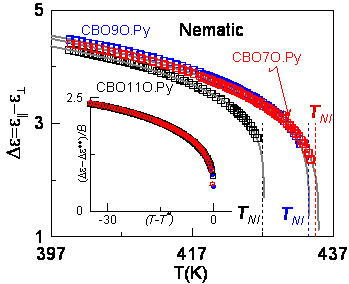
3.1. Specific Heat Measurements

| CBOnO.Py | TN–I (K) | ΔHN–I (kJ·mol−1) | Ref. |
|---|---|---|---|
| CBO11O.Py | 426.9 432.2 | 0.5 ± 0.1 1.8 | [46] [45] |
| CBO9O.Py | 431.95 434.15 | 0.33 ± 0.07 1.2 | This work [45] |
| CBO7O.Py | 433.35 437.15 | 0.32 ± 0.08 1.0 | This work [45] |
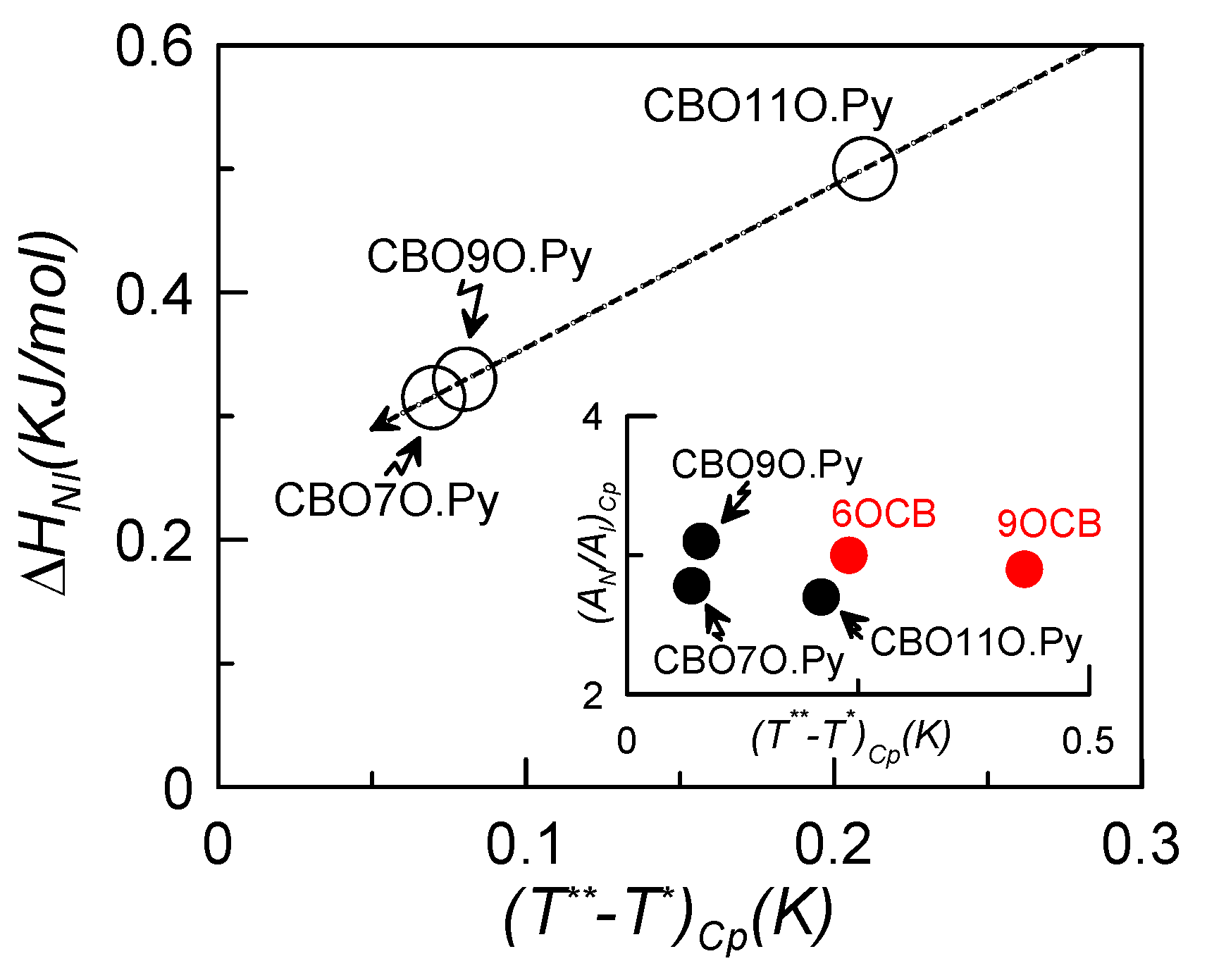
| α | T** − T* (K) | AN/AI | χ2 × 104 | Measurement | |
|---|---|---|---|---|---|
| CBO11O.Py | 0.51 ± 0.05 0.51 ± 0.05 | 0.21 0.42 | 2.7 ± 0.1 −0.90 ± 0.02 | 2 0.4 | Specific-heat Dielectrics |
| CBO9O.Py | 0.50 ± 0.02 0.5 ± 0.1 | 0.08 0.24 | 3.1 ± 0.1 −2.5 ± 0.1 | 1 1 | Specific-heat Dielectrics |
| CBO7O.Py | 0.50 ± 0.01 0.50 ± 0.01 | 0.07 1.53 | 2.8 ± 0.1 −1.70 ± 0.01 | 0.7 0.05 | Specific-heat Dielectrics |
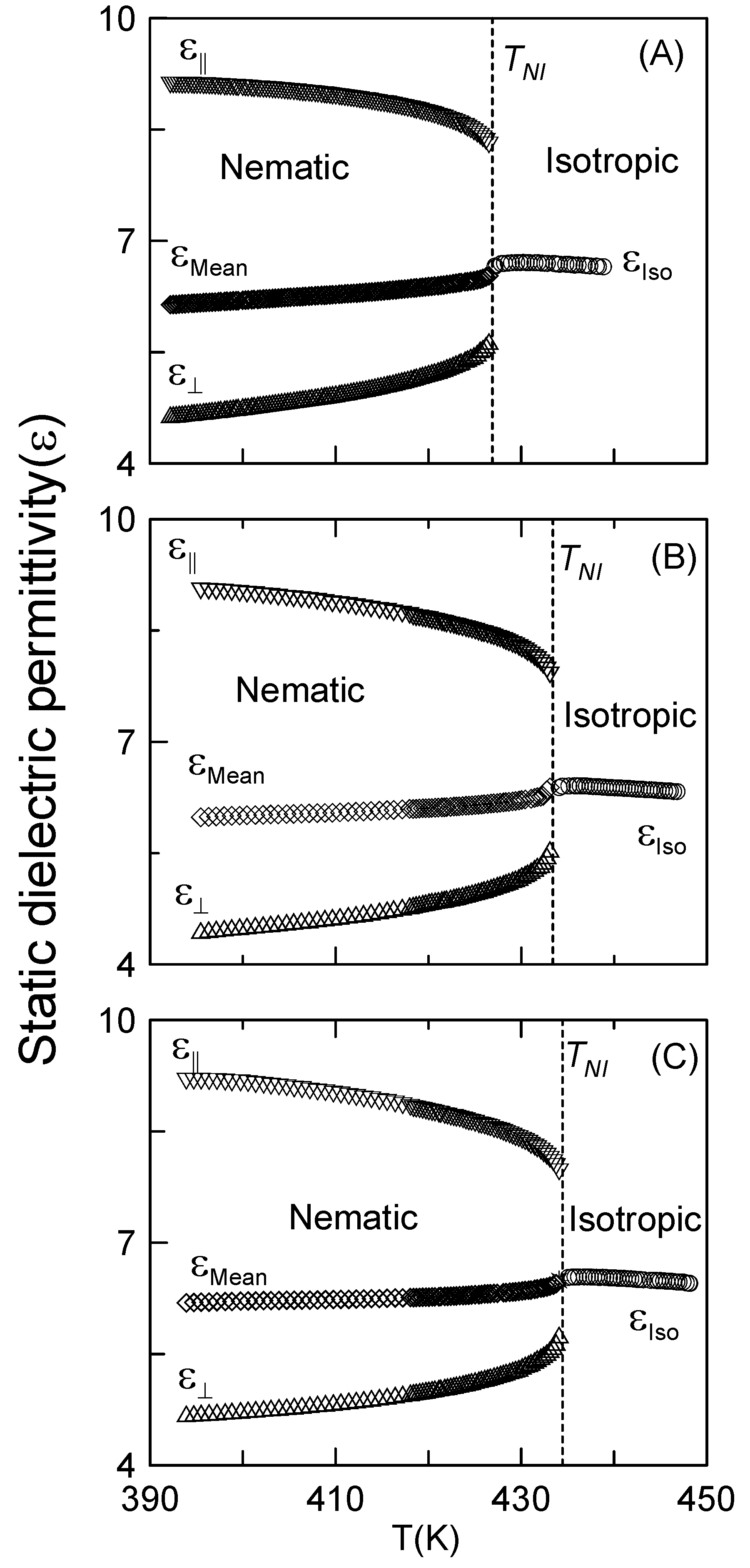
3.2. Dielectric Measurements
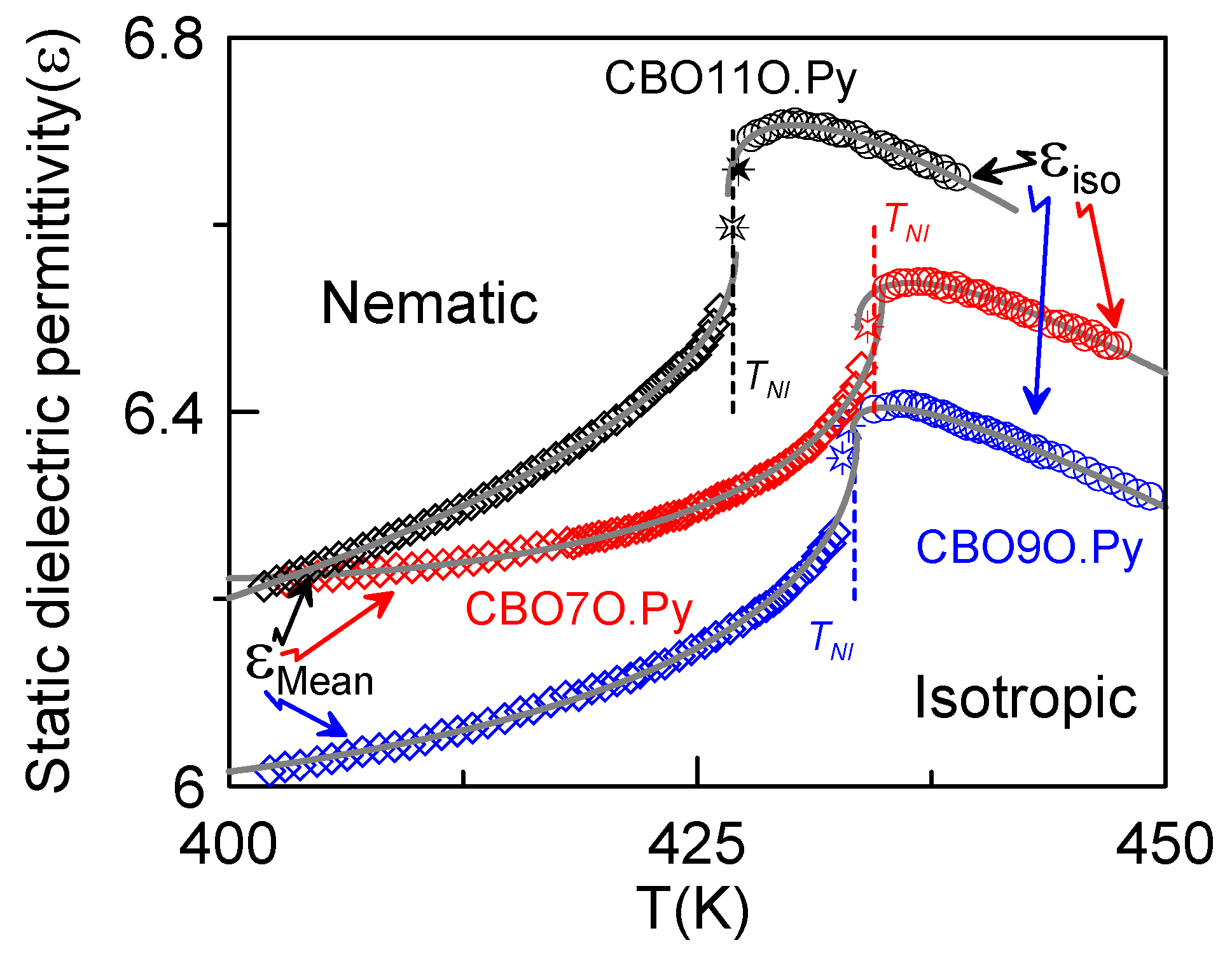
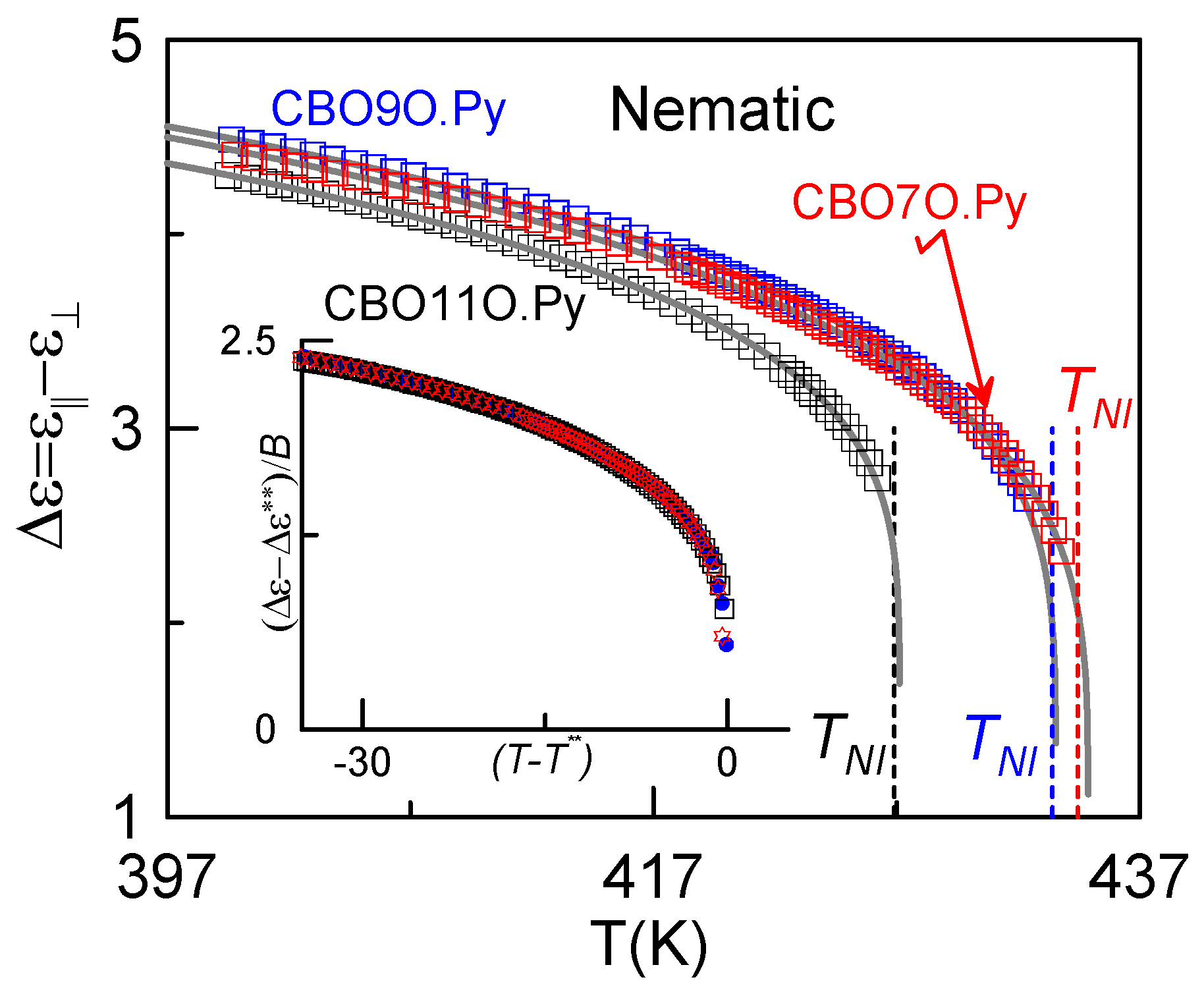
4. Conclusions
Acknowledgments
References
- Handbook of Liquid Crystals Vol. 1: Fundamentals; Demus, D.; Goodby, J.W.; Gray, G.W.; Spiess, H.W.; Vill, V. (Eds.) Wiley-VCH: Weinheim, Germany, 1998; Volume 1.
- Imrie, C.T.; Luckhurst, G.R. Liquid crystal dimers, oligomers. In Handbook of Liquid Crystals Vol. 2B: Low Molecular Weight Liquid Crystals; Demus, D., Goodby, J.W., Gray, G.W., Spiess, H.W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 1998; Volume 2, pp. 801–833. [Google Scholar]
- Ferrarini, A.; Luckhurst, G.R.; Nordio, P.L.; Roskilly, S.J. Understanding the unusual transitional behaviour of liquid crystal dimmers. Chem. Phys. Lett. 1993, 214, 409–417. [Google Scholar] [CrossRef]
- Tamba, M.G.; Kosata, B.; Pelz, K.; Diele, S.; Pelzl, G.; Vakhovskaya, Z.; Kresse, H.; Weissflog, W. Mesogenic dimers composed of a calamitic and a bent-core mesogenic unit. Soft Matte. 2006, 2, 60–65. [Google Scholar] [CrossRef]
- Dunmur, D.A.; Luckhurst, G.R.; de la Fuente, M.R.; Diez, S.; Pérez-Jubindo, M.A. Dielectric relaxation in liquid crystalline dimmers. J. Chem. Phys. 2001, 115, 8681–8690. [Google Scholar] [CrossRef]
- Stocchero, M.; Ferrarini, A.; Moro, G.J.; Dunmur, D.A.; Luckhurst, G.R. Molecular theory of dielectric relaxation in nematic dimmers. J. Chem. Phys. 2004, 121, 8079–8097. [Google Scholar] [CrossRef] [PubMed]
- Coles, H.J.; Pivnenko, M.N. Liquid crystal ‘blue phases’ with a wide temperature range. Nature 2005, 436, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Takanishi, Y.; Toshimitsu, M.; Nakata, M.; Takada, N.; Izumi, T.; Ishikawa, K.; Takezoe, H.; Watanabe, J.; Takahasi, Y.; Iida, A. Frustrated smectic layer structuresin bent-shaped dimer liquid crystalsas studied by X-ray microbeam diffraction. Phys. Rev. E 2006, 74, 051703:1–051703:10. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A. Liquid crystal dimers and higher oligomers: Between monomers and Polymers. Chem. Soc. Rev. 2007, 36, 2096–2124. [Google Scholar] [CrossRef] [PubMed]
- Sepelj, M.S.; Baumeister, U.; Diele, S.; Nguyen, H.L.; Bruce, D.W. Intercalated liquid-crystalline phases formed by symmetric dimers with an α,ω-diiminoalkylene spacer. J. Mat. Chem. 2007, 17, 1154–1165. [Google Scholar] [CrossRef]
- Ferrarini, A.; Greco, C.; Luckhurst, G.R. On the flexoelectric coefficients of liquid crystal monomers and dimers: A computational methodology bridging length-scales. J. Mater. Chem. 2007, 121, 1039–1042. [Google Scholar] [CrossRef]
- Aziz, N.; Kelly, S.M.; Duffy, W.; Goulding, M. Banana-shaped dopants for flexoelectric nematic mixtures. Liq. Cryst. 2008, 35, 1279–1292. [Google Scholar] [CrossRef]
- de la Fuente, M.R.; López, D.O.; Pérez-Jubindo, M.A.; Dunmur, D.A.; Diez-Berart, S.; Salud, J. Cylindrical sub-micrometer confinement results for the odd-symmetric dimer α,ω-bis[(4-cyanobiphenyl)-4’-yloxy]undecane (BCB.O11). J. Phys. Chem. B 2010, 114, 7864–7873. [Google Scholar] [CrossRef] [PubMed]
- Cestari, M.; Diez-Berart, S.; Dunmur, D.A.; Ferrarini, A.; de la Fuente, M.R.; Jackson, D.A.; López, D.O.; Luckhurst, G.R.; Pérez-Jubindo, M.A.; Richardson, R.M.; Salud, J.; Timini, B.A.; Zimmermann, H. The phase behaviour and properties of the liquid crystal dimer α,ω-bis[(4-cyanobiphenyl)-4’-yl]-heptane: A novel twist-bend nematic? Phys. Rev. E 2011, in press. [Google Scholar]
- Lagerwall, J.P.F.; Giesselmann, F.; Wand, M.D.; Walba, D.M. A chameleon chiral polar liquid crystal: Rod-shaped when nematic, bent-shaped when smectic. Chem. Mater. 2004, 16, 3606–3615. [Google Scholar] [CrossRef]
- Umadevi, S.; Sadashiva, B.K.; Murthy, H.N.; Raghunathan, V.A. Mesogenic dimers composed of bent-core molecules with flexible alkylene spacer. Soft Matter 2006, 2, 210–214. [Google Scholar] [CrossRef]
- Umadevi, S.; Sadashiva, B.K. Liquid crystal properties and dependence of transition temperatures on the length of the flexible alkylene spacer of symmetric dimers composed of bent-core units. Liq. Cryst. 2007, 34, 673–681. [Google Scholar] [CrossRef]
- Marcelja, S. Chain ordering in liquid crystals. I. Even-odd effect. J. Chem. Phys. 1974, 60, 3599–3604. [Google Scholar] [CrossRef]
- Emsley, J.W.; Luckhurst, G.R.; Shilstone, G.N. The orientational order of nematogenic molecules with a flexible core. A dramatic odd even effect. Mol. Phys. 1984, 53, 1023–1028. [Google Scholar] [CrossRef]
- Ferrarini, A.; Luckhurst, G.R.; Nordio, P.L.; Roskilly, S.J. Prediction of the transitional properties of liquid crystal dimers. A molecular-field calculation based on the surface tensor parametrization. J. Chem. Phys. 1994, 100, 1460–1469. [Google Scholar] [CrossRef]
- Luckhurst, G.R. The Marcelja-Luckhurst molecular-field for uniaxial nematics composed of flexible molecules. A variational derivation. Mol. Phys. 1994, 82, 1063–1073. [Google Scholar] [CrossRef]
- Luckhurst, G.R.; Romano, S. Computer simulation studies of anisotropic systems. 26. Liquid crystal dimers: A generic model. J. Chem. Phys. 1997, 107, 2557–2572. [Google Scholar] [CrossRef]
- Berardi, R.; Zannoni, C. Do thermotropic biaxial nematics exist? A montecarlo study of biaxial Gay-Berne particles. J. Chem. Phys. 2000, 113, 5971–5979. [Google Scholar] [CrossRef]
- Luckhurst, G.R. Biaxial nematic liquid crystals: Fact or a fiction? Thin Solid Films 2001, 393, 40–52. [Google Scholar] [CrossRef]
- Galerne, Y. Comment on “Thermotropic biaxial nematic liquid crystals”. Phys. Rev. Lett. 2006, 96, 219803. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.A.; Dingemans, T.J.; Nakata, M.; Samulsky, E.T. Comment on “Thermotropic biaxial nematic liquid crystals”. Phys. Rev. Lett. 2006, 96, 219804. [Google Scholar] [CrossRef]
- Luckhurst, G.R. Liquid crystals. A missing phase found at last? Nature 2004, 430, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Luckhurst, G.R. V-shaped molecules: New contenders for the biaxial nematic phase. Angew. Chem. Int. Ed. 2005, 44, 2834–2836. [Google Scholar] [CrossRef]
- Luckhurst, G.R. Biaxial nematics composed of flexible molecules: A molecular field theory. Liq. Cryst. 2009, 36, 1295–1308. [Google Scholar] [CrossRef]
- Maier, W.; Saupe, A. Eine einfache molekulare theorie des nematischen kristallinflussigen zustandes. Z. Naturforsch. 1958, 13, 564–566. [Google Scholar]
- Maier, W.; Saupe, A.Z. Eine einfache molekular-statistische theorie der nematischen kristallinflussigen phase.1. Naturforsch 1959, 14, 882–889. [Google Scholar]
- Maier, W.; Saupe, A.Z. Eine einfache molekular-statistische theorie der nematischen kristallinflussigen phase. 2. Naturforsch 1960, 15, 287–292. [Google Scholar]
- Mukherjee, P.K.; Mukherjee, T.B. Critical region of the nematic-isotropic phase-transition in the epsilon expansion. Phys. Rev. B 1995, 52, 9964–9969. [Google Scholar] [CrossRef]
- Wang, Z.H.; Keyes, P.H. Critical and multicritical fluctuations of nematic liquid crystals. Phys. Rev. E 1996, 54, 5249–5262. [Google Scholar] [CrossRef]
- Mukherjee, P.K. The TN–I − T* puzzle of the nematic-isotropic phase transition. J. Phys. 1998, 10, 9191–9205. [Google Scholar]
- Freiser, M.J. Ordered states of a nematic liquid. Phys. Rev. Lett. 1970, 24, 1041–1043. [Google Scholar] [CrossRef]
- Boccara, N.; Mejdani, R.; de Seze, L. Solvable model exhibiting a 1st order phase-transition. J. Phys. 1977, 38, 149–151. [Google Scholar] [CrossRef]
- De Gennes, P.G. The Physics of Liquid Crystals; Oxford Science Publications: Oxford, UK, 1994. [Google Scholar]
- Anisimov, M.A. Critical Phenomena in Liquids and Liquid Crystals; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 1991. [Google Scholar]
- De Matteis, G.; Virga, E.G. Tricritical points in biaxial liquid crystal phases. Phys. Rev. E 2005, 71, 061703:1–061703:8. [Google Scholar] [CrossRef]
- Bisi, F.; Romano, S.; Virga, E.V. Uniaxial rebound at the nematic biaxial transition. Phys. Rev. E 2007, 75, 041705:1–041705:11. [Google Scholar] [CrossRef]
- Allender, D.; Longa, L. Landau-de Gennes theory of biaxial nematics re-examined. Phys. Rev. E 2008, 78, 011704:1–011704:11. [Google Scholar] [CrossRef]
- Tschierske, C.; Photinos, D.J. Biaxial nematic phases. J. Mat. Chem. 2010, 20, 4263–4294. [Google Scholar] [CrossRef]
- Salud, J.; Cusmin, P.; de la Fuente, M.R.; Pérez-Jubindo, M.A.; López, D.O.; Diez-Berart, S. Study of the critical behavior and scaling relationships at the N–to–I phase transition in hexyloxycyanobiphenyl. J. Phys. Chem. B 2009, 113, 15967–15974. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.S.; Imrie, C.T. Low molar mass liquid-crystalline glasses: Preparation and properties of the α-(4-Cyanobiphenyl-4’-oxy-) ω-(1 -pyreniminebenzylidene-4’-oxy)alkanes. Chem. Mater. 1992, 4, 1246–1253. [Google Scholar] [CrossRef]
- Sebastián, N.; de la Fuente, M.R.; López, D.O.; Pérez-Jubindo, M.A.; Salud, J.; Diez-Berart, S.; Ros, M.B. Dielectric and thermodynamic study of the liquid cristal dimer α-(4-cyanobiphenyl-4’-oxy)-ω-(1-pyreniminebenzylidene-4’-oxy) undecane (CBO11O.Py). J. Phys. Chem. B 2011, 115, 9766–9775. [Google Scholar] [CrossRef] [PubMed]
- Sied, M.B.; Salud, J.; López, D.O.; Barrio, M.; Tamarit, J.L. Binary mixtures of nCB and nOCB liquid crystals. Two experimental evidences for a smectic A-nematic tricritical point. Phys. Chem. Chem. Phys. 2002, 4, 2587–2593. [Google Scholar] [CrossRef]
- Puertas, R.; Rute, M.A.; Salud, J.; López, D.O.; Diez, S.; Van Miltenburg, J.C.; Pardo, L.C.; Tamarit, J.L.; Barrio, M.; Pérez-Jubindo, M.A.; de la Fuente, M.R. Thermodynamic, crystallographic, and dielectric study of the nature of glass transitions in cyclo-octanol. Phys. Rev. B 2004, 69, 224202:1–224202:9. [Google Scholar] [CrossRef]
- Cusmin, P.; de la Fuente, M.R.; Salud, J.; Pérez-Jubindo, M.A.; Diez-Berart, S.; López, D.O. Critical behavior and scaling relationships at the SmAd–N and N–I transitions in nonyloxycyanobiphenyl (9OCB). J. Phys. Chem. B 2007, 111, 8974–8984. [Google Scholar] [CrossRef] [PubMed]
- Keyes, P.H.; Shane, J.R. Tricritical exponents for the isotropic-nematic transition: Experimental-verification. Phys. Rev. Lett. 1979, 42, 722–725. [Google Scholar] [CrossRef]
- Cordoyiannis, G.; Apreutesei, D.; Mehl, G.H.; Glorieux, C.; Thoen, J. High-resolution calorimetry study of a liquid crystalline organo-siloxane tetrapode with a biaxial nematic phase. Phys. Rev. E 2008, 78, 011708:1–011708:5. [Google Scholar] [CrossRef]
- Merkel, K.; Kocot, A.; Vij, J.K.; Korlacki, R.; Mehl, G.H.; Meyer, T. Thermotropic biaxial nematic phase in liquid crystalline organo-siloxane tetrapodes. Phys. Rev. Lett. 2004, 93, 237801:1–237801:4. [Google Scholar] [CrossRef]
- Sebastián, N.; de la Fuente, M.R.; López, D.O.; Pérez-Jubindo, M.A.; Salud, J.; Diez-Berart, S.; Ros, M.B. Dielectric studies of the odd liquid cristal dimers α-(4-cyanobiphenyl-4’-oxy)-ω-(1-pyreniminebenzylidene-4’-oxy) alkanes (CBOnO.Py). Unpublished results. 2011. [Google Scholar]
- Rzoska, S.J.; Ziolo, J.; Sulkowski, W.; Jadzyn, J.; Czechowski, G. Fluidlike behavior of dielectric permittivity in a wide range of temperature above and below the nematic-isotropic transition. Phys. Rev. E 2001, 64, 052701:1–052701:4. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastián, N.; López, D.O.; Diez-Berart, S.; De la Fuente, M.R.; Salud, J.; Pérez-Jubindo, M.A.; Ros, M.B. Effect of Molecular Flexibility on the Nematic-to-Isotropic Phase Transition for Highly Biaxial Molecular Non-Symmetric Liquid Crystal Dimers. Materials 2011, 4, 1632-1647. https://doi.org/10.3390/ma4101632
Sebastián N, López DO, Diez-Berart S, De la Fuente MR, Salud J, Pérez-Jubindo MA, Ros MB. Effect of Molecular Flexibility on the Nematic-to-Isotropic Phase Transition for Highly Biaxial Molecular Non-Symmetric Liquid Crystal Dimers. Materials. 2011; 4(10):1632-1647. https://doi.org/10.3390/ma4101632
Chicago/Turabian StyleSebastián, Nerea, David Orencio López, Sergio Diez-Berart, María Rosario De la Fuente, Josep Salud, Miguel Angel Pérez-Jubindo, and María Blanca Ros. 2011. "Effect of Molecular Flexibility on the Nematic-to-Isotropic Phase Transition for Highly Biaxial Molecular Non-Symmetric Liquid Crystal Dimers" Materials 4, no. 10: 1632-1647. https://doi.org/10.3390/ma4101632