Abstract
The last five years’ achievements in the synthesis and investigation of thermotropic ionic liquid crystals are reviewed. The present review describes the mesomorphic properties displayed by organic, as well as metal-containing ionic mesogens. In addition, a short overview on the ionic polymer and self-assembled liquid crystals is given. Potential and actual applications of ionic mesogens are also discussed.
1. Introduction
The liquid crystalline state is a state of matter in which orientational order is maintained, but, similarly to liquids, gases and amorphous solids, positional order in the molecular arrangement is lost [1]. Nowadays, materials forming liquid crystalline phases (mesophases) have found wide applications in the manufacturing of displays [2], spatial light modulators [3], optical connectors and switches [4], molecular sensors and detectors [5,6], and in many other topics [7]. A thermotropic mesophase is formed by heating a solid or cooling an isotropic liquid (or another mesophase), while lyotropic mesophases are prepared by dissolving an amphiphilic mesogen (a compound that displays liquid crystalline behavior) in suitable solvents, under appropriate conditions of concentration and temperature [1]. The conventional design of mesogenic molecules, exhibiting liquid crystalline (mesomorphic) properties, could be modified by an introduction of ionic groups, leading to ionic liquid crystals [8]. Actually, ionic liquid crystals are closely related to ionic liquids, which have attracted a growing interest as solvents with easily tunable physical and chemical properties [9]. Due to the presence of ionic units in the mesophase, the typical feature of ionic liquid crystals is ion conductivity, and this phenomenon can be used for the construction of materials with anisotropic electric current conductivity (see below) [10]. In general, ionic interactions tend to stabilize lamellar mesophases, due to an ion-ion stacking and electrostatic interactions [11]. This has already recently been observed for ionic liquids. Detailed small angle neutron scattering of N-alkyl-N-methylimidazolium-based hexafluorophosphate ionic liquids displayed the presence of local anisotropy in the bulk, isotropic and ionic liquid phases [12]. The strength and intensity of the diffraction peak correlated with the length of the alkyl substituent in the imidazolium cation. Therefore, it has been concluded that there is still no long-range molecular aggregation and local order (ca. 1-2 Å) results from increasing anisotropy of the long-chain-substituted amphiphilic imidazolium cation [12]. Ujiie and coworkers have obtained the first experimental evidence, showing a significant stabilization of the thermotropic ionic mesophase, compared with a conventional LC (liquid crystalline) molecular arrangement [13]. They observed a slight decrease of the melting points and, at the same time, strong increase of the clearing points of the azobenzene-derived ammonium mesogens over their neutral analogs [13]. A similar phenomenon was recently described for triphenylene-based ionic systems. Incorporation of imidazolium ion functionalities into the paraffinic side-chain termini of a triphenylene derivative resulted in the stabilization of the columnar mesophase (see below) [14]. Another unique feature of the liquid crystals, formed by ionic amphiphilic molecules, is their spontaneous homeotropic alignment on a glass surface. It has been claimed that this spontaneous self-organization of the ionic mesophase is caused by interactions between cationic head groups and the surface and by an arrangement of hydrophobic tails, thus creating a monodomain of similarly oriented molecules of the ionic mesogen [15].
According to the type of order by which molecules are arranged in the liquid crystalline state, several mesophase types can be distinguished: nematic (N), smectic (Sm), columnar (Col) and cubic (Cub) [16,17]. A nematic (N) mesophase is usually formed by rod-like (calamitic) molecules. In the nematic type of molecular organization, molecules are arranged in random positional, but directionally correlated order. They are aligned in a general direction, defined by a unit vector ñ, the so-called director axis (A, Scheme 1). Chiral calamitic structures form chiral nematic (N*) mesophases, in which molecules are arranged in a helical manner (B, Scheme 1). In smectic order, calamitic molecules are organized in lamellar supramolecular assemblies and all oriented along the vector ñ. There are several types of smectic mesophases. In a smectic A arrangement (SmA), molecules are assembled in layers. In a layer, molecules are positionally random, but directionally ordered with their long axes normal to the plane of the layer (C, Scheme 1). In a smectic C phase (SmC), molecules have the same lamellar arrangement like in a SmA phase, but the vector ñ is tilted to the plane of a layer (D, Scheme 1) [16,17]. In an ordered smectic B (SmB) phase, there is short-range positional order within the layer. The neighbor molecules are arranged in six-fold bond-orientational order, which is lost within few intermolecular distances (E, Scheme 1).
In the conventional design, the ionic amphiphilic mesogenic molecule consists of a positively charged cationic head and long-chain hydrophobic alkyl substituent. The driving forces, leading to the formation and stabilization of the ionic mesophase, in a first approximation, are cation-cation repulsion, the Van-der Waals hydrophobic interactions between long alkyl tails and hydrogen bonding between anions and cations [18]. Microsegregation of incompatible units, aggregation of compatible units and the minimization of volume in bulk of ionic mesogene lead at suitable temperature conditions to appearance of a lamellar thermotropic ionic mesophase [11]. Due to a combination of these repulsive and attractive electrostatic forces, Van-der Waals hydrophobic interactions and hydrogen bonding contacts, unique smectic type molecular arrangements are found in ionic liquid crystals. A smectic T arrangement consists of specific order, exhibited by ammonium mesogens (see below) and characterized by tetragonal layers, separated by hydrophobic long alkyl chains (F, Scheme 1). A bilayered SmA2 mesophase is typical for phosphonium salts (see below). In a SmA2 phase, cations and anions are assembled in bilayers, separated by a double layer of alkyl chains (G, Scheme 1). The cubic phases (Cub) are molecular arrangements, having cubic symmetry (see below).
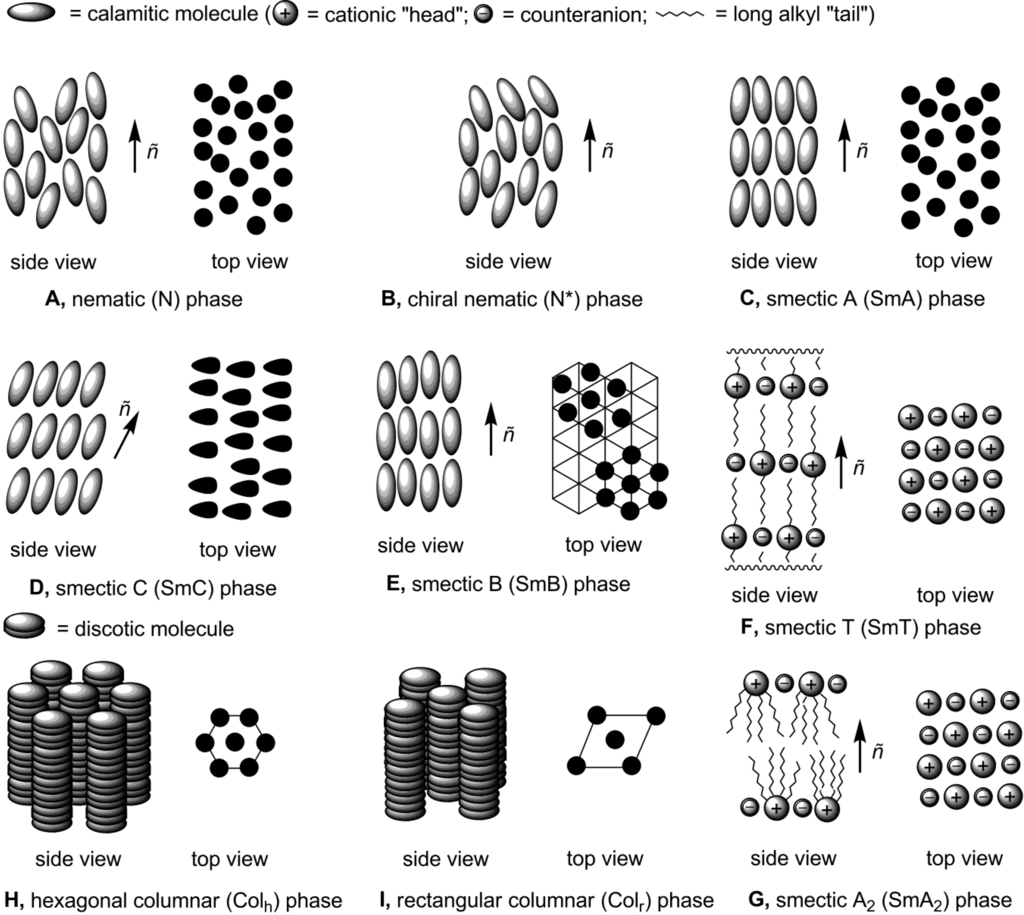
Scheme 1.
Discotic molecules usually form columnar mesophases. In this supramolecular organization, molecules are stacked on top of each other into columns, which are arranged either in hexagonal Colh or rectangular Colr order (H and I, Scheme 1, respectively). The discotic molecules can also form nematic phases: a nematic D phase, with short molecular axes oriented in one preferential direction, and a columnar nematic phase (Ncol), in which molecules are stacked into columns, arranged in nematic order [11].
Until today, there is no conventional theory that could predict, starting from a known structure of a mesogenic ionic liquid, which type of mesophase this compound would form, and how stable this mesophase would be. Recently, employing density functional theory, a new theoretical approach has been developed, attempting to explain the influence of the anisotropic charge distribution on mesophase stability in ionic liquid crystals [19]. Ionic mesogenic molecules have been represented as ellipsoidal particles with length L, width R and charges located in the center or on the tails (with distance D from the center of the molecule, Scheme 2). Attractive interactions were taken into account in terms of the Gay-Berne pair potential. Finally, it has been concluded that nematic order should be stable for the molecules, in which the single charge is located in the center or two like charges are each positioned in a distance D = 1.4 R from the center (Scheme 2). Stability of the nematic phase could be improved with an increase in strength of electrostatic Coulomb interactions. When D = R, the phase with smectic A molecular arrangement is only stable. In certain cases, increasing the length of a charged particle could have a stabilizing effect on the smectic A phase. Moreover, it must lead to growth of the layer spacing in the smectic A phase. The developed methodology provided theoretical insight into the underlying mechanisms responsible for the formation of bulk liquid crystalline phases from ionic liquids [19].
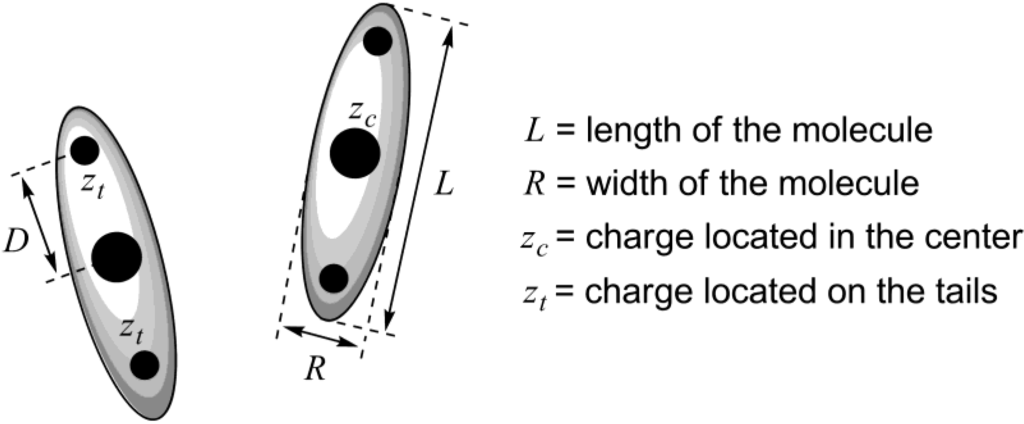
Scheme 2.
A detailed review on ionic liquid crystals has been published by Binnemans in 2005 [11]. In this respect, our main goal for this contribution was to shed light upon further developments of the topic during the last five years. Despite such a relatively short period, a literature search revealed an enormous amount of publications dealing with ionic mesogens. In order to keep our review concise, we chose to focus here on the properties of thermotropic ionic mesogens, leaving out lyotropic ionic liquid crystals. Excellent reviews on the topic of lyotropic mesogens can be found elsewhere [20].
In our Schemes, we used conventional notation of thermotropic liquid crystalline properties. Phase symbol is followed by the upper temperature limit, as measured during the heating and not during the cooling cycle. For selected examples, temperatures of enantiotropic phase transitions are given. Monotropic transitions, which could be observed only upon cooling, appear in parenthesis. The isotropic liquid state is indicated with the symbol (I). The melting point refers to the temperature, when the crystalline phase melts into the mesophase (or directly into the isotropic liquid). The clearing point is the temperature of the transition between the mesophase and the isotropic liquid [1].
Due to the huge variety of mesogens considered in this review, it was rather difficult to create a unified numbering system. The basic structures are numbered through the text and Schemes by conventional arabic numbers. If an alkyl substituent with n carbon atoms is attached to this basic framework, it is displayed by coefficient right underline after the number of the structure. The counteranion is shown by its formula (with the negative charge omitted for clarity) in square brackets right after the number of the basic core (or n coefficient). For example, 212[Br] means that this compound has the basic structure 2 (Scheme 3), which bears C12H25-side-chains and has Br– as a counteranion. For dendrimeric mesogens, the coefficient after the brackets displays a ratio between an amine dendrimeric core and an acidic second component in a self-assembled system (see Scheme 24).
2. Ammonium-Based Mesogens
Ammonium salts are simple amphiphilic molecules consisting of a substituted hydrophilic cationic nitrogen center and a hydrophobic hydrocarbon long-chain tail. Since synthesis of ammonium salts is simple and could easily be carried out from the corresponding amines via nucleophilic quaternization with alkyl halides [21,22], ammonium-based ILC (ionic liquid crystalline) compounds have been known for a long time. They form thermotropic or, in combination with a solvent, lyotropic mesophases. In addition, ammonium salts are widely used to build up a large variety of ionic self-assembled ordered structures (see below). In the absence of solvent, ammonium salts, in general, exhibit smectic ordered thermotropic mesophases [11]. One of the main reasons is that most of the popular ILCs are linear molecules consisting of a single ionic head group, connecting with one or multiple long aliphatic tails. It is generally accepted that the microsegregation of incompatible units, the aggregation of compatible units and the minimization of volume are the main driving forces that give rise to a general tendency for lamellar or columnar structures.
Scheme 3.
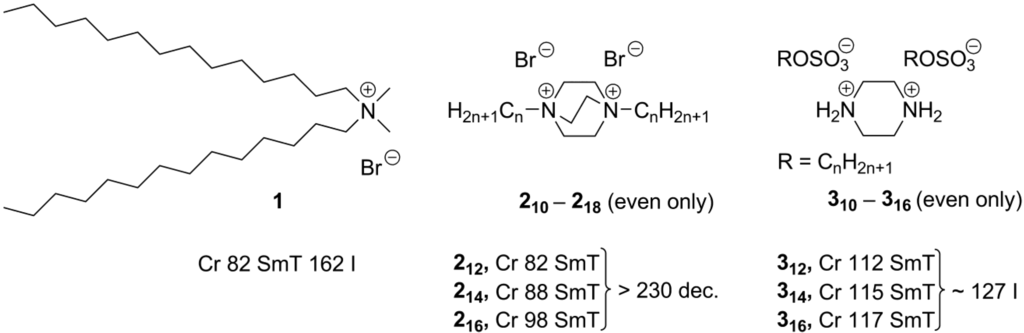
Recently, for ionic ammonium compounds a new type of liquid crystalline order—smectic T phase—has been reported [23]. In a smectic T phase, ammonium head groups and halide counteranions are packed into tetragonal lattices separated from each other by randomly oriented long alkyl tails (F, Scheme1).Typically, the molecules with two long alkyl chain substituents exhibit such smectic T phases (1–3, Scheme 3) [23,24,25].
New pyrrolidinium-based mesogens 48-420 (Scheme 4) have been prepared by quaternization (Menschutkin reaction) of 1-methylpyrrolidine with long alkyl chain bromides [26,27]. The bromide anions can further be exchanged by noncoordinated or complex metal-containing counteranions. The pyrrolidinium compounds 48-420 show rich mesomorphism, depending on the length of the N-alkyl substitutuent and size of the counteranion. They melted into a LC phase in a range of 27–92 °C, and clearing points were observed between 171 and 267 °C. It was found that a minimum alkyl chain length of 11 carbon atoms is required for the pyrrolidinium bromide salts to exhibit mesomorphism. The tetrabromouranyl salts 58-520, with a chain length of at least 14 carbon atoms, showed liquid crystalline SmE and SmA phases, while no liquid crystalline behavior was observed for the compounds 6[NTf2] and 6[Eu(tta)4], containing NTf2– (NTf2– = N(SO2CF3)2–) or Eu(tta)4– anions, respectively (Scheme 4) [26].
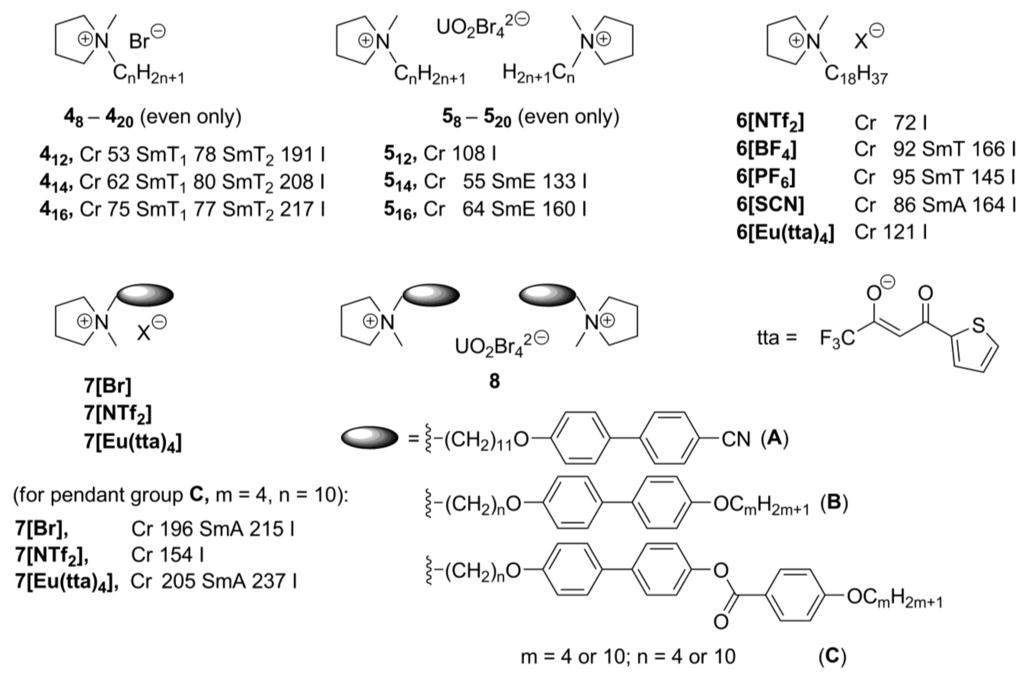
Scheme 4.
In the further improvement of the basic N-alkyl pyrrolidinium structure, mesogenic pendant biphenyl-derived groups were connected with the pyrrolidinium moiety via a flexible alkyl link (7[Br], 7[NTf2], 7[Eu(tta)4], Scheme 4). Except the pyrrolidinium derivative 7[NTf2], bearing NTf2– anions, these compounds feature a wide range of low and high ordered smectic phases (SmA, SmC as well as E, G, J, H, or K) [27]. The salts, which bear longer spacer and terminal alkyl substituent, showed lower melting points than their short-chain analogs. The europium-containing salts 7[Eu(tta)4] were not liquid-crystalline. It has been stated that the Eu(tta)4– anion is perhaps too bulky to be efficiently counterbalanced by the mesogenic units that were used [27]. However, by mixing the europium-containing salts with their bromide analogs 7[Br], luminescent liquid-crystalline mixtures could be obtained. The related pyrrolidinium systems have been recently investigated with the purpose to enhance electrochemical stability of an ionic liquid crystalline molecular arrangement. These new salts exhibited rich mesomorphism and formed mesophases that were stable over a wide range of temperatures [28].
In order to explore whether ionic liquid crystals based on other aliphatic heterocycles could exhibit unusual mesophases, piperidinium, piperazinium, and morpholinium-based salts 8–11 (Scheme 5) were investigated [29]. It was also attempted to lower the melting points of the compounds to such an extent that they might exhibit mesophases at ambient temperatures. Consequently, sulfosuccinates, substituted with long alkyl chains, were combined with cationic ammonium mesogenic cores, apart from the more classical anions, like Br–, BF4–, PF6–, or NTf2– (Scheme 5). Diverse mesophases have been observed for these compounds. The piperidinium salts 8[X] and 9[X] (X = Br–, BF4– or PF6–), bearing one or two long alkyl groups (Scheme 5), feature SmT or SmE/SmT phases, while salts 814[X] and 9[X] (X = DOS sulfate anion, Scheme 5) formed hexagonal columnar phases around 100 °C. In general, morpholinium-based structures behaved similarly and exhibited SmE and/or SmT phases (at higher temperatures less ordered SmA) [18]. All morpholinium-sulfosuccinate salts 10[SU] (Scheme 5)showed hexagonal columnar phases already at room temperature and had clearing points between 120 and 147 °C. For the piperazinium-based compounds 11, only those, bearing sulfosuccinate anions, displayed liquid crystalline properties and most of them formed smectic phases (SmA or SmE and SmT) at temperatures below 100 °C [29].
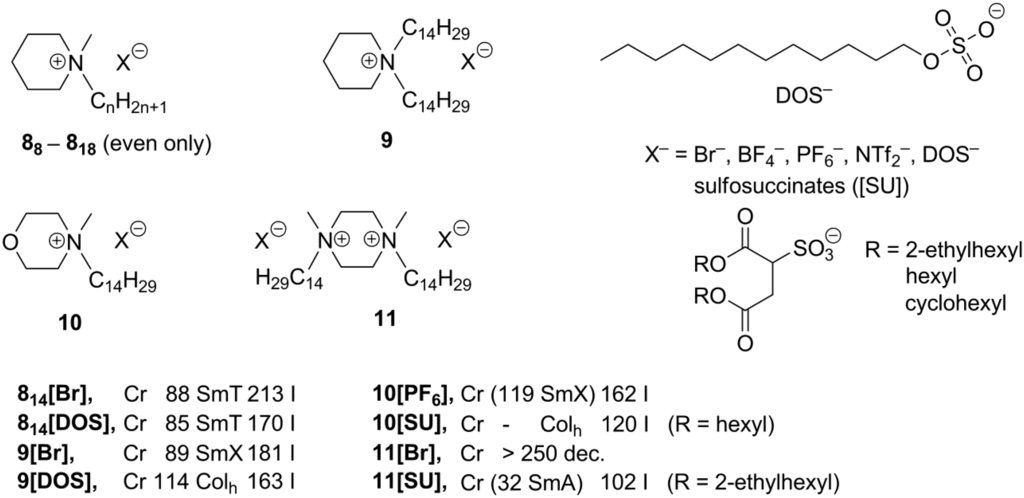
Scheme 5.
Recently, new mesogenic ammonium salts 126-1212 (Scheme 6), containing a calamitic azobenzene core with lateral substituents, have been reported [30]. Very unusual for ammonium mesogens, the derivatives 126 and 128 displayed a nematic mesophase. In order to realize nematic ILCs, a series of branched (close to T-shaped) quaternary ammonium salts have been designed, by connecting an azobenzene unit with a benzoate link to a lateral ammonium bromide group (126-1212, Scheme 6). Depending on the length of a terminal alkyl group, the compounds 126-1212 formed nematic (n = 6, 8) or SmA (n = 10, 12) mesophases with melting points between 23 and 32 °C and clearing points in the range of 36–92 °C [30].
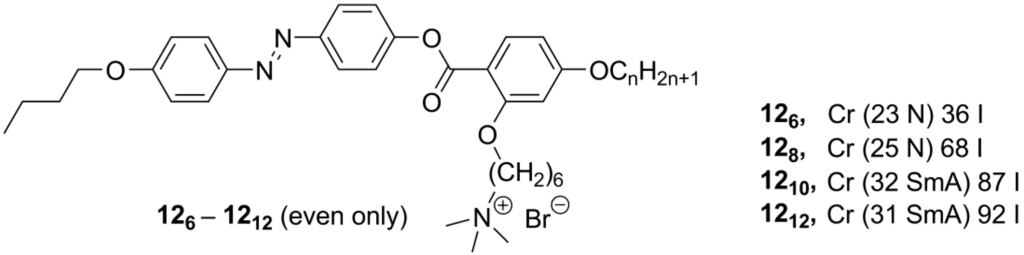
Scheme 6.
It can be concluded that in the last five years, the variety of ammonium-based mesogens has been expanded by involvement of new mesogenic groups—cyclic ammonium cations. The stability of mesophases, formed by ammonium molecules, is mainly depended on the lengths of N-alkyl substituents and used counterions. It should be noted that the highly polarized NTf2– and metal-containing anions disfavor the formation of a mesophase. Conversely, stable smectic and columnar arrangements were obtained in the presence of bulky organic sulfates. Nematic mesophases have been observed for the mesogens constructed in a form of T-shape. Evidently, besides the counteranion and length of N-alkyl group, the position of a positively charged head group relative to a mesogenic tail is equally important for mesomorphic properties of an ionic mesogen (see also reference [19]), and this could be successfully used for the tuning of molecular order in an ionic mesophase.
3. Phosphonium-Based Mesogens
Due to the limited availability and increased chemical reactivity of alkylphosphines, the corresponding phosphonium salts find not so extensive application as ionic liquids, compared with their ammonium analogs [11]. Typically, phosphonium centers can be formed by quaternization reaction of trialkylphosphines with alkyl bromides. In order to avoid the presence of halides in the ionic product, alkyl sulfates or phosphates can be used as alkylating agents [31]. Although phosphines are less basic than the corresponding amines, their larger radii and more polarizable ionic pair make them more nucleophilic and, therefore, more reactive in alkylation reactions [32,33]. As it was already reviewed earlier [11], the phosphonium-based mesogens preferably form smectic A2 phases. Such SmA2 phases are composed of double layers of n-alkyl chains separated by ionic planes with the molecular long axes, which are orthogonal to the layer planes (as in a SmA phase). The A-type designation results from disorganized arrangements of the alkyl chains within a layer, although the positively charged head groups and their counterions are probably more regularly packed within ionic planes (G, Scheme 1) [34]. The most remarkable feature of the thermotropic phosphonium-based mesogens 13 (Scheme 7) is their ability to broaden their LC temperature range and to diminish their onset temperatures upon the addition of one or more equivalents of a protonic solvent, such as water or an alcohol [35]. It has been stated that noncovalent interactions between hydroxyl groups of a solvent and charged phosphorus centers induce mesomorphism and attenuate ion pairing [36,37]. Recently, new phosphonium mesogens 14, containing β-hydroxyethenyl groups, have been prepared (Scheme 7) [38]. Due to intermolecular OH∙∙∙P+ interactions in these compounds, they displayed self-enhanced amphotropic behavior, similarly to it was earlier observed upon the addition of methanol to simple tetraalkylphosphonium mesogens 13. In the absence of solvents, the phosphonium salts 13 showed melting points around 100 °C and clearing points between 106.2 and 114.0 °C, while phosphonium mesogens 14, with embedded hydroxyl groups and the methanol solvates 13∙CH3OH (m = 1, X– = Cl–, Br–; Scheme 7), featured considerably lower melting points (at 43.5–85.0 °C; for 13∙CH3OH 49.7–54.9 °C) and clearing points (73.4–99.0 °C; for 13∙CH3OH 27.5–82.5 °C) [38]. In another approach, the phosphonium-“ate” zwitterionic structures 15 have been designed (Scheme 5), with an idea to weaken cation-anion contacts within an ionic layer and, at the same time, to establish new weak interactions between counteranions and “ate” groups in the phosphonium cations [39]. It was believed that this could help to maintain smectic lamellar order upon melting of the crystalline phase. However, none of the synthesized zwitterionic salts 15 showed liquid crystalline behavior. It has been proposed that the increase in disorder within lamellae of these salts frustrates melting into a liquid crystalline phase [39].
A number of new phosphonium salts 16 and 17 bearing perhalogenated carborane anions has been prepared (Scheme 7) [40]. Unfortunately, the authors did not report any mesomorphic properties of the new phosphonium products. It was only mentioned that the use of longer alkyl substituents resulted in a remarkable decrease of the melting points (17, m.p. 53 °C vs. 16, m.p. 239 °C, respectively).
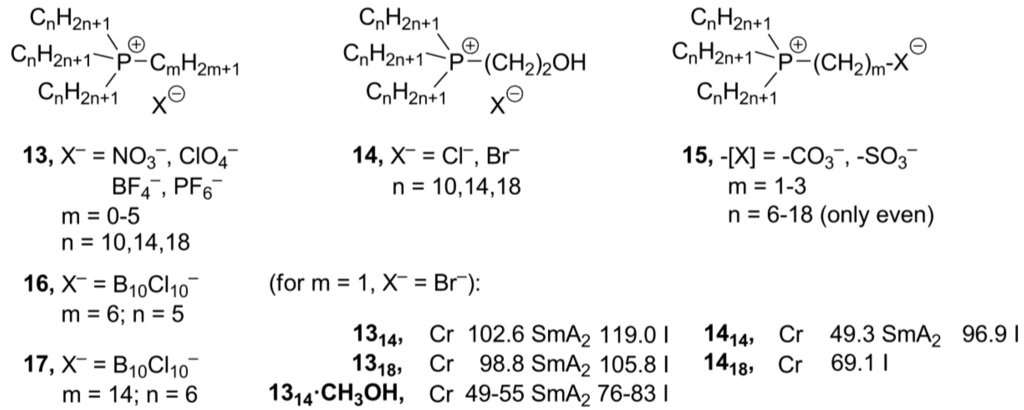
Scheme 7.
Phosphonium salts with long-chain alkyl substituents gave smectic liquid crystalline phases. Polar OH groups from the solvent or modified long alkyl chains enhance the stability of liquid crystalline order. It seems that the large phosphonium cation is easily accessible for the polar OH groups, placed in its close vicinity, and relatively strong hydrogen-bonding R4P+···HO contacts could be established, thus stabilizing a mesophase.
4. Imidazolium-Based Mesogens
Most of the so far studied systems are imidazolium-derived ionic liquid crystals. This is connected with the extensive use of imidazolium salts for the synthesis of cheap and environmentally friendly ionic liquids [41]. Besides that, imidazolium compounds are easily accessible and stable precursors for the generation of late transition metal N-heterocyclic carbene complexes, employed for selective catalytic processes [42]. For imidazolium-based ionic liquids, the in situ formation of metal-carbene complexes has been attributed to be one of the key factors in the improvement of their catalytic behavior [43,44]. Reported to date liquid crystalline materials, based on imidazolium, can be classified into two types in terms of their molecular structure (Scheme 8) [11].
Scheme 8.
In the first type (A, Scheme 8), the imidazolium group acts as a mesogenic core, which is substituted by one or multiple long aliphatic tails. In most of such cases, alkyl substituted imidazolium salts exhibit smectic mesophases [41]. Here, the molecules are arranged into layers due to a combination of electrostatic interactions in the head group region and weaker van der Waals forces in the hydrophobic tails [12,46]. Due to a flexibility of the imidazolium core, even in the presence of large counteranions, the system is able to compensate in a smectic A rearrangement the difference between the cross-section of the tails and the ionic lattice area by the gathering of the ionic sublayer and by folding of the tails. In this situation, hydrophobic interactions between alkyl chains and hydrogen bonding interactions are important factors to induce and stabilize the mesophase [47]. For this type of imidazolium mesogens, the temperature range of the observed mesophase rapidly increases with the increasing alkyl chain length, although the alkyl chain length at which liquid crystalline mesophases appear depends on the anionic species [11,12,46,47]. Recently, by means of ab initio computer simulations, the structure and supramolecular interactions have been modeled for dialkyl-substituted imidazolium ionic liquids in different states of aggregation, from crystals to liquids and clusters. It has been concluded that a balance between Coulomb, van der Waals, and, sometimes, moderate hydrogen-bonding interactions, determines the macroscopic properties and behavior of imidazolium ionic liquids. This balance is crucial, when describing structures of reduced dimensionality, such as surfaces, interfaces, and clusters [48]. Small angle neutron scattering (SANS) methods have been applied for structural studies of the imidazolium ionic liquids, having alkyl chains with an intermediate length (A, m = 1; n = 4, 6, 8; X– = PF6–, Scheme 8). They revealed structural features [49], which connect the close-packed cation-cation radial distribution observed for 1,3-dimethylimidazolium hexafluorophosphate, to the bilayer spacing of the long-chain liquid crystalline ionic liquids [12,47]. The model compounds (A, m = 1; n = 2, 4, 6; X– = NTf2–, Scheme 8) were investigated by a combination of physical-chemical methods, such as X-ray diffraction, adiabatic calorimetric measurements and temperature-dependent IR spectroscopy, supported by quantum-chemical calculations. As a result, new approaches for the structural identification of an ionic liquid mesophase by the use of temperature-dependent IR spectroscopy have been developed [50].
Alternatively, the imidazolium group could be connected via a flexible linkage to a conventional liquid crystal mesogen on the tail ends (B, Scheme 8). In these types of imidazolium-based materials, the liquid crystalline properties originate from their strong amphiphilic character. The ionic interactions of the imidazolium groups stabilize both SmA and SmE phases. Stable columnar phases have been obtained for the compounds, where imidazolium groups have been attached on the tail ends of discotic molecules (see below).
The type A imidazolium compounds could easily be modified with a variety of chemical methods, therefore synthesis and studies of mesomorphic behavior of new imidazolium salts still attract considerable attention. Recently, detailed studies of [C16mim]Cl (A, m = 1; n = 16; X– = Cl–, Scheme 8) have been carried out to reveal a lamellar double-layer structure of the ABACAB type, which could be useful for further synthesis of mesoporous SiO2[51]. It is believed that, due to hydrogen bonding interactions, the presence of water could improve stability of a liquid crystalline phase formed by imidazolium mesogens [11]. As an example, [C12mim]Br·H2O hydrate (1812[Br]·H2O) showed a slightly increased temperature range for the smectic A mesophase, compared with the anhydrous analogs 1812[Br] (Scheme 9) [52].
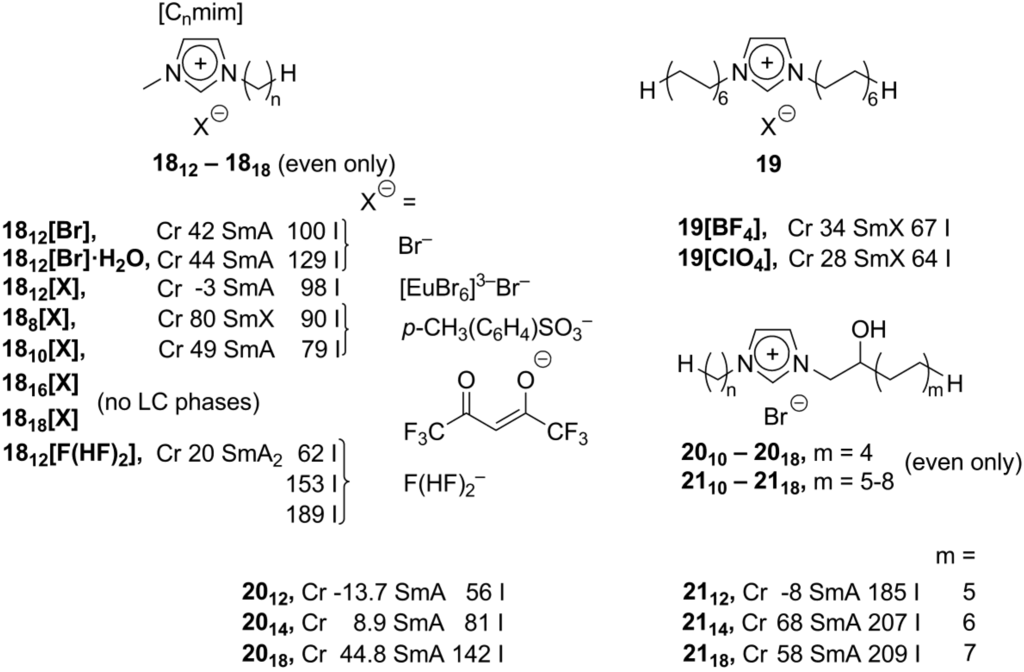
Scheme 9.
The basic [Cnmim]+X– structure could be simply modified by the use of new suitable counteranions X−. This approach provided a new series of easily accessible imidazolium mesogens, having wide spectra of interesting and applicable properties. With the complex large perhalogenated carborane anions, B10Cl102– and B12Cl122–, imidazolium [Cnmim] salts (n = 2, 16, 18; Scheme 9) exhibited smectic phases with high transition and clearing temperatures [40]. By an incorporation of Eu-containing counteranions, imidazolium-based luminescent mesophases 18[X] (X = [EuBr6]3–Br–, Scheme 9) have been formed in the temperature range of −3 to 98 °C and determined as smectic phases [53]. It has been recently discovered that the [C8mim] and [C10mim] imidazolium salts 188[X] and 1810[X] (X = tosylate anion, Scheme 9) generate lamellar mesophases being induced by shearing (or water traces) at room temperature. Conversely, bulk samples of these salts, investigated by DSC methods, display Cr-SmA(SmX) transitions between 50 and 80 °C [54]. Lately, utilizing the hexafluoroacetylacetonate counteranion, new hydrophobic ionic liquids 18[X] (X = hexafluoroacetonate) have been synthesized and investigated (Scheme 9). While they did not show liquid crystalline behavior, it is worth to mention here that, remarkably, they were able to extract by tight complexation ions of late transition metals from water phase [55]. A series of new ionic mesogens [Cnmim]+F(HF)2− (18[F(HF)2]), bearing very polar fluorohydrogenate anion F(HF)2−, showed thermotropic smectic A phases in a broad temperature range (depending on the alkyl substituent, Scheme 9) and pronounced anisotropy in ionic conductivity [56].
In an alternative approach, the alkylimidazolium core could easily be modified by incorporation of the second long chain alkyl substituent [57] or small polar groups on the side-chain of the imidazolium mesogen [58]. These changes preserve the rod-like shape of the alkylimidazolium cation, and, consequently, keep smectic supramolecular order in the liquid crystalline state. At the same time, they allow tuning of the physico-chemical properties (temperature range, viscosity, etc.) of the ionic liquid crystal phase. The imidazolium salts 19[BF4] and 19[ClO4], having two long dodecyl side-chain alkyl groups (Scheme 9), form smectic ordered mesophases at remarkably low temperatures of around 30 °C [57]. In the liquid crystalline state, these doubly substituted imidazolium salts exhibit non-Newtonian viscosity behavior (dependency of viscosity on shear rate), which is very unusual for ionic liquids and ionic liquid crystals. Above the liquid crystalline state, the viscosity of these compounds is independent of the shear rate. Consequently, non-Newtonian viscosity behavior of 19[BF4] and 19[ClO4] could be switched on and off, by keeping the temperature below or above of the phase transition point [57]. The presence of a small and polar hydroxyl group in the β-position to the imidazolium core (20 and 21, Scheme 9) leads to a considerable decrease of the Cr-LC transition temperature and to an expansion of the liquid crystalline temperature range. For a series of dialkylimidazolium salts 21 with two long alkyl substituents and one of them with an attached β-hydroxyl group (Scheme 9), an extremely wide mesophase temperature range has been observed (ca. 200 °C) [58]. Obviously, the hydrogen bonding interactions between hydroxyl substituents are weak enough to allow rearrangement of the molecules from solid to liquid crystalline state already at ambient or subambient temperatures. At the same time, these OH···OH contacts are strong enough to keep organized hydrophobic side-chains in layered smectic liquid crystalline order over a wide temperature interval.
When the imidazolium cation is connected to a rigid (usually aromatic) mesogenic group, liquid crystalline properties of the ionic salt are defined by the structure of the rigid mesogenic skeleton (B, Scheme 8). Depending on the shape of mesogenic cores, imidazolium liquid crystals exhibit a huge variety of mesophases, from smectic to highly ordered columnar or even cubic phases. Calamitic benzyl groups, bearing long-chain alkoxy substituents in the p-position, could be simply connected with the imidazolium core by the reaction of corresponding benzyl bromides with imidazole. The bromide anion could further be exchanged by an anion metathesis reaction, which gave a series of benzylimidazolium salts 22 (Scheme 10) [59]. These compounds displayed low Cr-LC transition temperatures and high clearing temperatures. Small-sized anions stabilize the liquid crystalline phase (stabilization order: Br− > BF4− > SCN− > PF6−). Lately, this series of imidazolium salts has been expanded by synthesis of new members with variable chain length of the p-alkoxy substitutuent in the benzyl ring (2210-2216, Scheme 10). All these structures 228-2216 exhibited smectic A order in the liquid crystalline state [60].
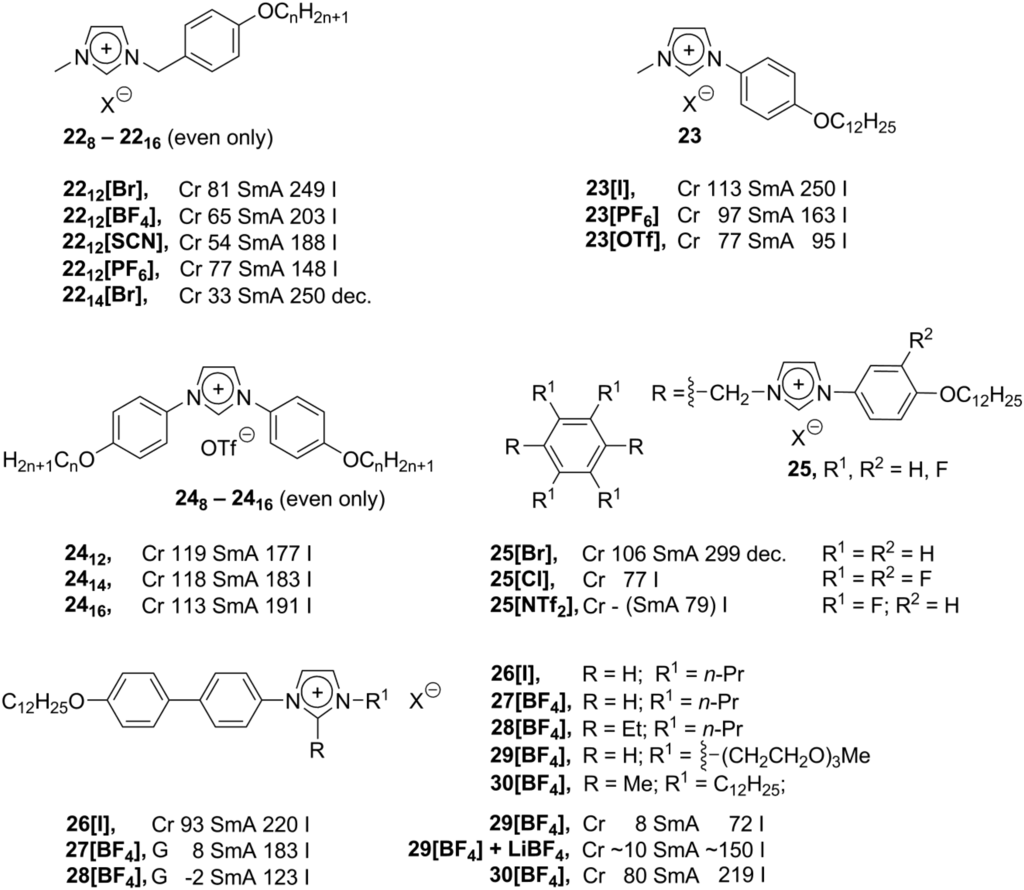
Scheme 10.
Due to flexibility of the benzyl substituents in 228-2216, such benzylimidazolium structures possess a lot of conformational freedom. In order to create the rigid calamitic imidazolium molecules, the imidazolium cation and the aromatic mesogenic group should be connected directly. Unfortunately, aromatic halogenides are much less reactive towards nucleophiles than their aliphatic analogs; therefore, phenyl substituted imidazolium salts are barely accessible. Recently, based on transition metal-catalyzed coupling reactions, synthesis of new phenylimidazolium structures has been developed. Long-chain alkoxyphenyl-p-iodides have been reacted with imidazole under harsh conditions (Cu(II)NaY as catalyst, 72 h, 180 °C). After treatment of the products with MeI, new phenylimidazolium mesogens 23 (Scheme 10) have been obtained [61]. These compounds show typical smectic A phases, and it has been found that in the presence of highly polarized anions the clearing point and stability of the mesophase remarkably decrease. In another synthetic approach, the diphenylsubstituted imidazolium core 24 (Scheme 10) has been constructed from corresponding aromatic glyoxaldiimines. Their reaction with chlormethylpivaloate and AgOTf (AgOTf = AgOSO2CF3) afforded structures 24 with a high yield and purity [62,63]. The original compound 2412 showed smectic A molecular order and quite a high Cr-SmA transition temperature (119 °C) [62]. An increase in the length of the alkoxy substituent led to an increase of the clearing temperature (2414 and 2416, Scheme 10). The measured charger carrier mobility in the liquid crystalline state was in the range of 10−4 cm2/(V·s) [63].
Recently, synthesis and liquid crystalline properties of the phenylimidazolium salts 25 have been reported, where two imidazolium moieties were connected via the -CH2- bridges to the fluorinated aromatic core (Scheme 10). These compounds were synthesized with the purpose to study the influence of the fluorine groups on liquid crystalline properties. It has been recognized that four factors are important for liquid crystalline characteristics: the length of the alkoxy substituent, the type of the counteranion, and the position and number of the fluorine groups [64]. The complex multisubstituted imidazolium mesogens 26–30 (Scheme 10) have been synthesized via a Cu-mediated coupling between 2-alkyl-substituted imidazoles and a series of p-alkoxybiphenylbromides, p-alkoxyphenylbromides or p-alkoxybenzoatophenylbromides. On the other side of the imidazolium unit (in 3-N position), various substituents, including chiral branched systems, have been introduced [65]. Complex mesomorphic behavior of these compounds has been investigated. It has been found for the BF4– anion-derived salts 27[BF4] and 28[BF4] that, compared with other analogs (26[BF4], for example), the melting points decrease (Scheme 10). Moreover, in the presence of 1.0 eq. of LiBF4, the mesophase temperature range of salts 29 can be expanded, and smectic liquid crystalline order can be observed in the whole spectrum of temperatures from 0 °C to 150 °C. The compounds with larger cores display more stable SmA mesophases, while branching (either in a racemic or chiral form) in the alkyl substituent has a minimal influence on phase behavior. Short alkyl substituents enhance the formation of a SmA2 phase, and large lateral substituents destabilize the mesophase [65].
Despite the number of known synthetic methods employed to connect an aromatic mesogenic group directly with an imidazolium moiety, these structures are still not easy to access and modify. Simultaneously with synthesis and studies of the rigid-core imidazolium-based mesogens, a lot of attention was given to the preparation of complex flexible structures, where a mesogenic group was connected with the imidazolium core vi a along-chain flexible linker. The incorporation of the linker between an imidazolium and mesogenic group was provided using simple synthetic transformations, such as esterification or Williamson reaction. In such a way, new biphenyl-based 31 and cholesteric 32 imidazolium salts have been prepared (Scheme 11). Unexpectedly, along with the anticipated appearance of chiral SmA* phases for 32, the formation of nematic phases has been observed upon cooling of an isotropic liquid of 31 [66]. In a similar approach, the azobenzene mesogens 33, 34 have been connected with the imidazolium core via a long flexible alkoxy bridge (Scheme 11) [67,68]. The special feature that makes the azobenzene moiety particularly attractive is that the materials containing azobenzene chromophores undergo photoinduced modification of their absorbtion characteristics through reversible trans-cis isomerization by irradiation with the linearly polarized light [67]. The monocationic imidazolium salts 33 reveal smectic A liquid crystalline phases with the Cr–SmA transition points over 100 °C and a narrow temperature range of an LC phase (20–60 °C) [67]. The dicationic imidazolium compounds 34 formed ordered monolayered smectic C phases [68].
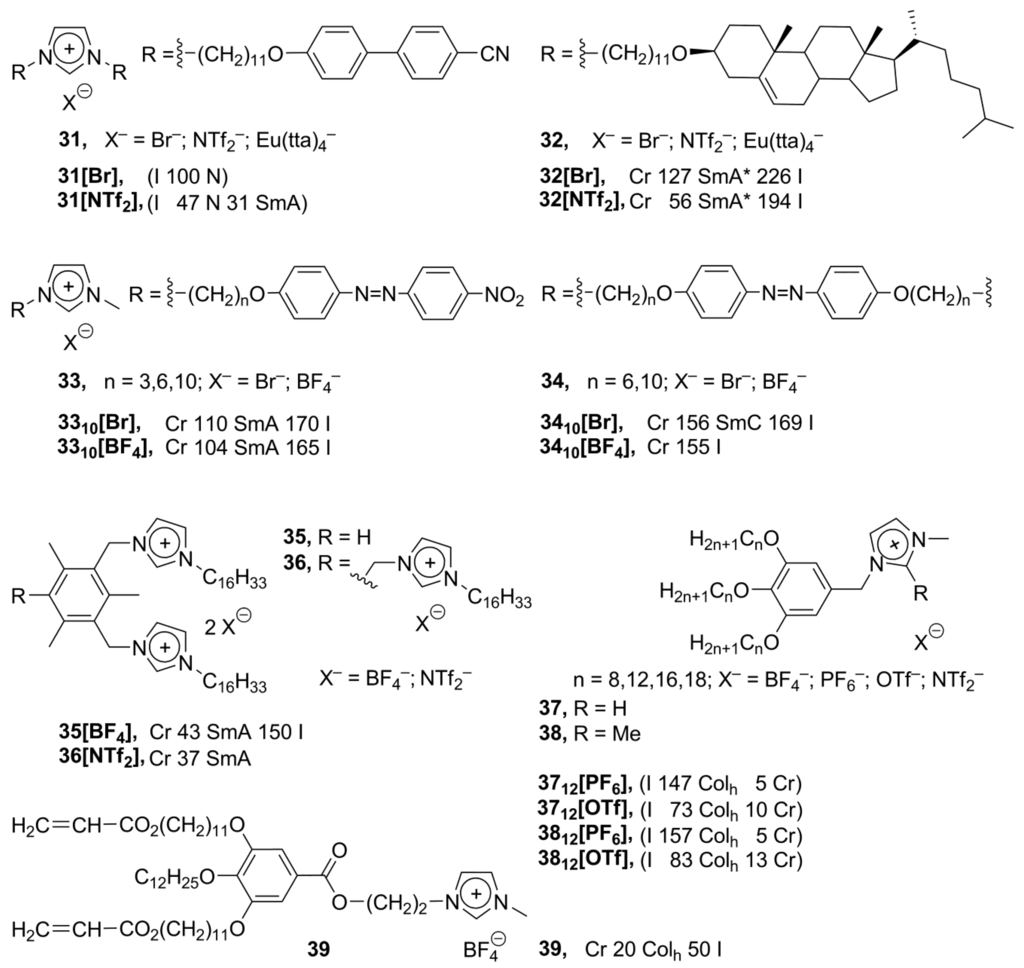
Scheme 11.
Bis- and triscationic imidazolium salts 35 and 36 (Scheme 11), bearing long-chain hexadecyl substituents, have been synthesized based on a mesitylene spacer [70]. These compounds showed low melting temperatures and formed smectic A phases in a wide temperature range. The salts with NTf2–anions tend to supercool before crystallization [70]. Recently, it has been shown that imidazolium-based discotic alkyl-substituted polyphenoles can form complex highly ordered hexagonal columnar phases. Being aligned on a surface within an enantiotropic columnar phase, these structures displayed anisotropic electric current conductivity [10]. In order to improve the conductivity of these compounds, new imidazolium derived polyphenol ethers 37 have been synthesized. Among a series of salts 37 (Scheme 11), it has been observed that the melting points show an increasing trend as the chain length increases. The counteranions in the salts 37 and 38 have order of the stabilization effect: BF4− > PF6− > OTf− > NTf2−, typical for imidazolium mesogens [71]. The aligned and self-ordered on a surface hexagonal columnar organization with formed ionic channels can be fixed in 39 by a polymerization of the acrylate-derived alkoxysubstituents. As a result, stable 1D ion-conductive polymeric films are formed with enhanced ion-conductive properties [72].
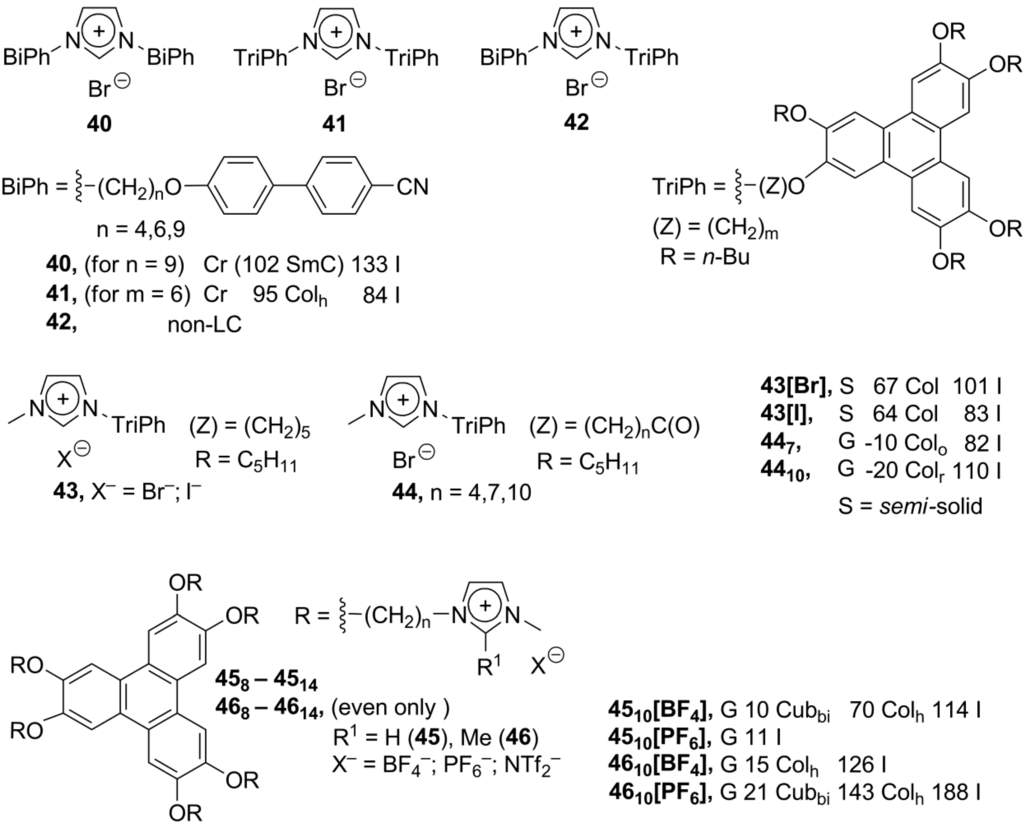
Scheme 12.
Besides polyphenol ethers, the well-known triphenylene group was used as a discotic mesogenic core for imidazolium ionic liquid crystals. With a series of biphenol-based calamitic and triphenylene discotic substituents, the calamitic-calamitic, discotic-discotic and calamitic-discotic imidazolium-based structures 40–42 have been synthesized by a microwave-assisted technique [73]. A combination of substituents, connected to the imidazolium cation, defines liquid crystalline properties of the investigated molecules. The calamitic-calamitic structures 40 exhibited tilted smectic C phases, while the discotic-discotic molecules 41 were organized into rectangular columnar phases (with the calamitic-discotic combination 42 being non-liquid crystalline) (Scheme 12).
Originally, the ether link (-O-) is typically used to connect the alkylimidazolium and triphenylene core (43, Scheme 12) [74,75]. Alternatively, the discotic imidazolium salts 44 have been obtained, where imidazolium and triphenylene moieties were bound via the ester spacer (-C(O)-O-) [76]. The mesogens 43 exhibited subambient Cr-Col transition temperatures, but narrow temperature windows for liquid crystalline columnar phases; while for the related compounds 44, the lower limit of the liquid crystalline phase extended to −20 °C [74,75]. Lately, the multisubstituted triphenylene ionic mesogens 45 and 46, bearing six charged imidazolium groups, have been designed (Scheme 12). In a comparison to the parent triphenylene and neutral imidazole-triphenylene analog, the compounds 45 and 46 exhibited a liquid crystalline phase with increased stability. With the externally added imidazolium salt [C6mim][BF4], the columnar phase is maintained over the temperature range from 4 °C to 117 °C. Obviously, ionic interactions within the mesophase stabilize the columnar assembly of the triphenylene moieties [14]. In more detailed synchrotron studies, it was realized that the optically “isotropic” phase, formed upon cooling of the parent columnar liquid crystals of 45 and 46 is in reality not amorphous but a mesophase with Cubbi geometry. Columnar triphenylene arrays within cubic geometry are expected to enhance electric conductivity of the liquid crystalline substrate. It is believed that the bicontinuous cubic (Cubbi) geometry contains a three-dimensionally interconnected network of π-electron channels [77].
An additional way to modify the imidazolium core is to use related heterocyclic systems instead. In one approach, the halogenide substituents were introduced into an imidazolium ring. It was believed that they might stabilize the mesophase by a “halogen bonding effect”, interactions between an antibonding orbital of C-Hal groups as an acceptor and a Lewis base as an electron pair donor [78]. In the solid crystalline state, the compounds 47 and 48 (Scheme 13) feature a relatively short connection (around 2.9 Å) between the halogen imidazolium substituents and oxygen atoms of triflate counteranions. Unfortunately, none of the salts 47 and 48 revealed any of liquid crystalline properties.
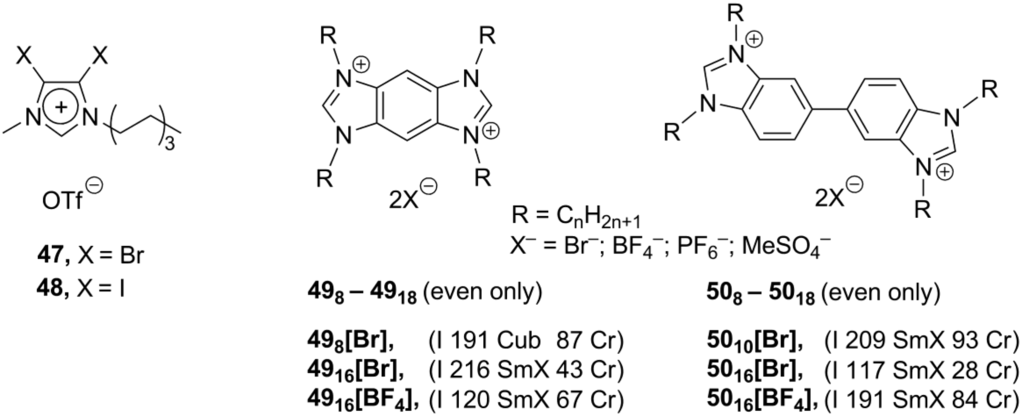
Scheme 13.
The extended aromatic systems can theoretically stabilize a mesophase by enhanced ionic and π-π type interactions [79]. With this purpose, the benzobis- 49 and bibenzimidazolium 50 systems (Scheme 13) have been constructed [80]. The compounds exhibited high thermal stability and form smectic or cubic liquid crystalline phases in a wide range of temperatures (ca. 34–220 °C). Mesophase stability of salts 49 rises with an increase in the length of the N-alkyl substituent up to C15, after which stability decreases, probably, due to plasticization effects. This trend was not observed for the bibenzimidazolium derivatives 50. It seems that a free rotation in the dicationic core of 50 may disturb the liquid crystalline organization [80].
The cationic imidazolium core can be easily synthesized and further modified by simple chemical transformations. This ease of access places the imidazolium structures as one of the most applicable mesogenic groups so far, used for preparation of ionic liquid crystals. The mesomorphic characteristics of the imidazolium-based systems can be easily tuned by simple modifications of the substitution pattern around the imidazolium core. This led to a huge family of imidazolium ionic mesogens, which display diverse liquid crystalline phases, from smectic (alkylimidazolium salts) to highly ordered organization with molecular arrangements of columnar or cubic geometries (benzyl- or triphenylene-imidazolium mesogens). The highly ordered phases potentially could be used as anisotropic ion conductors (see below). Small, highly polarized anions, due to strong electrostatic and hydrogen bonding cation-anion interactions, display a stabilizing effect on the mesophase, while large “soft” anions, like SCN– or I–, or “noncoordinating” anions, like PF6, rather destabilize liquid crystalline order. Together with a suitable counteranion, the presence of polar OH groups, even from traces of water, provide stable ionic mesophases, similarly to that observed for phosphonium mesogens (see above). From this point of view, the incorporation of –OH groups in the vicinity of an imidazolium core is a very promising synthetic strategy to build mesophases, stable over wide temperature intervals.
5. Pyridinium-Based Mesogens
Pyridinium-derived mesogens exhibit properties similar to the related imidazolium mesogens. The driving forces for the formation of pyridinium and imidazolium ionic liquid crystals are the same: hydrophobic interactions of the long alkyl substituents and ionic, dipole-dipole, cation-π interactions as well as π-π stacking of the aromatic cationic core groups. Consequently, in most of cases, smectic liquid crystalline phases are expected for pyridinium salts [11]. Among organic cations, mesomorphism of pyridinium salts has been known since 1938 [81]. Later, it has been demonstrated that N-methylation (or more generally N-alkylation) is necessary for these salts to show mesomorphic behavior [82,83]. Recently, new imidazolium 51 and pyridinium salts 52 (Scheme 14), bearing N-alkyl substituents with a chiral center in the position four to the nitrogen atom, have been synthesized and investigated [84]. Both series, imidazolium and pyridinium salts, form smectic A phases and exhibit similar trends in the mesophase stabilization effect of alkyl groups and counteranions. Mesophase stability was observed to decrease in order, for 51: Br− > OAc− > I− > BF4− > SCN− > PF6−; for 52: Br− > OAc− > BF4− > I− > SCN−. However, compared with their imidazolium analogs 51, thepyridinium derivatives 52 have much lower solid-LC transition temperatures and clearing points [84].
Besides the alkyl long chains, other mesogenic groups have been employed for the design of new pyridinium-based mesogens (53–57, Scheme 14). It has been observed that, although the length of the N-alkyl group is important for stability of the mesophase, in general, the incorporation of a phenyl group is destabilizing the liquid crystalline state. The mesophase temperature range for the compounds 5418[Br] and 5518[Br] has been found to be much narrower than that recorded for the p-methylpyridinium analogs 5318[Br] and 5318[I] (Scheme 14) [85]. In the stilbazolium-based derivatives 57, the presence of a donor methoxy group in the 4'-position of the aromatic system in the combination with the electron accepting pyridinium ring induces a large dipole moment in the molecule. This leads to a stabilization of a liquid crystalline phase (Scheme 14). All compounds 53–57 exhibit smectic A phases, with the head-to-tail arrangement of molecules within a layer. Counteranions are localized between positively charged pyridinium rings [85].
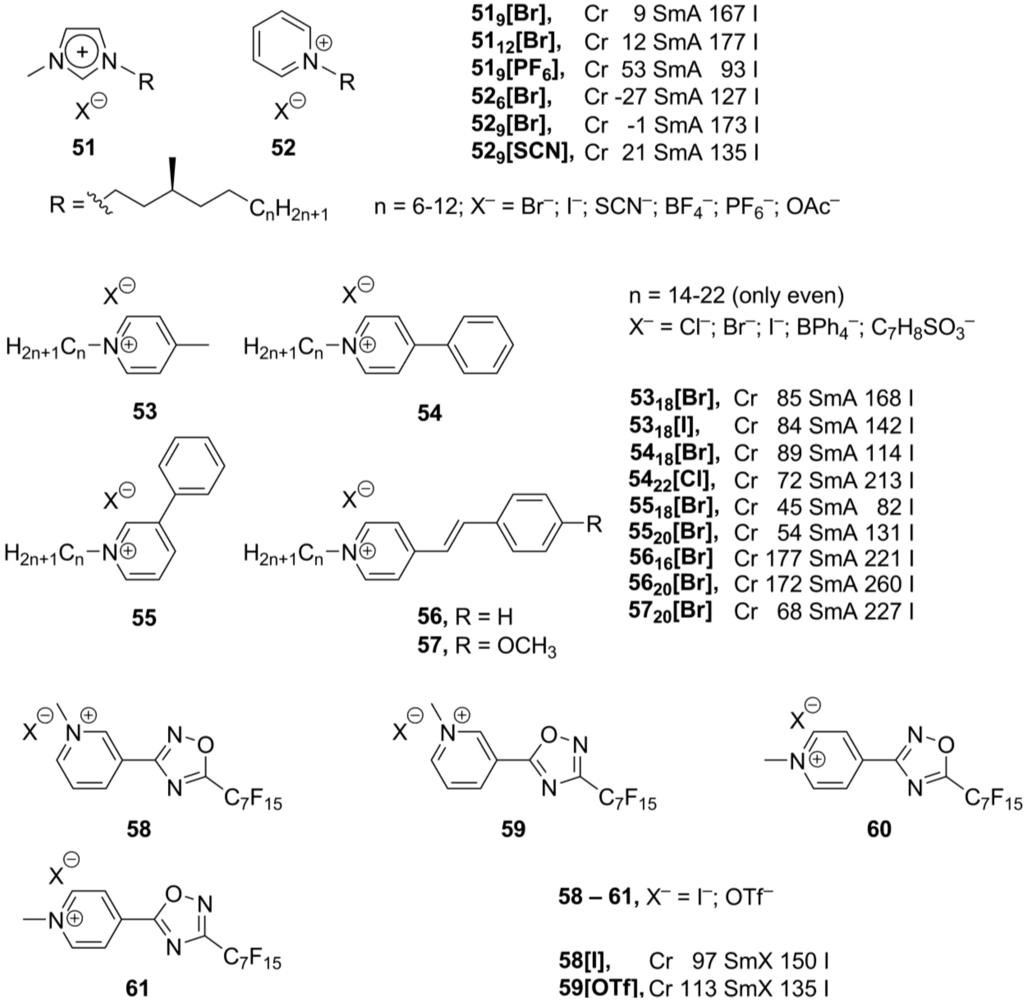
Scheme 14.
In a similar approach, the pyridinium core was modified by a connection with the 1,2,4-oxodiazole ring (58–61, Scheme 14). In order to maintain stability of a mesophase, long linear perfluorinated substituents have been attached to the mesogenic core in 58–61 [86]. It has previously been shown that the presence of a perfluorinated group in an appropriate position improves liquid crystalline properties [87]. Moreover, the linear substituents have better stabilizing effect over branched ones [88]. The 3’-substituted derivatives 58 and 59 displayed liquid crystalline properties, though in a narrow temperature range, while the 4’-oxodiazole salts 60 and 61 melt directly from solid state into isotropic liquids. To explain such different behavior of the 3’- and 4’-derivatives 58–61, it has been proposed that the positive charge is delocalized over the whole cationic aromatic pyridinium-oxadiazole system, thus weakening cation/anion electrostatic interactions. The formation of smectic order in a liquid crystalline phase was established. In the solid crystalline phase, the hydrophobic perfluorinated tails are packed in extended bilayers in an end-to-end fashion [86].
Recently, new ionic derivatives 62–64 (Scheme 15) with an axial chirality were reported [89]. Most of the compounds 62–64 exhibited different liquid crystalline phases, depending on the structure of the rod-like cation and the nature of counteranion. In these salts, two long-chain substituents in the cation are required to maintain a stable mesophase. Cholesteric (N*) phases have been observed for the enantiomerically pure salts, while racemic mixtures formed nematic or smectic achiral phases [89].
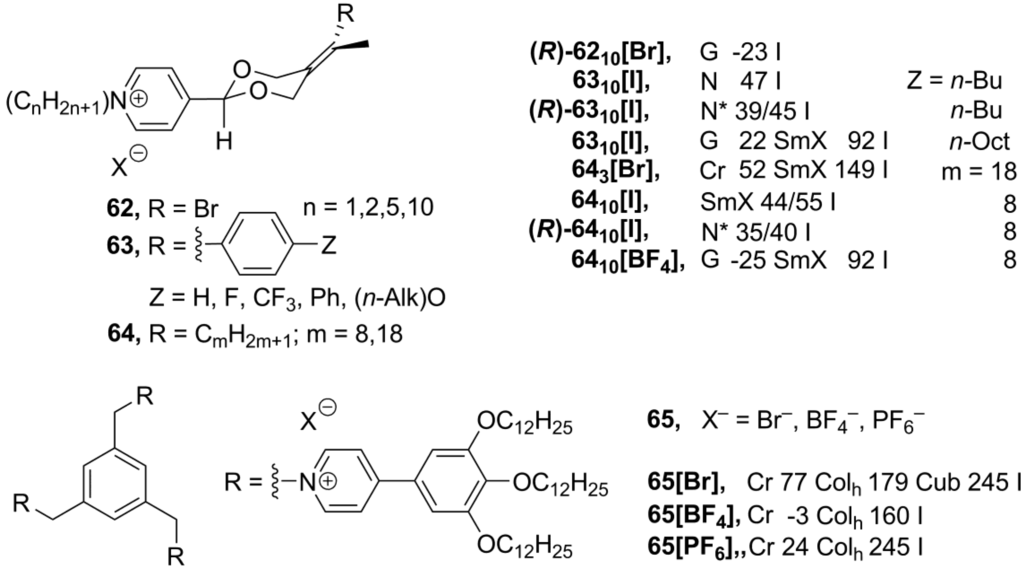
Scheme 15.
The imidazolium frameworks, based on discotic mesogenic cores, form extensive networks consisting of ionic molecules organized in a supramolecular columnar arrangement [11,14,73,74,75,76,77]. Similar ionic liquid columnar networks have been obtained from discotic pyridinium-derived structures 65 (Scheme 15). These tripodal discotic structures exhibit rectangular or hexagonal columnar liquid crystalline phases over wide temperature ranges, including room temperature. At higher temperatures, compound 65[Br] forms micellar cubic phase, where each micelle is assumed to contain approximately 16 clustered molecules on average. Perhaps, the small-sized counteranions allow closer packing of ionic pyridinium cores and, consequently, favor the formation of a cubic phase over a columnar rearrangement [90].
The pyridinium-based mesogens display mesomorphic properties, similar to related imidazolium salts. Consequently, pyridinium-based structures can be used in practical applications as alternatives to imidazolium analogs, in particular for the fabrication of anisotrpic ion conductors. Stability of a mesophase is also depenent on the counteranions and the substitution pattern around the pyridinium core. Pyridine is a chemically stable structure, providing on the other side a reactive nitrogen center, which is easily accessible for synthetic transformations into a substituted mesogenic cation. Together with the substituent on the nitrogen center, the p-position at pyridine ring is also important to retain stability of a liquid crystalline phase. Placed in the p-position, donor alkyl (methylpyridinium), substituted donor aryl (structure 65) or flexible vinylphenyl (p-stilbazolium) groups provide a positive stabilizing effect (compared with p-phenylpyridinium-derived mesogens).
6. Miscellaneous Organic Ionic Mesogens
Above, we have considered ionic compounds, formed from classical ionic organic mesogenic groups: ammonium, phosphonium, imidazolium and pyridinium. Despite a huge variety of designed structures, the choice of the ionic organic mesogenic core is still rather limited. In order to overcome this problem, research toward the discovery of new applicable inexpensive ionic mesogenic units is highly important. Recently, a new family of liquid crystalline salts 66 (Scheme 16), based on the alkyl-substituted caprolactam cation, has been reported [91]. Caprolactam is available in a large scale from industry and reacts easily with alkylating sulfate reagents. The obtained caprolactam cationic compounds 66 (Scheme 16), substituted with long-chain alkyl groups, exhibited enantiotropic mesomorphism with the formation of smectic A phases, unfortunately only in very narrow temperature intervals.
The combination of the triphenylene core and new ionic mesogenic moiety—guanidinium group leads to rectangular columnar enantiotropic liquid crystalline phases 67 (Scheme 16), formed in a relatively broad temperature range between 42 °C and 115 °C (for comparison, see [14,73,74,75,76,77,90]). Temperature- and chain-length-depended transitions between a common Colr phase with C/2m symmetry and a new P2m symmetric Colr mesophase have been observed within these new systems [92]. The calamitic guanidinium-based biphenyl ionic mesogens 68 form bilayered smectic A mesophases with hydrogen bonding between the guanidinium cation and counteranion. Stability of the mesophase and the temperature interval of the liquid crystalline state depend more on the employed counteranion than on the length of the p-alkoxy substituent in the biphenyl unit [93]. Detailed studies displayed that the counteranion is positioned along the N-H axes of the guanidinium cation and has pronounced hydrogen-bonding interactions with the N-H group of the cation. As a result, large counteranions disturb anisotropy of the molecule and destabilize the liquid crystalline phase. In the alkylated guanidinium compounds 69, in which hydrogen bonding is no longer possible, stability of the mesophase is reduced severely [94]. The highly polarized and yet overall neutral character of zwitterionic sydnone systems 70–72 (Scheme 16) reveal them as promising structures for ionic chiral liquid crystals. Bearing chiral cholesteryl groups, salts 72 exhibited chiral smectic A mesophases. Although detailed structural investigations were prevented, due to unsatisfactory thermal behavior of the compounds 71, a partially bilayered phase with either SmC* or hexatic structures was preliminary suggested [95].
As an alternative to the pyridinium charged core, the viologen-based compounds have lately been employed for the construction of ionic mesogens. It has been found that the symmetrically substituted viologen salts 73 (Scheme 16) with sufficiently long alkyl chains exhibit smectic B phases over a wide range of temperatures (from 0 °C to 140 °C). More specially, with slightly induced asymmetry in the molecular structure, the solid–LC transition temperatures could be lowered, and liquid crystalline properties of compounds 73 can be tuned by changes in the alkyl side-chains, attached to the viologen core [96]. In the further development, the complex viologen-derived discotic molecules 74 (Scheme 16) have been synthesized. They exhibit rectangular or hexagonal columnar phases, which are stable up to 160 °C. Additionally, redox behavior of salts 74 has been investigated [97].
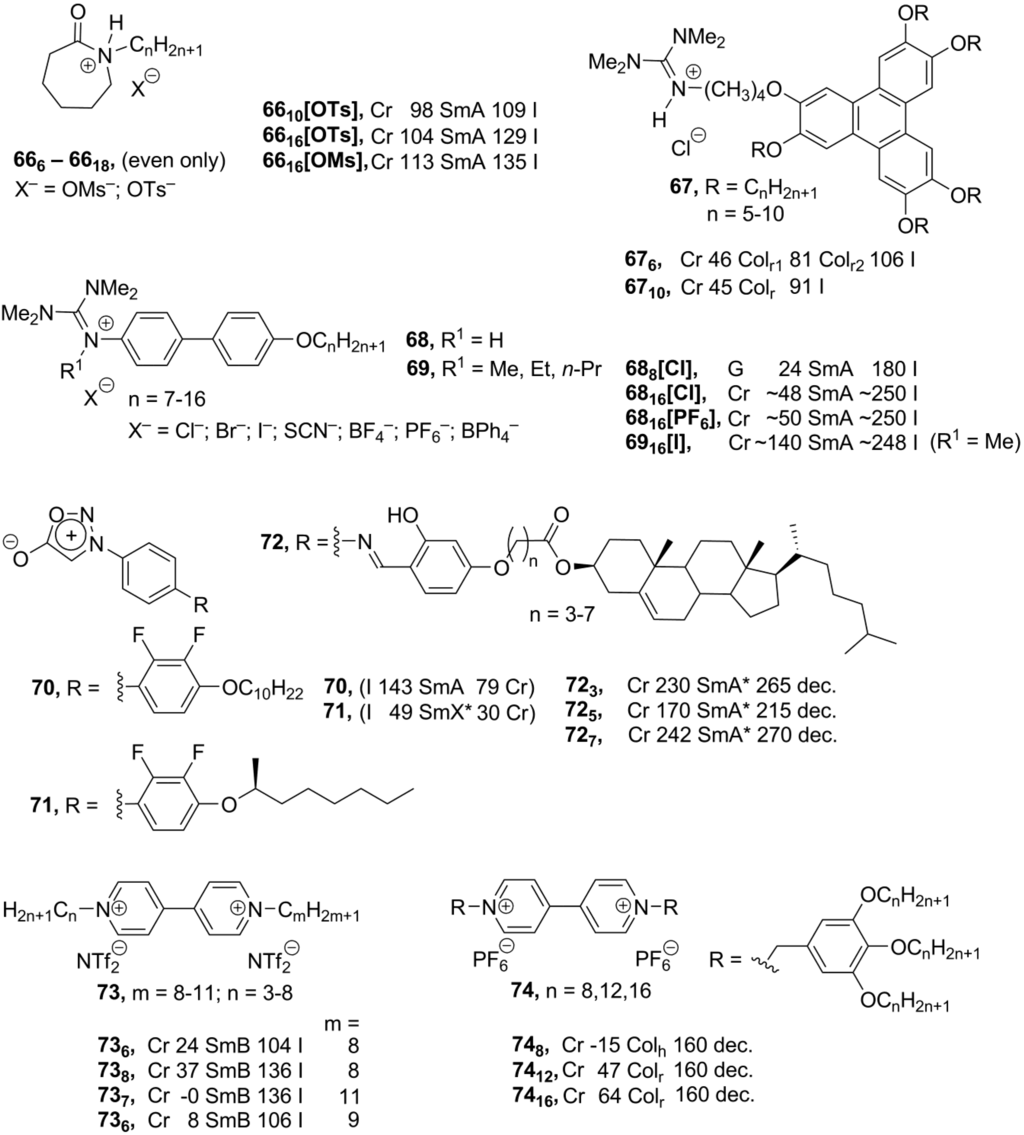
Scheme 16.
The search for new unexpensive, stable, but yet easily chemically convertible cationic mesogenic groups is essentially important for the preparation of new mesogens possessing practically applicable properties. While the caprolactam-based systems did not form stable mesophases, the guanidinium- or viologen-derived salts appeared to be promising candidates for practically usefull mesogens. Viologen compounds could provide, for example, redox liquid crystalline systems (see also below). Guanidinium-based mesogens formed stable mesophases in wide temperature intervals, which could be expanded further by the right choice of counteranion and suitable cationic substitution pattern.
7. Metallomesogens
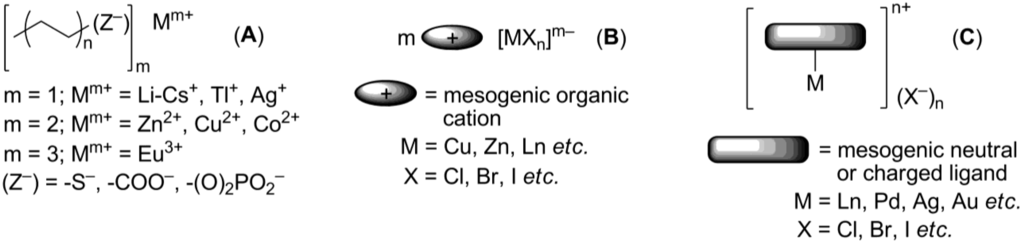
Ionic metallomesogens are metal complexes bearing ionic groups and displaying liquid crystalline properties. The incorporation of a metal center gives a great possibility to combine variable physical and physico-chemical properties of metal complexes with anisotropy of the aligned liquid crystalline arrangement [98]. This makes metallomesogens (both, neutral and ionic) attractive materials for broad practical applications, particularly as anisotropic magnetic liquid crystals [99], redox systems [100], luminescent liquid crystals for OLEDs [101] or solar sensitizers [102]. In this chapter, we focus mainly on the mesomorphic properties of ionic metal complexes. The possible and practical applications of metal-containing ionic crystalline materials will be discussed in a separate chapter below. There are several ways to introduce a metal center into the potentially mesogenic molecule [11]. In the simplest approach, positively charged metal particles, typically alkali or monovalent heavy metal cations, are combined with mesogenic “ate”-anions (usually, phosphates and carboxylates), bearing long hydrophobic hydrocarbon tails (Structure A, Scheme 17). Conversely, mesogenic large organic cations could form mesophases together with negatively charged metal-containing anions (Structure B, Scheme 17). Few examples, the uranium and europium-containing mesogenic salts 58-520, 6[Eu(tta)4] and 7[Eu(tta)4], 18[X] (X = [EuBr6]3–Br–) have been already discussed above. Alternatively, a metal center could be connected with a multidentate mesogenic ligand to form a large complex cation, leaving small counteranions on the outer coordination sphere (Structure C, Scheme 17).
Mesogenic “ate” metal salts (A, Scheme 17) have been known for a long time [103]. They tend to form lyotropic mesophases (soaps) in the presence of solvents. Nevertheless, in the absence of a solvent they also exhibit thermotropic liquid crystalline forms. Recently, in a continuation of early studies [11], anisotropic electroconductivity of Na hexanoate (A, Mm+ = Na+, (Z–) = -COO−, Scheme 17) and Co decanoate (A, Mm+ = Co2+, (Z–) = -COO−, Scheme 17) have been measured [104]. It has been observed that due to a smectic anisotropic molecular arrangement, electric current conductivity along the cation-anion layers is larger by four orders of magnitude than that in the perpendicular direction. Tl pentanoate (A, Mm+ = Tl+, (Z−) = -COO−, Scheme 17) exhibits a smectic mesophase in a range of 180–214 °C, which could be stabilized by the addition of Li pentanoate [105]. Long-chain Eu alkanoates (A, (Z−) = -COO−, n = 6–9, Scheme 17) display lamellar structures, where Eu ionic layers are separated by perpendicularly oriented alkanoate chains arranged in a hexagonal packing [106]. Unfortunately, the characteristic emission of Eu3+ is weakened by chelating carboxylate groups. The combination of the K+ cation and cholesteryl-derived sulfate anion gives a SmA mesophase in a temperature range of 139–238 °C [107].
Mesomorphism of the type B ionic metallomesogens (Scheme 17) is defined by the nature and size of mesogenic cation. These compounds find their application as luminescent molecules or precursors for inorganic nanoparticles (see below). For the latter purpose, the pyridinium-based salts 5312 [CuCl4] (see structures 53[X] in Scheme 14) have been synthesized [108]. It shows a smectic mesophase between 50 and 76 °C. Electrolysis of imidazolium liquid crystals 22[X] (X− = Ag(CN)2− or Au(CN)2−, see structures 22[X] in Scheme 10) produced Ag and Au nanoparticles, respectively [109]. These Ag and Au salts 22[Ag(CN)2] and 22[Au(CN)2] exhibit smectic A liquid crystalline phases, started around 60 °C. The silver complex 22[Ag(CN)2] forms more stable mesophase than the gold analog 22[Au(CN)2] (clearing points for 22[Ag(CN)2] and 22[Au(CN)2] are 157 °C and 112 °C, respectively). The imidazolium salt [C12mim]3[DyBr6] exhibits a smectic A liquid crystalline molecular arrangement at subambient temperatures and luminescence, characteristic for lanthanide complexes [110]. Between 127 °C and 224 °C, a smectic A mesophase has been observed for the pyridinium based salts bearing ZnCl42– anion [111]. Employed as a mesogenic group, the β-diketone moiety, bearing an alkoxyphenyl substituent, was attached to the o-position of the pyridinium ring.
Scheme 17.
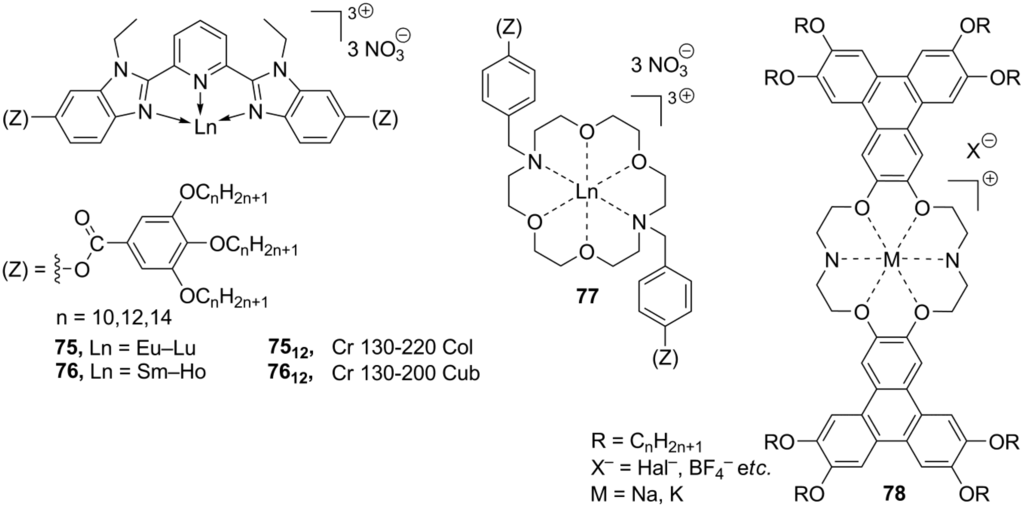
Together with structures B, considerable attention has been given to synthesis and studies of ionic metal complexes bearing a mesogenic ligand (C, Scheme 17). Recently, both types of metallomesogens, containing imidazolium groups, have been reviewed [112]. In the last decade, significant efforts have been directed toward synthesis of lanthanide mesogenic complexes, which combine luminescence with anisotropy in physico-chemical properties, induced by liquid crystalline molecular order. For most of so far synthesized lanthanide mesogenic complexes, counteranions (monodentate Cl− or bidentate NO3− anions) are found in the inner coordination sphere connected with the metal center [113,114]. Consequently, these compounds cannot be considered as true ionic mesogens with the complete separation of positive and negative charged species. For this reason, we give here only a general overview. The design of transition metal-based, especially lanthanide, mesogenic complexes is not easy [115]. Typically, a simple incorporation of a lanthanide metal center with the extended coordination core into a mesogenic ligand framework leads to molecules that do not form liquid crystalline phases [98]. In order to overcome this problem and preserve the mesomorphic properties after coordination with metal, several strategies have been developed. Those include a wrapping of the metal atom into a “cocoon” of aromatic rings, a connection of the ligand core with a large amount of flexible long-alkyl chains, an incorporation of rigid aromatic subunits in a periphery of the metal-ligand bulky core (“spatial ligand decoupling”) [114]. On account of these structural requirements and restrains, bulky ligands based on a bis(benzimidazole)pyridine core 75–76 (Scheme 18) or substituted Shiff base structures have been designed [112,113,114,115,116]. Other mesogenic lanthanide complexes have been obtained via the complexation of lanthanide salts with crown-ether derivatives (77, Scheme 18). They exhibited hexagonal columnar mesophases at temperatures above 60 °C [117]. In a similar approach, the liquid crystalline state could be generated in crown ether structures via incorporation into the ligand cavity of alkali metal cations [118,119]. For the crown-ether-derived ligands, bearing rigid lateral aryl or terphenylene subunits (78, Scheme 18), the influence of the alkali metal cation, counteranion and mesogenic substituents around the ligand-metal core on the structure of liquid crystalline phases and their stability at diverse temperature conditions have been investigated [120,121,122,123,124].
Scheme 18.
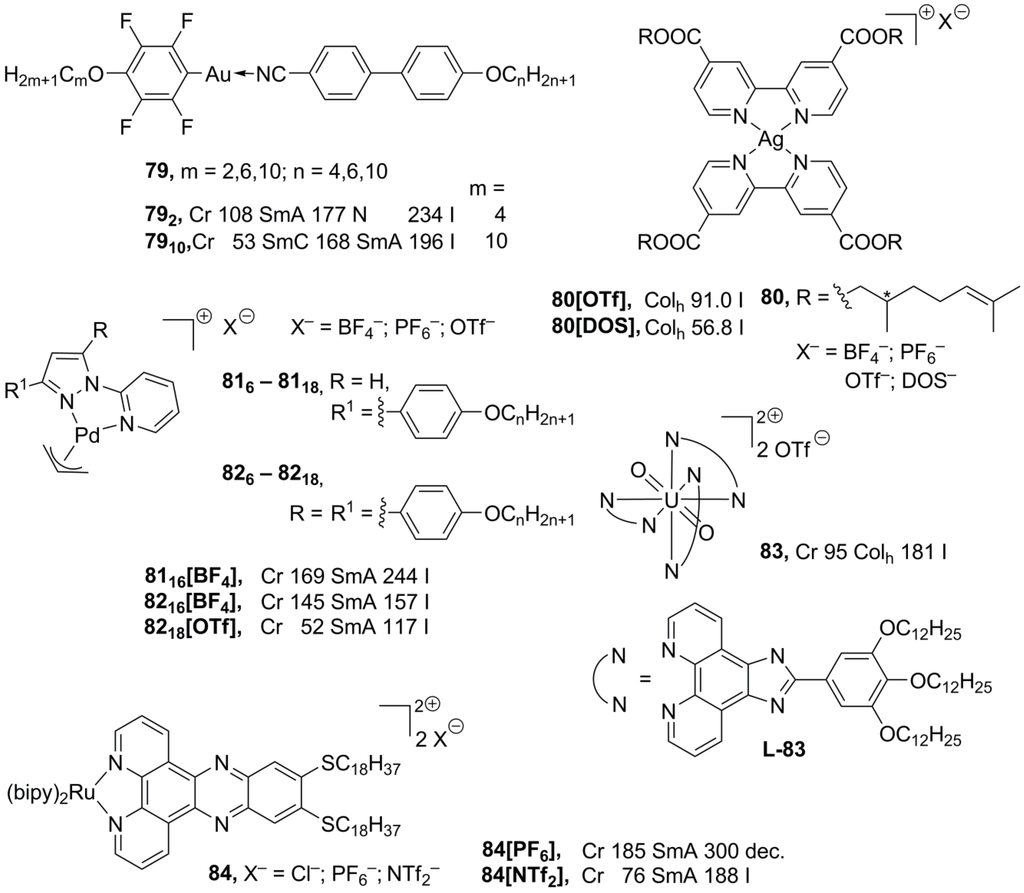
The rod-like geometry of gold(I) complexes favors the formation of lamellar mesophases. In addition, it is believed that weak intermolecular Au···Au interactions stabilize molecular liquid crystalline molecular organization [125]. Moreover, luminescence of gold linear complexes is attributed to weak intermolecular Au···Au contacts [126]. The structures 79 (Scheme 19), combining liquid crystalline properties and luminescence, have been recently reported [127]. These compounds exhibit rich mesomorphism: nematic, smectic A and C mesophases have been observed, depending on the substitution in the ligand. Surprisingly, Au···Au contacts have not been found in the solid state, and most possibly are absent in the liquid crystalline phase. Instead, the complexes 79 display very short intermolecular F···F interactions, which could be a driving force for a stabilization of the mesophase [127]. Coordination of silver with bipyridyl-derived ligands leads to the planar mesogenic silver complexes (80, Scheme 19). These structures 80 form hexagonal columnar mesophases, when OTf– or DOS– ions are employed as counteranions [128]. New bipyridino-derived silver mono- and polynuclear mesomorphic complexes have been recently prepared [129]. It has been observed that in the presence of a strongly coordinated anion (like NO3– or saccharinate) these silver complexes do not form polymeric structures and do not possess liquid crystalline properties. Conversily, in the case of weakly or noncordinated counteranions (OTf– or ClO4–) a lamellar-columnar mesophase is induced upon complexation with silver(I) [129].
Palladium metallomesogens 81, 82 (Scheme 19), with a planar arrangement of ligands around the metal center, have been prepared from the substituted pyrazolylpyridine ligand precursors. The presence of a positive charge on the palladium-ligand core together with long lateral side-chain substituents is essential that the complexes 81, 82 show mesomorphic properties [130,131]. The neutral dichloropalladium complexes melt directly into isotropic liquids around 170 °C, while the charged allylpalladium species form SmA phases with an interdigitaled bilayering organization. The Pd mesogens 8218[OTf], with two lateral long-chain substituents and the OTf– counteranion, exhibit liquid crystalline phases at subambient temperatures, in the range of 50–90 °C [130], but the monosubstituted complexes 81 form smectic mesophases at relatively harsh conditions above 150 °C [131].
Scheme 19.
A new propeller-like design for metallomesogens with an octahedral configuration around the metal center has recently been presented [132]. The ionic complex 83 (as mixture of Λ and Δ isomers), in which a charged uranyl moiety was surrounded by three imidazolphenanthroline-based ligands, displayed a hexagonal columnar mesophase between 95 °C and 181 °C. In order to keep efficient packing order within the mesophase of 83 (Scheme 19), few consecutive stacking enantiomers should randomize a pile of molecules with an identical absolute configuration around the metal center, stacked on top of each other into polar aggregates. In general, most stable mesophases with imidazolphenanthroline ligands are shown by nonplanar lanthanide and uranyl complexes, with a ratio between the metal and ligand as 2:1 or 3:1 [133]. The phenanthroline-derived framework have also been employed for the design of new class of metallomesogens, highly luminescent (bipy)2Ru complexes 84 [134]. Regardless of the counteranion, the most of the complexes displayed smectic A mesophases at quite harsh temperatures, with an exception of the NTf2− derivative 84[NTf2], forming a SmA phase already at 76 °C (Scheme 19).
Metallomesogens are very attractive compounds with regard to various practical applications. However, the presence in mesogen of a three-dimensional metal-ligand complex disturbs in most cases the stability of the mesophase. Conversely, in-plane coordinated metal centers stabilize the mesophase. Metals, such as palladium, silver or gold, are an excellent choice. They usually form planar complexes with T-shaped or square coordination of the metal center. In addition, in silver and gold complexes, weak argentophilic interactions or Au···Au contacts help to retain stability of liquid crystalline order. Consequently, these complexes exhibit liquid crystalline properties over a wide temperature range. In order to create a stable mesophase from nonplanar trasition metal complexes, a new “cocoon” synthetic strategy has been developed. By this new synthetic approach, it seems possible to get access to a variety of mesogenic three-dimensional metal complexes, which could find applications in different areas, from catalysis to optical electronics.
8. Self-Assembled Ionic Liquid Crystalline Systems
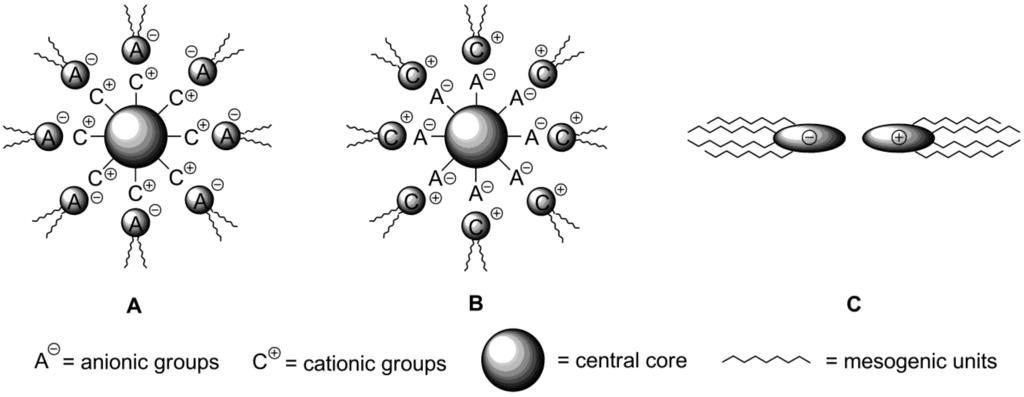
In the conventional design of an ionic liquid crystalline moiety, an ionic core is connected with mesogenic groups via chemical covalent bonding [11]. Alternatively, in ionic compounds, strong electrostatic interactions between a cation and counteranion can be used to build up liquid crystalline order on a supramolecular level [135,136]. By the connection of two or more structurally relatively simple charged particles via noncovalent forces, the tedious synthetic work could be avoided. By this general approach, called ionic self-assembly (ISA), a variety of attractive interactions, such as electrostatic contacts, hydrogen bonding, π-π interactions and van der Waals forces, has been employed for connecting of positively and negatively charged mesogenic cores together and creating a highly organized ionic liquid phase with supramolecular order [137]. All these interactions differ by strength, species or groups involved. Van-der-Waals and “amphiphilic” interactions are weak (up to 51 kJ·mol−1), non-directional, non-selective and operate on a short range. H-bonding is also rather weak interaction (up to 65 kJ·mol−1), but directional and selective [135]. The H-bonding interactions have been successfully used to create dimeric mesomeric structures (benzoic acids) or self-assembling multi-component systems [138,139]. In these structures, however, the problem of molecular recognition arises. The presence of complementary functional groups is required, which limites the choice of suitable molecules. The electrostatic forces are rather strong (up to 250 kJ·mol−1), but non-selective and non-directional. In this respect, a wide range of substrates can be used for the formation of ionic self-assemblies; the only requirements are the presence of cationic and anionic groups. However, the ionic self-assembly mechanism differs from the simple coulombic formation of salts. It has cooperative nature and, usually, includes the combination of many kinds of interactions. The cooperative character means that first bonds stimulate further binding, which propagates towards the final self-assembled structures [135]. In the last few years, the amount of published contributions in this subject increased dramatically with the purpose to build up the structures suitable for practical applications [140]. It was already described above that imidazolium based discotic molecules of substituted polyphenol ethers 37–39 (Scheme 11) could create supramolecular liquid crystalline phases via ionic self-assembly [10,71,72]. Recently, a new analogous system has been designed, in which an imidazolium unit is connected with polyphenols by a L-glutamic acid link [141]. When Br− is used as a counteranion, after an initial columnar liquid crystalline molecular arrangement the formation of a cubic mesophase has been observed.
Scheme 20.
All self-assembled mesogens could basically be classified within three structural types. Structures A (Scheme 20) are constructed from a small sized discotic multicharged cationic core bounded by electrostatic forces with mesogenic anionic species. Conversely, the central core can be negatively charged and surrounded by mesogenic cations (B, Scheme 20). Alternatively, both cationic and anionic species may contain mesogenic units, then simple cationic-anionic liquid crystalline pairs can be build up (C, Scheme 20). The systems of A-type (Scheme 20) are usually designed starting from a branched flexible polyamine core, which is further protonated in situ by mixing with mesogenic polyphenol-based benzoic acids [141]. The counterparts form different liquid crystalline phases, depending on the ratio of the amine 85 and used benzoic acids 86–89 (Scheme 21). When compounds 85 and 86 are mixed in a ratio of 1:2, SmA mesophases have been observed, while hexagonal columnar molecular liquid crystalline order was obtained at a ratio between 85 and 86 = 1:4. Mixtures of 85 and 87 also exhibit Colh phases. The melting and clearing temperatures can be tuned by changing the length of alkoxy-groups in polyphenolbenzoate moieties [142,143]. Micellar cubic mesophases with Pm3n symmetry have been obtained when branched or partially fluorinated alkoxy-substituents have been attached to anionic units, derived from acids 88 and 89 [144].
In order to build up the structures B (Scheme 20), the rigid aromatic carboxylate polyanions are used as the central negatively charged cores (e.g., see structures 90–93, Scheme 21). Ammonium or imidazolium salts are typically employed as cationic counterparts (e.g., see structures 94, 95, Scheme 21). The benzenehexacarboxylate 90 was mixed with six equivalents of the ammonium cations 94 with variable alkyl chain lengths [145]. Surprisingly, no self-assembled columnar molecular arrangement has been observed. Instead, the mixture of 90 and 94 exhibited a bilayered smectic A2 mesophase at temperatures, started from −20 °C. In this molecular arrangement, the molecules of carboxylate-anion 90 are ordered with their planes parallel to layers. Within the second layer, alkyl chains from ammonium counterparts 94 are oriented perpendicular to the layer planes [145].
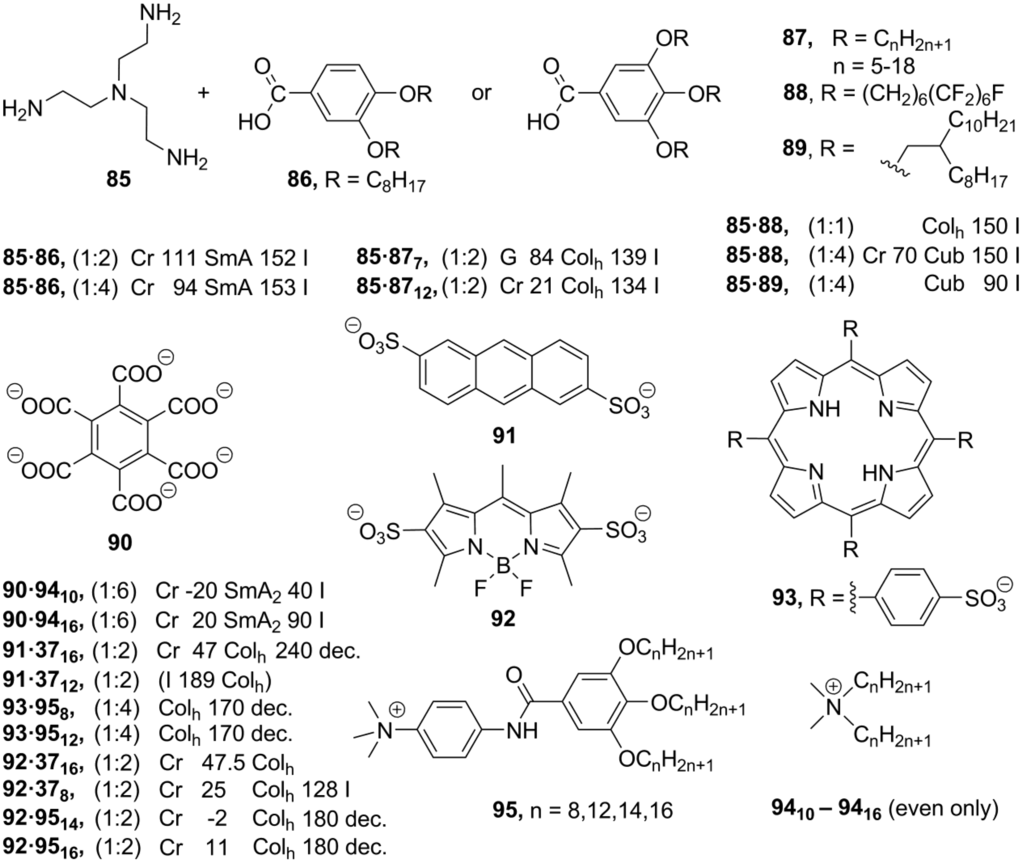
Scheme 21.
The self-assembled structures, based on the anionic luminescent disulfatoanthracene core 91, have been constructed in a similar way. As cationic mesogens, ammonium and imidazolium-derived units 37, 94, 95 (Scheme 21) have been employed. Whereas ammonium-anthracenedisulfate systems did not form liquid crystalline phases, imidazolium-anthracenedisulfate mixtures displayed hexagonal columnar phases [146]. The ionic self-assembly method is an elegant solution for synthesis of luminescent ionic liquid crystals. Besides anthracenedisulfate 91, the BODIPY- (boron-dipyrromethene) and porphyrine-derived anions have been applied as luminescent anionic cores for the preparation of self-assembled liquid crystalline structures. The BODIPY-modified fluorophore 92 (Scheme 21), bearing the polyalkoxybenzyl-substituted imidazolium cations 37 (in 1:2 ratio), exhibit hexagonal columnar fluorescent mesophases. In a solid state (and in a mesophase) emission is strongly red-shifted and broadened, showing a considerable degree of aggregation [147].
Alternatively, ammonium counterparts 95 have been mixed with BODIPY- and porphyrine-modified fluorophores 92 and 93 (Scheme 21). The mixtures build up hexagonal columnar mesophases, which are stable over a large temperature interval (from room temperature to 180 °C). It has been observed that the amido subunits in the ammonium parts are arranged in a hydrogen-bonded supramolecular network, stabilizing the thermotropic mesophase [148].
The simple combination of the [closo-1-CB9H10]– carborane-anions 96–103, substituted by mesogenic benzoate or azobenzene groups, with the heptyloxy-substituted N-butylpyridinium unit 104 provided partly interdigitated monolayer SmA mesophases (C, Scheme 20 and Scheme 22) [149]. Instead of electrostatic interactions keeping charged counterparts together, the hydrogen bonding can stabilize an ionic self-assembled supramolecular organization. Recently, it has been shown that, depending on the amount of alkoxy groups in compounds 106–108, different mesophases can be created by mixing of pyridine-substituted imidazolium salts 105 and polyalkoxybenzoic acids 106–108 (Scheme 22). The imidazolium part served as H-bonding acceptor, while benzoic acids were used as H-bonding donor molecules. The p-monoalkoxybenzoic acids 106 together with the salt 105 form smectic C phases, while mixtures with p,m-dialkoxybenzoic acids 107 exhibit rectangular columnar phases. Finally, the treatment of 105 with p,m,m-trialkoxybenzoic acids 108 led to a cubic liquid crystalline phase [150].
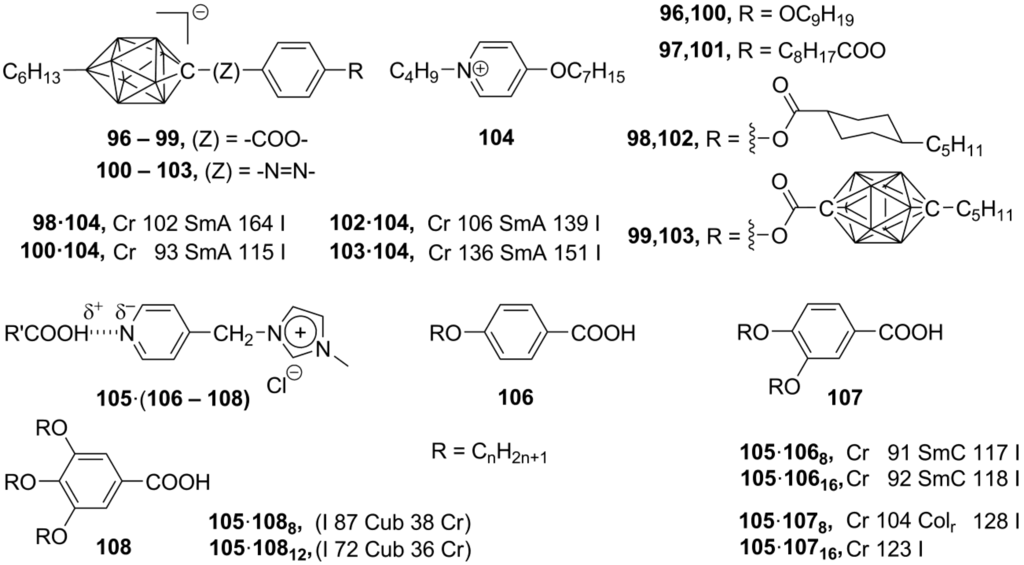
Scheme 22.
By the ionic self-assembly (ISA) approach, a wide range of ionic liquid crystalline phases, having a complex structure and including different cores, e.g., the bio-compatible BODIPY fluorescent framework, can be easily obtained, avoiding tedious synthetic work. Usually, the assembly of ionic species occurs under a cooperative binding mechanism. The highly ordered complex mesophases with columnar or continuous cubic geometries, which could be potentially applicable for the construction of anisotropic ionic conductive materials, are formed by the ISA-method from simple components. In this respect, the consideration and use of other binding forces, such as e.g., “fit interactions” or H-bonding, together with electrostatic interactions could give new perspectives and further development of the ISA-synthetic approach [150].
9. Ionic Polymers and Dendrimers
Incorporation of charged mesogenic groups into a polymer chain provides a new class of ionic liquid crystals, ionic polymer mesogens. In the presence of charged highly polarized ionic mesogenic groups attached to a polymer chain, the ion-ion electrostatic interactions become an important factor affecting the morphology of the polymer and supramolecular organization of the charged polymer species [151]. Ionic mesogenic substituents are able to provide tight contacts with solvent molecules (especially with highly polarized protic and aprotic solvents) or allow an ionic self-assembly of charged polymer chains into highly organized microdomains [152]. Consequently, ionic polymers can easily form lyotropic mesophases in the presence of solvent molecules or thermotropic liquid crystals in the absence of a solvent. Since the ionic polymers have been already reviewed by Binnemans in 2005 [11], we mainly focus here on the recent developments during the last five years. Due to easy synthesis and variability, imidazolium-mediated mesogens have widely been employed for the design of ionic polymers. The conventional design of imidazolium-based monomers (Structure A, Scheme 6) has been modified by variations of alkyl substituents and the incorporation of polymerizable acrylate groups (109, Scheme 23). The lyotropic mesomorphism of the imidazolium monomers and 3D bicontinuous cubic lamellar phases, created upon polymerization, have been investigated [153]. The polymerization of the vinylimidazolium salts having a thiophene group, attached via a flexible alkyl spacer (110, Scheme 23), gave electrochromic polymer films consisting of liquid crystalline domains. The detailed investigation revealed that the polymerization occurred on both thiophene and vinyl sides. In the lamellar supramolecular structure, the sheets of thiophene polymer are oriented perpendicular to the polymerized vinylimidazolium networks [154]. The vinylimidazolium monomers 111 and 112 (Scheme 23), bearing coumarin groups or connected to the p,p’-dioxy-biphenyl core, respectively, were polymerized in the presence of the AIBN initiator. The monomer 112 gave the polymer, exhibiting a liquid crystalline phase above 127 °C (no information given about the structure and clearing point) [155]. In an alternative approach, negatively charged sulfate groups were used to transform nonpolar hydrophobic hydrocarbon polymer chains into ionic polymer structures. The cholesteryl-based neutral ester and anionic sulfate units (113, Scheme 23) have been attached to the polysiloxane backbone via a Pt-mediated graft polymerization.
The incorporation of a photosensitive azobenzene unit [69] into an ionic polymer chain provided photoactive ionic liquid crystalline polymers. The polymers showed smectic and cholesteric molecular arrangements in the liquid crystalline state at temperatures above 66 °C [156]. A cross-linked polymerization of polysiloxane and calamitic azobenzene molecules (derived from “brilliant yellow”) resulted in liquid crystalline elastomers, displaying smectic molecular order upon heating to 180 °C [157]. The supramolecular material with photoinduced anisotropy properties was obtained viaionic self-assembly of small negatively charged p-(N,N-dimethyl)-azobenzene sulfate units to a hydrocarbon polymer chain with positively charged pyridinium side-substituents. These supramolecular assemblies build up lamellar layered structures, and after irradiation with an UV laser show a very pronounced anisotropic orientation [158]. Alternatively, long azobenzene-derived rod-like ammonium cations have been assembled with a poly(acrylic) acid polymer containing free carboxylate groups. After irradiation with a laser, azobenzene chromophores have been observed to become oriented in the direction perpendicular to the laser polarization [159]. The poly(methacrylic) acid-based polymers with piperidinium azobenzene units as end groups exhibit smectic A phases in a range of 35–155 °C. This temperature interval is much higher than that observed for nonionic analogs, and this was attributed to the ionic aggregation in the charged polymer [160].
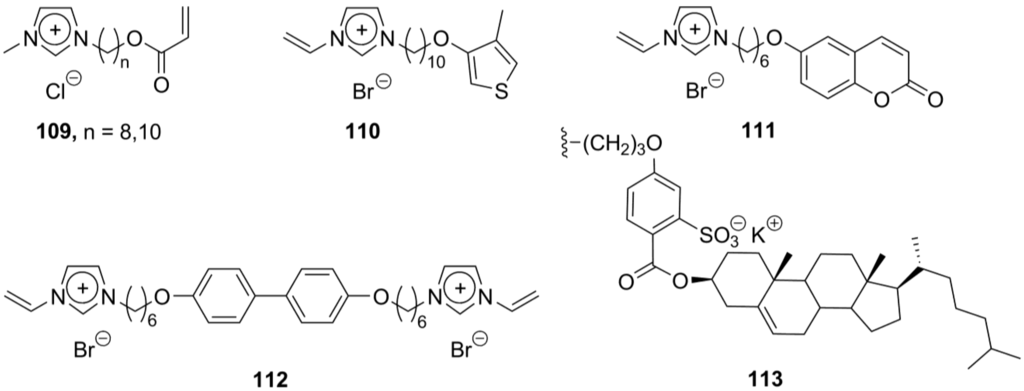
Scheme 23.
The conventional design of dendrimer liquid crystals includes a central dendrimer core linked to periphery mesogenic groups via a hydrocarbon spacer. The formation of a LC phase is a result of the molecular arrangement at the supramolecular level, which is determined by the microsegregation of the three molecular regions with very diverse characteristics: a central dendrimer core, mesogenic units and terminal flexible chains [161]. In ionic dendrimer mesogens, liquid crystalline order arises from the segregation of charged groups in molecules, therefore, in this case, the presence of mesogenic groups is not crucial. The typical way to generate the ionic dendrimer molecules is to modify the basic amino groups on a periphery of a polyamino dendrimeric core by the reaction with long-chain carboxylic acids. By this approach, PAMAM- (polyamidoamine) and PPI-based (polypropyleneimine) ionic mesogenic dendrimers have recently been synthesized (114, Scheme 24).
These systems exhibit smectic A phases, except for the PPI-derived dendrimers of 5th generation, forming columnar self-assemblies [162]. When polyalkoxysubstituted acids 106–108 (Scheme 22) have been used for the protonation of the PPI-dendrimeric substrate, the formation of smectic (with 106 and 107), hexagonal and rectangular columnar (with 108) phases has been observed [163]. It was later discovered that these dendrimer molecules are luminescent in solution and these emission properties were retained in the liquid crystalline state [164].
The in situ protonation of diaminobutane-derived (DAB) dendrimeric cores (1st–5th generations) with facial amphiphilic carboxylic acids (115 and 116, Scheme 24) led to the mesogenic ionic self-assemblies. The mesophase structures are mainly depended upon a ratio of a dendrimer to carboxylic acid and the length of the lateral polymer spacer to carboxylate groups in counterparts 115 and 116. The increased degree of the molecular organization in the mesophases, depending on the ratio of reagents and structure of the acid counterpart, was observed in a sequence: SmA, ChLh (hexagonal channeled layer phase), Colh, Colsqu (p4gm symmetry), Colsqu (p4mm symmetry) [165].
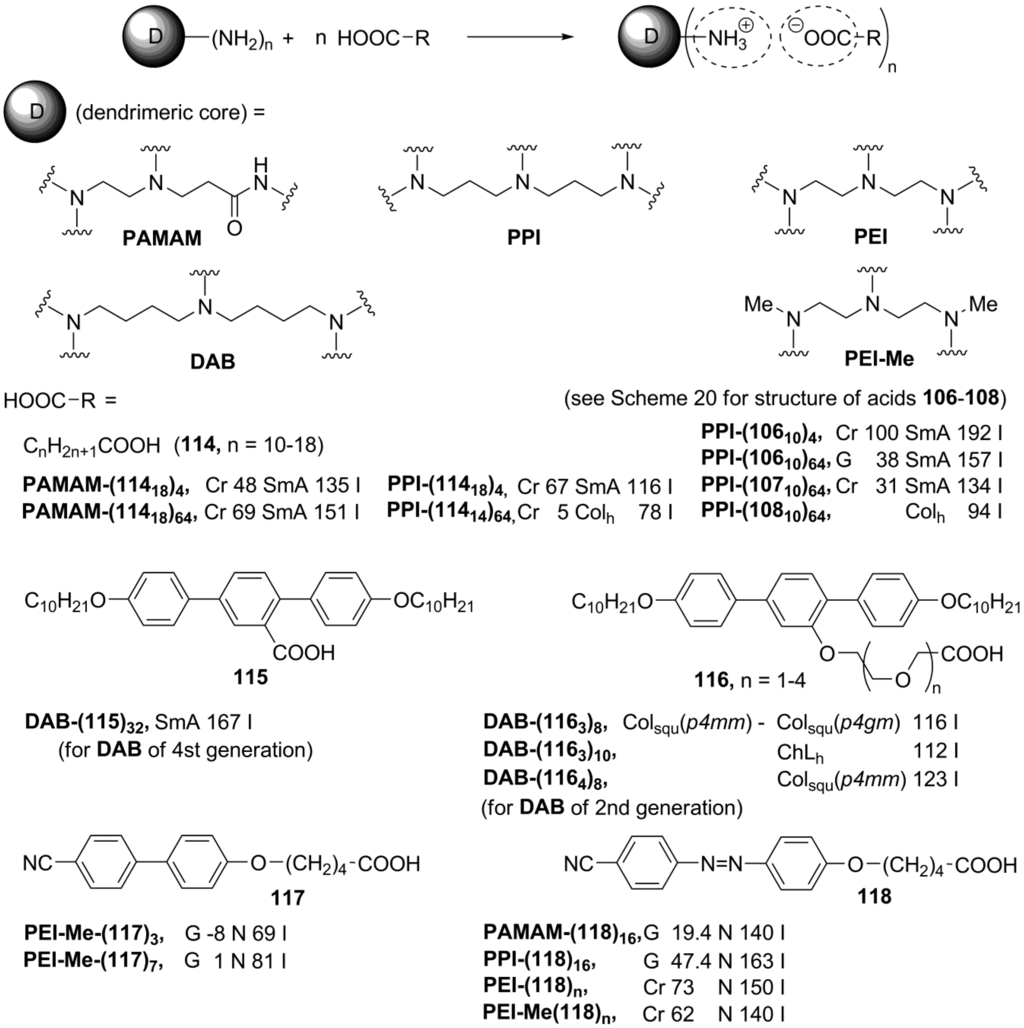
Scheme 24.
Unexpectedly, the self-assembled ionic systems, based on PEI-Me (methylated polyethylenimine) dendrimers and p-cyanobiphenylpentanoic acid 117 (Scheme 24), displayed the formation of nematic phases. The temperature mesophase intervals (−12–80 °C) were moderately affected by changes in a ratio of the reagents [166]. The further modification of an acid counterpart provided photosensitive ionic dendrimers, forming nematic phases. The carboxylic acid 118, containing an azobenzene moiety (Scheme 24), was assembled with PAMAM, PPI, PEI and PEI-Me based dendrimer cores. Stable birefringence was induced within the obtained nematic thermotropic systems using the linearly polarized light [167].
The physical properties, in particular the mesomorphic behavior of ionic polymers differ considerably from those revealed by small ionic mesogens. The formation of continuous polymer chains or networks allows creation of homeotropically aligned polymeric monodomains on a surface and, consequently, retains continuous liquid crystalline order along a mesophase. This is very important concerning, for example, 1D transport of ions. Alternatively, a construction of polymeric sheets from thiophene monomers can enhance hole conductivity in a liquid crystalline phase. This phenomena can be used for construction of hole conductors with organized liquid crystalline structure for OLEDs or molecular electronics.
The ionic self-assembly of dendrimeric molecules give complex structures with unique mesomorphic properties, in particular stable discotic nematic D phases. The incorporation of the azobenzene groups to these nematic phases is one perspective direction, which could lead to new photoactive materials with nematic liquid crystalline organization.
10. Applications of Ionic Mesogens
In this chapter, we give an overview on the actual trends in potential and practical applications of ionic mesogens. Ionic liquid crystals have the typical characteristics for ionic liquids, and, at the same time, an organized structure of a liquid crystalline phase. This leads to the unique combination of ionic conductivity and high polarizability, displayed by ionic liquids, and anisotropic physical and chemical properties, revealed by liquid crystalline materials [11]. Nowadays, these kinds of liquid crystals are expected to serve as anisotropic ion-conductors, ordered reaction media or templates for synthesis of zeolites, mesoporous materials and nanomaterials. Additionally, ILCs also possess a high potential as electrolytes in dye-sensitized solar cells.
Recently, it has been discovered that the reductive decomposition of pyridinium-based salts 5312 [CuCl4], bearing CuCl42– anion, led to the CuCl nanoplatelets with a well-developed crystal habit and a tunable particle size. In the original procedure, CuCl nanoparticles were obtained at elevated temperatures by the reaction of 5312 [CuCl4] with the reducing agent, 6-O-palmitoyl ascorbic acid [168]. The morphology of CuCl nanoparticles was a result of the layered molecular arrangement in the liquid crystalline precursor. The particle size, thickness and connectivity can be tuned by varying the reaction temperature [169]. In detailed studies, following the original contribution, it has been observed that the reduction of the CuCl-precursor 5312[CuCl4] started at temperatures far below 100 °C even in a solution phase, giving the inorganic CuCl macroporous network [108]. The two-component system of 5312[CuCl4] and 6-O-palmitoyl ascorbic acid showed complex decomposition behavior [170], therefore further research was directed toward the preparation of single-component ionic precursors for inorganic particles. According to this methodology, electrolysis of the imidazolium liquid crystalline salts 22[Ag(CN)2] and 22[Au(CN)2] gave Ag and Au nanoparticles, respectively. The size of the particles was controlled by a variation of potential and current density [109]. Alternatively, gold nanoparticles have been obtained by photolysis of the imidazolium salt 5312[AuCl4]. Upon mechanistic studies, it has been concluded that photoreduction of AuCl4- anions leads to Au(0) atoms embedded into a liquid crystalline matrix of 5312[AuCl4]. Depending on the viscosity of the system at different temperatures, either Au nanoplatelets or spherical particles can be produced [171]. Blue- and green-luminescent ZnO nanocrystals, supported with imidazolium mesogens bearing carboxylate group, have been obtained via hydrolysis of intermediate Zn-carboxylate products [172].
Due to an identical orientation of molecules in a mesophase (orientational order), liquid crystals possess anisotropic properties, in particular, anisotropic conductivity of charge carriers, such as holes, electrons or ions [137]. Ionic mesogens are consisted of cation-anionic pairs; therefore, ionic liquid crystals are most suitable materials to provide ionic anisotropic conductivity [173]. The ionic self-assembly approach opened a way to new ionic liquid crystalline supramolecular arrangements, with use as 1D or 2D ionic conductors [137]. Ion conductive mesophases of columnar or cubic morphology, which are most efficient for the ion mobility, have been constructed by a self-assembly of imidazolium and pyridinium-based mesogens [140]. Recently, new 1D ion conductive columnar mesophases have been prepared, utilizing a “noncovalent” supramolecular design. The mesogens, were assembled via noncovalent interactions from two components, ionic imidazolium salts [C4mim]+X− 119 and polar multi-substituted diols 120 (Scheme 25). Depending on the ratio of components, the systems 119·120 exhibited columnar phases in the temperature range of 0–80 °C. Compared with the related imidazolium salts 37 and 38 (Scheme 11), the systems 119·120 showed highest conductivity along the columnar axes (σ|| = 3.9 × 10−3 S cm−1 for 119·120 vs. 10−5–10−8 S cm−1 for 37 and 38), and displayed anisotropy of ionic conductivity (σ|| /σ﬩) in a range of 8–87, depending on the length of alkoxy-substituents [174].
Conductivity of neutral triphenylene mesogens have been compared with that provided by the ionic columnar and cubic mesophases, created by the compounds 45 and 46 (Scheme 12). The studies showed better characteristics for compounds 45 and 46 over their neutral analogs [77]. Interestingly, for the L-glutamic-based imidazolium salt with Br– anion, ionic conductivity displayed some decrease upon the columnar-micellar cubic transition [141]. For a stabilization of the continuous liquid crystalline molecular organization in the homeotropically aligned mesophases, polymerazable substituents have been introduced into an ionic mesogenic core. After polymerization, stable ordered conductive molecular thin films have been obtained [175]. The imidazolium salts 39 (Scheme 11), bearing acrylate groups, create one-dimensional ion conductive polymeric films under photopolymerization conditions. Prior to the polymerization procedure, the self-assembled molecular columnar substructures of the mesophase can be homeotropically aligned either perpendicular to the substrate surface or, by shearing, with the parallel orientation. Anisotropy of conductivity (σ|| /σ﬩) reached 260–1400 for the polymerized samples at different temperatures [72]. In the new imidazolium-based salts 121–123, reported recently, ionic conductivity was combined with an electronic charge transport by an incorporation of a trithiophyl subunit into an imidazolium mesogen (Scheme 25). The compounds 121–123 exhibited smectic A phase, in which high ionic conductivities were observed. In addition, these compounds displayed efficient electrochromism in the liquid crystalline bulk state [176]. The viologen-based mesogens 73 (Scheme 16) have been used to fabricate carbon composite electrodes, with the purpose to improve their characteristics [177].
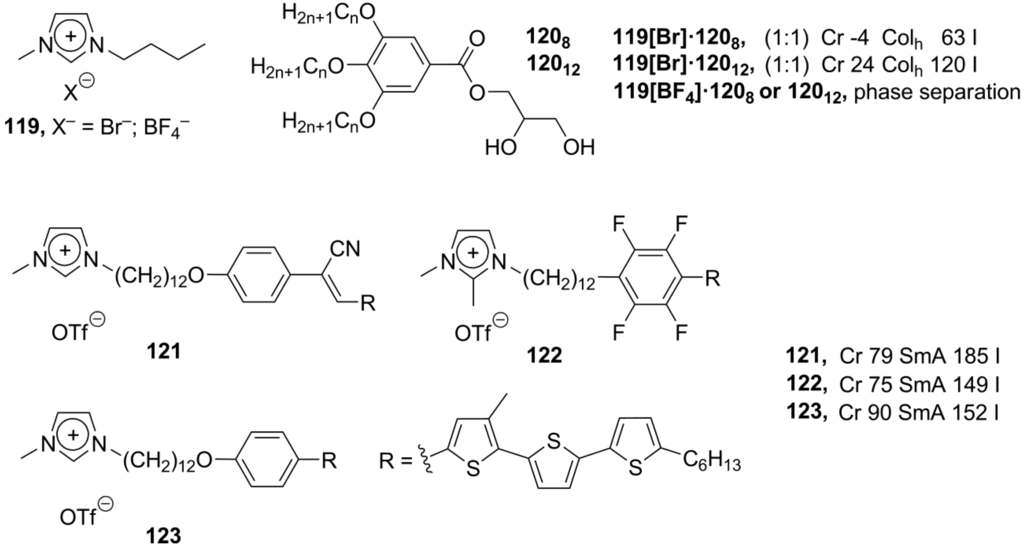
Scheme 25.
The liquid crystalline materials easily and reversibly respond to electric and magnetic stimuli. These properties have successfully been applied for the construction of LCD displays. However, due to the use of the dichroic sheet polarizers and absorbing color filters, the viewing angles and brightness of LCD displays are rather insufficient. In order to omit additional polarizers and color filters and, consequently, optimize the optical parameters, the application of luminescent LCs as bright light emitters is highly desirable [178]. In this respect, lanthanide mesogenic complexes have been proposed as potential candidates for bright luminescent emitters [179]. Lanthanide metals are well known for their ability to display emission spectra with very narrow emission bands, giving the high purity color of the luminescent light [180]. Upon an excitation, electron transfers to an excited singlet state (S1) then to a triplet (T1) state of the ligand, and, finally, to an excited 4f-state of the metal center. Upon relaxation, the sharp spectroscopic bands originate from the f-f transitions within the metal center (A, Scheme 26) [179,181]. The ligand structure has a slight influence on the shift of the emission wavelength, but a pronounced effect on the molar extinction coefficient and, consequently, on the intensity of an emitted light [181]. A lanthanide-containing mesophase could easily be obtained by doping of the non-luminescent ionic liquid crystalline substrate with a photoactive lanthanide complex. The ionic liquid crystalline phase, formed from [C12mim]Cl (A, m = 1; n = 12; X− = Cl−, Scheme 8) and 1 mol% of a phenanthroline-based lanthanide complex exhibited intense NIR (near IR) luminescence at room temperature [182]. The mesophase is stable in a range of 10–100 °C, and the intensity of the emitted light is enhanced in a liquid crystalline phase, compared with parent complexes in solid state or solution. As an example, the measured quantum yields of the Yb-containing material were 1.5 times higher than for the parent [(tta)4Yb(phenanthroline)] (2.1% vs.1.6% (as a solid) vs. 1.4% (as a toluene solution)) [182]. The synthesis of new ligands, based on a “cocoon” design (see above and Scheme 18, Scheme 19), made a preparation of the lanthanide mesogens possible, which exhibited liquid crystalline phases at room and subambient temperatures [113,114,115,116,117,133]. The lanthanide complexes 77 (Scheme 18), based on aza-crown-ether ligands, displayed columnar hexagonal mesophases in wide temperature windows and retain their luminescent properties almost up to the clearing point [117].
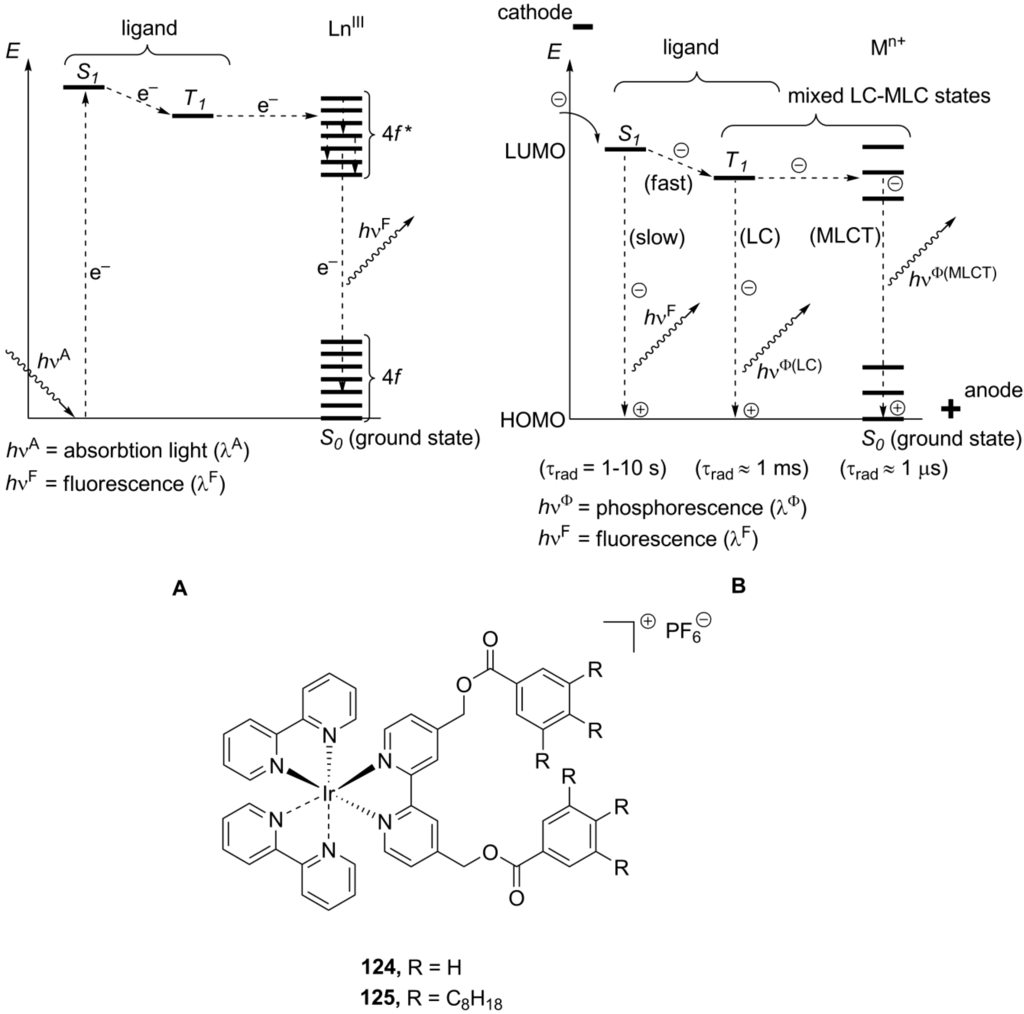
Scheme 26.
A schematic representation of luminescence process in lanthanide complexes (A) and electron-hole phosphorescent recombination in late-transition metal complexes (B).
Lanthanide complexes with the phenanthroline-derived ligand L-83 (Scheme 19) formed a luminescent cubic mesophases above 215 °C [133]. Unfortunately, the molar extinction coefficient of intraconfigurational f-f transitions within a lanthanide ion is rather low, usually less than 10 L·mol−1·cm−1, leading to weak luminescence of lanthanide complexes [183]. In this respect, parallel with the design of new lanthanide-based mesogens, the new liquid crystalline metal complexes have been investigated. The recently synthesized silver complexes 80 exhibited hexagonal columnar phases, consisted of the dimers with bridged triflate or DOS– anions. These mesophases displayed unusual blue luminescence, probably due to excimers arising from argentophilic interactions in the columns of stacked dimeric structures [128].
Within the past decade, new highly efficient triplet emitters, based on late-transition metal complexes, have become highly attractive, in particular, due to their applicability in organic light-emitting diodes (OLEDs) [184]. Under an electrical excitation, both, singlet and triplet, excited states are generated in an emitter. In a conventional organic-based emitter, the singlet-triplet (S1-T1) transitions between excited states are spin-forbidden, and the fluorescence occurs entirely from a relaxation of an excited singlet state (S1-S0), giving low quantum yields (theoretical maximum is only 25%) [185]. In late-transition metal complexes, the S1-T1 transitions are allowed by a spin-orbit coupling induced by the presence of the metal center. In this mechanism of the relaxation, electron transfers from an initial excited singlet state S1 to a triplet state T1, located on the LUMO of the ligand, and then to the HOMO of the ligand (LC-transfer) or the metal center (MLCT-transfer), causing phosphorescence of the complex (B, Scheme 26) [186]. Due to a triplet harvesting phenomena, the theoretical quantum efficiency of phosphorescence in late-transition metal complexes reaches 100% [187]. Among a huge variety of studied metal complexes, derivatives of Ir, Os, Ru appeared to be most suitable for potential applications in OLEDs [184]. The alignment of the phosphor molecules in orientational order of a liquid crystalline phase could simplify the hole transport from an ITO anode to a doped emission layer (EML) of an OLED device. As a potential application, the ionic metallomesogenic phosphors could be used to fabricate emitting ordered liquid crystalline films. The high polarization of ionic molecules and high ion conductivity should enhance the hole transport, as well, consequently, an efficiency of an OLED device. Recently, new ionic Ru-based complexes 84 (Scheme 19) have been reported, exhibiting smectic A phases up from 76 °C. Bright luminescence of the complexes 90 has been observed, and the color of luminescence can be tuned by changes of the counteranion [134].
In an alternative approach, new tris-piridyl-based iridium complexes 124 and 125 bearing linked polyalkoxybenzoate moieties have been synthesized (Scheme 26) [188]. The iridium derivative 125, substituted with C8-alkoxy groups, displayed liquid crystalline behavior and induced a columnar hexagonal mesophase by fast cooling of an isotropic liquid. The decrease of order on going from the crystalline to the frozen liquid-crystalline state in 125 is followed by a shift of the luminescence wavelength from green to yellow and only a slight decrease in the luminescence quantum yield. A further shift in the emission color to orange-red has been observed in formed by spin coating amorphous thin films. By heating these thin films, the solid crystalline order can be restored, giving back green luminescence, and this process is fully reversible [188].
In 1991, a photovoltaic solar cell of new type (so-called dye-sensitized solar cell, DSSC), was reported [189]. The heart of DSSC is a thin mesoporous oxide layer, composed of TiO2 nanoparticles, sinthered together to establish electronic conduction. The oxide layer is placed on a transparent conducting oxide (electrode, Scheme 27) and covered on the surface by a monolayer of a charge-transfer dye. A solar photoexcitation causes an injection of electron to the conduction band of the oxide, leaving the dye in its oxidized state. The subsequent migration of electron to an electrode and further on via an electric chain to a counterelectrode generates electric current. The dye is restored to its ground state by a redox electron transfer from an electrolyte, containing a redox system, which is subsequently regenerated on the surface of a counterelectrode (Scheme 27). Since the transfer of electron from the redox system to the dye is much slower than an injection of electron to the TiO2 connection band upon the photoexcitation, the DSSC-based devices are able to generate an electric current with overall theoretical efficiency limit of 34%. Practically achieved efficiency of this type of solar cells has reached 12%. All aspects concerning DSSCs are discussed in detail in a very recent review [190]. One of the essential components of a dye-cell is an electrolyte. The function of an electrolyte is the transfer of charged components of a redox system from the counterelectrode to the oxidized dye molecule, thus regenerating the ground state of the dye [191]. Originally [189] and nowadays [190], electrolytes, based on organic solvents, e.g., acetonitrile, and containing ionic additives, give best output efficiencies. During the last years, ionic liquids have been considered as an environmentally friendly alternative to conventional organic electrolytes [191,192]. The rate of the ion transport is one of the factors, limiting the productivity of the dye-sensitized solar cell. The ion mobility and, consequently, electrical energy conversion efficiency of the cell can be improved considerably by a decrease of the viscosity of an electrolyte. For example, the diffusion coefficient D of I3− anion in acetonitrile (viscosity 0.34 MPa s−1), used in record DSSCs, is 1.5 × 10−5 cm2 s−1, while D for [C3mim]I (viscosity 880 MPa s−1) amounts to 1.9 × 10−7 cm2 s−1 [190].
As discussed above, the ion conductivity of an ionic liquid electrolyte can be enhanced by assembling molecules into liquid crystalline order [137,140,173,174]. In this respect, new ionic liquid crystalline electrolytes, based on the imidazolium cation, have been synthesized [193]. The equimolar mixture of iodine and the imidazolium salt, [C12mim]I (A, m = 1; n = 12; X− = I−, Scheme 8), exhibited a smectic A mesophase between 27 °C and 45 °C. The dye-sensitized solar cell with this mixture displayed maximum short-circuit photocurrent Jsc around 7 mA cm−2, while the cell with the mixture of [C11mim]I/I2, which does not show liquid crystalline properties, gave Jsc around 6 mA cm−2. The measured diffusion coefficients for both electrolytes were 4.2 × 10−8 cm2 s−1 and 3.2 × 10−8 cm2 s−1, respectively. Homeotropically aligned samples of [C12mim]I/I2 exhibited anisotropy in ion conductivity for the directions, parallel and perpendicular to the director vector ñ. It has been postulated that in the smectic A molecular arrangement the I− and I3− anions are moved between smectic A layers, thus promoting the redox reaction between the dye and I- anion (Scheme 27) [193]. Later, it has been observed that the output characteristics of the DSSC, based on the amphiphilic Ru-dye 126 (Scheme 27), can be enhanced by the use of an ionic liquid crystalline mixture [C12mim]I/I2. Perhaps, the dye with C13 long chain substituents perfectly matches anisotropic orientation order of the ionic liquid crystalline electrolyte [194]. In addition, the maximum short-circuit photocurrent Jsc has been increased by forming the quasi-solid-state ionic liquid crystal DSSC (based on N719 Ru-dye) in the presence of the low molecular weight gelator 127 (Scheme 27) [195]. The imidazolium electrolyte is also operational in the solid crystalline state, though fabricated “all-solid-sate” DSS cells displayed lower electrical energy conversion efficiency then those constructed with ionic liquid crystalline electrolyte (2.6% vs. 3.51% for DSSCs based on dye 126) [196].
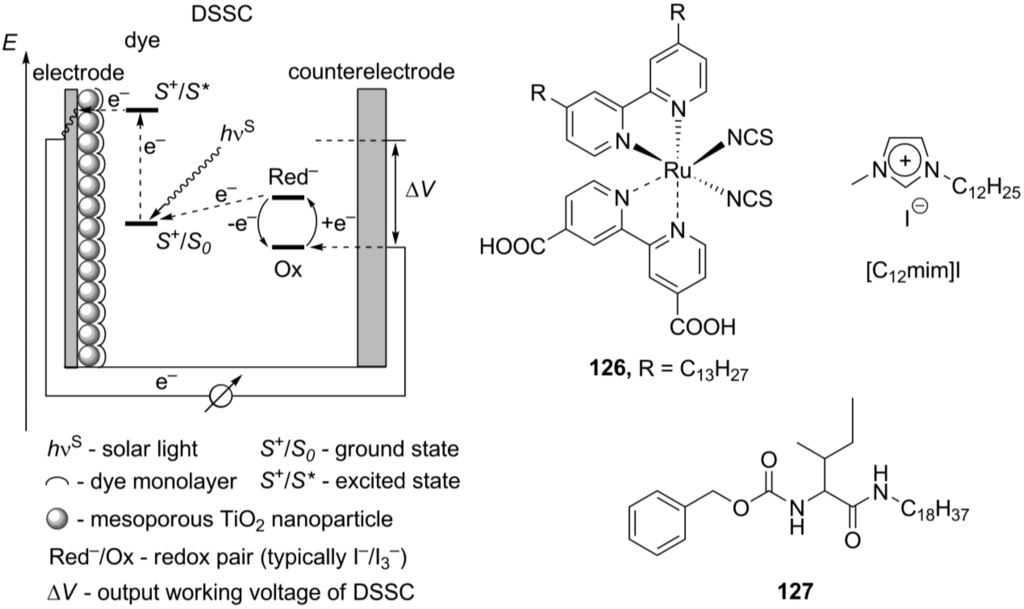
Scheme 27.
Schematic representation of a DSSC.
Ionic mesogens are also finding use in molecular biochemistry applications. As an example, new dialkoxy-substituted N-benzylimidazolium salts have been synthesized [197]. These compounds showed rich mesomorphism and formed smectic A (molecules with short alkyl tails) or hexagonal columnar phases (molecules with long chain substituents). The rare Ia3d bicontinuous cubic liquid crystalline phases have also been obtained and characterized. These ionic mesogenic molecules, when formulated with colipides, enhance the transport of active siRNA into a biological cell. The obtained results show a high transfection efficacy of formulated compounds and a high silencing efficiency with more than 80% inhibition of the targeted gene at 10 nM of siRNA concentration. Thus, the potency of amphiphilic imidazolium salts as a new generation of the RNA transport system has been demonstrated [197,198].
As it was demonstrated in this chapter, ionic liquid crystalline materials can find suitable applications in many areas of interest, from synthesis of inorganic nanoparticles to construction of OLEDs or solar cells of a new generation. One of the perspective directions could be an application of ionic-self assembled systems, possesing high anisotropic ion conductivity, as liquid crystalline electrolytes for fabrication of highly efficient dye-sensitized solar cells.
The late transition metallomesogens could be used for the preparation of highly ordered liquid crystalline emitting films for phosphorescent OLEDs. The self- or directed organization of metallomesogenic molecules into liquid crystalline orientational order could enhance hole transport directly to metal center and, consequently, the efficiency of a fabricated OLED device.
11. Summary and Outlook
The first thermotropic ionic liquid crystalline phases were obtained a long time ago. However, until recently, ionic mesogens have not received much attention. In the last two decades, the situation has changed dramatically. Due to the presence of charged particles in a mesophase, ionic liquid crystals reveal different mesomorphic behavior and phase morphology, as compared to neutral mesogens. The calamitic ionic molecules are constructed from a positively charged head and long hydrocarbon tails. This structure favors the formation of smectic lamellar phases via a combination of the electrostatic ion-ion interactions, stabilizing the structure of a layer, and Van-der Waals forces, organizing the arrangement of hydrophobic tails between ionic layers. Among a huge variety of so far obtained ionic mesogens, most of them are derived from the substituted cationic imidazolium framework, although ammonium, phosphonium, pyridinium and other charged mesogenic cores have also been employed for the design of ionic mesogenic molecules. In this field, new cationic mesogenic units, which could easily be accessed and further modified via simple synthetic transformations, are in high demand. As an illustration, the mesogens containing viologen or guanidinium-derived cationic groups displayed rich mesomorphism and form stable ionic mesophases. In an alternative synthetic approach, ionic self-assembly methodology (ISA) provides the possibility to build up ionic liquid crystalline complex structures from relatively simple charged components. The ionic self-assembled phases contain the highly branched network of channels, enhancing the ion transport and, consequently, ionic conductivity of a mesophase. Ionic mesogens are characterized by the ability to spontaneously create homeotropically aligned liquid crystalline phases with continuous orientational molecular order. The combination of enhanced ionic conductivity with liquid crystalline anisotropic properties makes ionic mesogens highly demanding materials for molecular electronics. Nowadays, the ionic liquid crystalline electrolytes are widely used for the fabrication of environmentally nonaggressive solar cells, having a reasonable electrical energy conversion efficiency and, at the same time, displaying a high efficiency over a very long operational period in harsh conditions, passing, for example, the stability test over 1000 h at 80 °C.
The synthesis of ionic metallomesogens is a highly challenging task, because the presence of a multicoordinated metal center typically disfavors the formation of a liquid crystalline phase. However, in the last years, a number of ionic mesogenic transition metal complexes, which are able to exhibit a mesophase at subambient temperatures, have been synthesized. This opens a way to the new luminescent and phosphorescent liquid crystalline materials possessing high ion conductivity. Potentially, the late transition metallomesogens could be employed for the preparation of highly effective phosphorescent OLEDs.
In conclusion, ionic liquid crystals display many interesting and useful features, among them, enhanced ion conductivity together with the ability to spontaneously create homeotropically aligned mesophases. Despite a huge variety of already synthesized ionic mesogenic systems, there is always room for further structural improvements, leading to interesting and variable mesomorphic properties, suitable for subsequent applications.
References and Notes
- Goodby, J.W.; Gray, G.W. Handbook of Liquid Crystals, 1st ed.; Demus, D., Goodby, J., Gray, G.W., Spiess, H.-W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany; New York, NY, USA, 1998; Volume 1, Chapter 2; pp. 18–19. [Google Scholar]
- Electro-Optical Displays, 1st ed.; Karim, M.A. (Ed.) Marcel Dekker, Inc.: New York, NY, USA, 1992.
- Clark, N.A.; Johnson, K.M. Liquid Crystal—Applications and Uses, 1st ed.; Bahadur, B., Ed.; World Scientific: Singapore, 1992; Volume 3, Chapter 17; pp. 209–252. [Google Scholar]
- De Bougrenet de la Tocnaye, J.L. Engineering liquid crystals for optimal uses in optical communication systems. Liq. Cryst. 2004, 31, 241–269. [Google Scholar]
- Hussain, A.; Pina, A.S.; Roque, A.C.A. Bio-recognition and detection using liquid crystals. Bisens. Bioelectron. 2009, 25, 1–8. [Google Scholar] [CrossRef]
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef] [PubMed]
- Goodby, J.W.; Gray, G.W. Handbook of Liquid Crystals, 1st ed.; Demus, D., Goodby, J., Gray, G.W., Spiess, H.-W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 1998; Volume 1–4. [Google Scholar]
- Paleos, C.M. Thermotropic liquid crystals derived from amphiphilic mesogens. Mol. Cryst. Liq. Cryst. 1994, 243, 159–183. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Mukai, T.; Ohno, H.; Kato, T. One-dimensional ion transport in self-organized columnar ionic liquids. J. Am. Chem. Soc. 2004, 126, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.E.; Hardacre, C.; Holbrey, J.D.; Johnston, S.; McMath, S.E.J.; Nieuwenhuyzen, M. Small-angle X-ray scattering studies of liquid crystalline 1-alkyl-3-methylimidazolium salts. Chem. Mater. 2002, 14, 629–635. [Google Scholar] [CrossRef]
- Ujiie, S.; IImura, K. Ammonium halide type thermotropic liquid-crystalline polyethylenimines and those low-mass model compounds. Chem. Lett. 1990, 19, 995–998. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Fukushima, T.; Aida, T. Discotic liquid crystals stabilized by interionic interactions: Imidazolium ion-anchored paraffinic triphenylene. Chem. Commun. 2005, 101–103. [Google Scholar] [CrossRef]
- Ujiie, S.; Iimura, K. Thermal properties and orientational behavior of a liquid-crystalline ion complex polymer. Macromolecules 1992, 25, 3174–3178. [Google Scholar] [CrossRef]
- Goodby, J.W. Handbook of Liquid Crystals, 1st ed.; Demus, D., Goodby, J., Gray, G.W., Spiess, H.-W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 1998; Volume 2A, Chapter 1; pp. 3–18. [Google Scholar]
- Chandrasekhar, S. Handbook of Liquid Crystals, 1st ed.; Demus, D., Goodby, J., Gray, G.W., Spiess, H.-W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 1998; Volume 2B, Chapter 8; pp. 750–752. [Google Scholar]
- Yu, W.; Peng, H.; Zhang, H.; Zhou, X. Synthesis and mesophase behaviour of morpholinium ionic liquid crystals. Chin. J. Chem. 2009, 27, 1471–1475. [Google Scholar] [CrossRef]
- Kondrat, S.; Bier, M.; Harnau, L. Phase behavior of ionic liquid crystals. J. Chem Phys. 2010, 132, 184901:1–184901:10. [Google Scholar] [CrossRef]
- Fairhurst, C.E.; Fuller, S.; Gray, J.; Holmes, M.C.; Tiddy, G.J.T. Handbook of Liquid Crystals, 1st ed.; Demus, D., Goodby, J., Gray, G.W., Spiess, H.-W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 1998; Volume 3, Chapter 7; pp. 341–392. [Google Scholar]
- Busico, V.; Cernicchiarro, P.; Corradini, P.; Vacatello, M. Polymorphism in anhydrous amphiphilic systems. Long-chain primary n-alkylammonium chlorides. J. Phys. Chem. 1983, 87, 1631–1635. [Google Scholar] [CrossRef]
- Menschutkin, N. Affinity coefficients of alkyl halogen compounds and of amines. Z. Phys. Chem. 1890, 6, 41–57. [Google Scholar]
- Alami, E.; Levy, H.; Zana, R.; Weber, P.; Skoulios, A. A new smectic mesophase with two dimensional tetragonal symmetry from dialkyldimethylammonium bromides: ST. Liq. Cryst. 1993, 13, 201–212. [Google Scholar] [CrossRef]
- Ohta, K.; Sugiyama, T.; Nogami, T. A Smectic T Phase of 1,4-dialkyl-1,4-diazoniabicyclo-[2.2.2]octane dibromides. J. Mater. Chem. 2000, 10, 613–616. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Tsiourvas, D.; Arkas, M.; Sideratou, Z.; Paleos, C.M. Thermotropic liquid crystalline behaviour of piperazinium and homopiperazinium alkylsulphates. Liq. Cryst. 2002, 29, 1547–1553. [Google Scholar] [CrossRef]
- Goossens, K.; Lava, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Driesen, K.; Görller-Walrand, C.; Binnemans, K.; Cardinaels, T. Pyrrolidinium ionic liquid crystals. Chem. Eur. J. 2009, 15, 656–674. [Google Scholar] [CrossRef]
- Goossens, K.; Lava, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Pattison, P.; Binnemans, K.; Cardinaels, T. Pyrrolidinium ionic liquid crystals with pendant mesogenic groups. Langmuir 2009, 25, 5881–5897. [Google Scholar] [CrossRef] [PubMed]
- Getsis, A.; Mudring, A.-V. Structural and thermal behaviour of the pyrrolidinium based ionic liquid crystals [C10mpyr]Br and [C12mpyr]Br. Z. Anorg. Allg. Chem. 2009, 635, 2214–2221. [Google Scholar] [CrossRef]
- Lava, K.; Binnemans, K.; Cardinaels, T. Piperidinium. piperazinium and morpholinium ionic liquid crystals. J. Phys. Chem. B 2009, 113, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.; Li, B.; Zhang, M.; Wu, L. Branched quaternary ammonium amphiphiles: Nematic ionic liquid crystals near room temperature. Chem. Commun. 2009, 35, 5269–5271. [Google Scholar] [CrossRef]
- Bradaric, C.J.; Downard, A.; Kennedy, C.; Robertson, A.J.; Zhou, Y. Industrial preparation of phosphonium ionic liquids. Green Chem. 2003, 5, 143–152. [Google Scholar] [CrossRef]
- Henderson, W.A., Jr; Streuli, C.A. The basicity of phosphines. J. Am. Chem. Soc. 1960, 82, 5791–5794. [Google Scholar] [CrossRef]
- Henderson, W.A., Jr; Buckler, S.A. The nucleophilicity of phosphines. J. Am. Chem. Soc. 1960, 82, 5794–5800. [Google Scholar] [CrossRef]
- Abdallah, D.J.; Robertson, A.; Hsu, H.-F.; Weiss, R.G. Smectic liquid-crystalline phases of quaternary group Va (especially phosphonium) salts with three equivalent long n-alkyl chains. How do layered assemblies form in liquid-crystalline and crystalline phases? J. Am. Chem. Soc. 2000, 122, 3053–3062. [Google Scholar] [CrossRef]
- Chen, H.; Kwait, D.C.; Gönen, Z.S.; Weslowski, B.T.; Abdallah, D.J.; Weiss, R.G. Phase characterization and properties of completely saturated quaternary phosphonium salts. Ordered, room-temperature ionic liquids. Chem. Mater. 2002, 14, 4063–4072. [Google Scholar] [CrossRef]
- Xu, W.; Cooper, E.I.; Angell, C.A. Ionic liquids: Ion mobilities, glass temperatures, and fragilities. J. Phys. Chem. B 2003, 107, 6170–6178. [Google Scholar] [CrossRef]
- Fraser, K.J.; Izgorodina, E.I.; Forsyth, M.; Scott, J.L.; MacFarlane, D.R. Liquids intermediate between “molecular” and “ionic” liquids: Liquid ion pairs? Chem Commun. 2007, 37(Oct. 7), 3817–3819. [Google Scholar]
- Ma, K.; Somashekhar, B.S.; Nagana Gowda, G.A.; Khetrapal, C.L.; Weiss, R.G. Induced amphotropic and thermotropic ionic liquid crystallinity in phosphonium halides: “Lubrication” by hydroxyl groups. Langmuir 2008, 24, 2746–2758. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Lee, K.-M.; Minkova, L.; Weiss, R.G. Design criteria for ionic liquid crystalline phases of phosphonium salts with three equivalent long n-alkyl chains. J. Org. Chem. 2009, 74, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuyzen, M.; Seddon, K.R.; Teixidor, F.; Puga, A.V.; Viñas, C. Ionic liquids containing boron cluster anions. Inorg. Chem. 2009, 48, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Dröge, T.; Glorius, F. NHC—Ligand der Ideen. Nachr. Chem. 2010, 58, 112–117. [Google Scholar] [CrossRef]
- Xiao, J.-C.; Twamley, B.; Shreeve, J.M. An ionic liquid-coordinated palladium complex: A highly efficient and recyclable catalyst for the Heck reaction. Org. Lett. 2004, 6, 3845–3847. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.R.; Rajagopal, R.; Srinivasan, K.V. Ultrasound promoted C-C bond formation: Heck reaction at ambient conditions in room temperature ionic liquids. Chem. Commun. 2001, 17, 1544–1545. [Google Scholar] [CrossRef]
- Bowlas, C.J.; Bruce, D.W.; Seddon, K.R. Liquid-crystalline ionic liquids. Chem. Commun. 1996, 14, 1625–1626. [Google Scholar] [CrossRef]
- Downard, A.; Earle, M.J.; Hardacre, C.; McMath, S.E.J.; Nieuwenhuyzen, M.; Teat, S.J. Structural studies of crystalline 1-alkyl-3-methylimidazolium chloride salts. Chem. Mater. 2004, 16, 43–48. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; Ionic liquids and ionic liquid crystals. J. Chem. Soc., Dalton Trans. 1999, 13, 2133–2139. [Google Scholar] [CrossRef]
- Del Pópolo, M.G.; Kohanoff, J.; Lynden-Bell, R.M.; Pinilla, C. Clusters, liquids, and crystals of dialkylimidazolium salts. A combined perspective from ab initio and classical computer simulations. Acc. Chem. Res. 2007, 40, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Hardacre, C.; Holbrey, J.D.; Mullan, C.L.; Youngs, T.G.A.; Bowron, D.T. Small angle neutron scattering from 1-alkyl-3-methylimidazolium hexafluorophosphate ionic liquids ([Cnmim][PF6], n = 4, 6, and 8). J. Chem. Phys. 2010, 133, 074510:1–074510:7. [Google Scholar] [CrossRef]
- Paulechka, Y.U.; Kabo, G.J.; Blokhin, A.V.; Shaplov, A.S.; Lozinskaya, E.I.; Golovanov, D.G.; Lyssenko, K.A.; Korlyukov, A.A.; Vygodskii, Ya.S. IR and X-ray study of polymorphism in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides. J. Phys. Chem. B 2009, 113, 9538–9546. [Google Scholar]
- Kaper, H.; Smarsly, B. Templating and phase behaviour of the long chain ionic liquid C16mimCl. Z. Phys. Chem. 2006, 220, 1455–1471. [Google Scholar] [CrossRef]
- Getsis, A.; Mudring, A.V. Imidazolium based ionic liquid crystals: Structure, photophysical and thermal behaviour of [Cnmim]Br·xH2O (n = 12, 14; x = 0, 1). Cryst. Res. Technol. 2008, 43, 1187–1196. [Google Scholar] [CrossRef]
- Getsis, A.; Tang, S.; Mudring, A.V. A luminescent ionic liquid crystal: [C12mim]4[EuBr6]Br. Eur. J. Inorg. Chem. 2010, 14, 2172–2177. [Google Scholar] [CrossRef]
- Godinho, M.H.; Cruz, C.; Teixeira, P.I.C.; Ferreira, A.J.; Costa, C.; Kulkarni, P.S.; Afonso, C.A.M. Shear-induced lamellar phase of an ionic liquid crystal at room temperature. Liq. Cryst. 2008, 35, 103–107. [Google Scholar] [CrossRef]
- Mehdi, H.; Binnemans, K.; Van Hecke, K.; Van Meervelt, L.; Nockemann, P. Hydrophobic ionic liquids with strongly coordinating anions. Chem. Commun. 2010, 46, 234–236. [Google Scholar] [CrossRef]
- Xu, F.; Matsumoto, K.; Hagiwara, R. Effects of alkyl chain length on properties of 1-alkyl-3-methylimidazolium fluorohydrogenate ionic liquid crystals. Chem. Eur. J. 2010, 16, 12970–12976. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Heinemann, F.W.; Yang, M.; Melcher, B.U.; Fekete, M.; Mudring, A.-V.; Wasserscheid, P.; Meyer, K. A new class of double alkyl-substituted, liquid crystalline imidazolium ionic liquids—A unique combination of structural features, viscosity effects, and thermal properties. Chem. Commun. 2009, 47, 7405–7407. [Google Scholar] [CrossRef]
- Chiou, J.Y.Z.; Chen, J.N.; Lei, J.S.; Lin, I.J.B. Ionic liquid crystals of imidazolium salts with a pendant hydroxyl group. J. Mater. Chem. 2006, 16, 2972–2977. [Google Scholar] [CrossRef]
- Dobbs, W.; Douce, L.; Allouche, L.; Louati, A.; Malbosc, F.; Welter, R. New ionic liquid crystals based on imidazolium salts. New J. Chem. 2006, 30, 528–532. [Google Scholar] [CrossRef]
- Dobbs, W.; Douce, L.; Heinrich, B. 1-(4-Alkyloxybenzyl)-3-methyl-1H-imidazol-3-ium organic backbone: A versatile smectogenic moiety. Beilstein J. Org. Chem. 2009, 5. [Google Scholar] [CrossRef]
- Fouchet, J.; Douce, L.; Heinrich, B.; Welter, R.; Louati, A. A convenient method for preparing rigid-core ionic liquid crystals. Beilstein J. Org. Chem. 2009, 5. [Google Scholar] [CrossRef]
- Suisse, J.M.; Bellemin-Laponnaz, S.; Douce, L.; Maisse-François, A.; Welter, R. A new liquid crystal compound based on an ionic imidazolium salt. Tetrahedron Lett. 2005, 46, 4303–4305. [Google Scholar] [CrossRef]
- Suisse, J.M.; Douce, L.; Bellemin-Laponnaz, S.; Maisse-François, A.; Welter, R.; Miyake, Y.; Shimizu, Y. Liquid crystal imidazolium salts: Towards materials for catalysis and molecular electronics. Eur. J. Inorg. Chem. 2007, 24, 3899–3905. [Google Scholar] [CrossRef]
- Li, X.; Bruce, D.W.; Shreeve, J.M. Dicationic imidazolium-based ionic liquids and ionic liquid crystals with variously positioned fluoro substituents. J. Mater. Chem. 2009, 19, 8232–8238. [Google Scholar] [CrossRef]
- Kouwer, P.H.J.; Swager, T.M. Synthesis and mesomorphic properties of rigid-core ionic liquid crystals. J. Am. Chem. Soc. 2007, 129, 14042–14052. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Nockemann, P.; Driesen, K.; Goderis, B.; Görller-Walrand, C.; van Hecke, K.; Van Meervelt, L.; Pouzet, E.; Binnemans, K.; Cardinaels, T. Imidazolium ionic liquid crystals with pendant mesogenic groups. Chem. Mater. 2008, 20, 157–168. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiao, L.; Shan, C.; Hou, P.; Chen, B.; Xu, X.; Niu, L. Synthesis and characterisation of novel imidazolium-based ionic liquid crystals with a p-nitroazobenzene moiety. Liq. Cryst. 2008, 35, 765–772. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, C.; Wang, X.; Chen, L.; Niu, L.; Chen, B. New ionic liquid crystals based on azobenzene moiety with two symmetric imidazolium ion group substituents. Liq. Cryst. 2008, 35, 1299–1305. [Google Scholar] [CrossRef]
- Lévesque, I.; Leclerc, M. Novel dual photochromism in polythiophene derivatives. Macromolecules 1997, 30, 4347–4352. [Google Scholar] [CrossRef]
- Trilla, M.; Pleixats, R.; Parella, T.; Blanc, C.; Dieudonné, P.; Guari, Y.; Man, M.W.C. Ionic liquid crystals based on mesitylene-containing bis- and trisimidazolium salts. Langmuir 2008, 24, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Ichikawa, T.; Shimura, H.; Kagata, T.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Columnar liquid-crystalline imidazolium salts. Effects of anions and cations on mesomorphic properties and ionic conductivities. Bull. Chem. Soc. Jpn. 2007, 80, 1836–1841. [Google Scholar] [CrossRef]
- Yoshio, M.; Kagata, T.; Hoshino, K.; Mukai, T.; Ohno, H.; Kato, T. One-dimentional ion-conductive polymer films: Alignment and fixation of ionic channels formed by self-organization of polymerizable columnar liquid crystals. J. Am. Chem. Soc. 2006, 128, 5570–5577. [Google Scholar]
- Pal, S.K.; Kumar, S. Microwave-assisted synthesis of novel imidazolium-based ionic liquid crystalline dimers. Tetrahedron Lett. 2006, 47, 8993–8997. [Google Scholar] [CrossRef]
- Kumar, S.; Pal, S.K. Synthesis and characterization of novel imidazolium-based ionic discotic liquid crystals with a triphenylene moiety. Tetrahedron Lett. 2005, 46, 2607–2610. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, S.K. The first examples of discotic liquid crystalline gemini surfactants. Tetrahedron Lett. 2010, 51, 5459–5462. [Google Scholar] [CrossRef]
- Cui, L.; Zhu, L. Ionic interaction induced novel ordered columnar mesophases in asymmetric discotic triphenylene salts. Liq. Cryst. 2006, 33, 811–818. [Google Scholar] [CrossRef]
- Alam, Md.A.; Motoyanagi, J.; Yamamoto, Y.; Fukushima, T.; Kim, J.; Kato, K.; Takata, M.; Saeki, A.; Seki, S.; Tagawa, S.; Aida, T. “Bicontinuous cubic” liquid crystalline materials from discotic molecules: A special effect of paraffinic side chains with ionic liquid pendants. J. Am. Chem. Soc. 2009, 131, 17722–17723. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Nishikawa, K. Synthesis and crystal structures of two ionic liquids with halogen-bonding groups: 4,5-Dibromo- and 4,5-diiodo-1-butyl-3-methylimidazolium trifluoromethanesulfonates. Solid State Sci. 2010, 12, 783–788. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Wiggins, K.M.; Kerr, R.L.; Chen, Z.; Bielawski, C.W. Design, synthesis, and study of benzobis- and bibenz(imidazolium)-based ionic liquid crystals. J. Mater. Chem. 2010, 20, 5709–5714. [Google Scholar] [CrossRef]
- Knight, G.A.; Shaw, B.D. Long-chain alkyl pyridines and their derivatives new examples of liquid crystals. J. Chem. Soc. 1938, 46, 682–683. [Google Scholar] [CrossRef]
- Sudhölter, E.J.R.; Engberts, J.B.F.N.; de Jeu, W.H. Thermotropic liquid-crystalline behavior of some single- and double-chained pyridinium amphiphiles. J. Phys. Chem. 1982, 86, 1908–1913. [Google Scholar] [CrossRef]
- Nusselder, J.J.H.; Engberts, J.B.F.N.; van Doren, H.A. Liquid-crystalline and thermochromic behaviour of 4-substituted 1-methylpyridinium iodide surfactants. Liq. Cryst. 1993, 13, 213–225. [Google Scholar] [CrossRef]
- Kohnen, G.; Tosoni, M.; Tussetschläger, S.; Baro, A.; Laschat, S. Counterion effects on the mesomorphic properties of chiral imidazolium and pyridinium ionic liquids. Eur. J. Org. Chem. 2009, 32, 5601–5609. [Google Scholar] [CrossRef]
- Ster, D.; Baumeister, U.; Lorenzo Chao, J.; Tschierske, C.; Israel, G. Synthesis and mesophase behaviour of ionic liquid crystals. J. Mater. Chem. 2007, 17, 3393–3400. [Google Scholar] [CrossRef]
- Lo Celso, F.; Pibiri, I.; Triolo, A.; Triolo, R.; Pace, A.; Buscemi, S.; Vivona, N. Study on the thermotropic properties of highly fluorinated 1,2,4-oxodiazolylpyridinium salts and their perspective applications as ionic liquid crystals. J. Mater. Chem. 2007, 17, 1201–1208. [Google Scholar] [CrossRef]
- Guittard, F.; Taffin de Givenchy, E.; Geribaldi, S.; Cambon, A. Highly fluorinated thermotropic liquid crystals: An update. J. Fluorine Chem. 1999, 100, 85–96. [Google Scholar] [CrossRef]
- Misaki, S.; Takamatsu, S.; Suefuji, M.; Mitote, T.; Matsumura, M. The synthesis of fluorine containing phenyl benzoates and their properties as liquid crystals. Mol. Cryst. Liq. Cryst. 1981, 66, 123–132. [Google Scholar] [CrossRef]
- Baudoux, J.; Judeinstein, P.; Cahard, D.; Plaquevent, J.C. Design and synthesis of novel ionic liquid/liquid crystals (IL2Cs) with axial chirality. Tetrahedron Lett. 2005, 46, 1137–1140. [Google Scholar] [CrossRef]
- Tanabe, K.; Yasuda, T.; Kato, T. Luminescent ionic liquid crystals based on tripodal pyridinium salts. Chem. Lett. 2008, 37, 1208–1209. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Zhu, L.; Zhang, S.; Li, J.; Zhang, X.; Deng, Y. Novel ionic liquid crystals based on N-caprolactam as cations. Chem. Mater. 2007, 19, 2544–2550. [Google Scholar] [CrossRef]
- Sauer, S.; Steinke, N.; Baro, A.; Laschat, S.; Giesselmann, F.; Kantlehner, W. Guanidinium chlorides with triphenylene moieties displaying columnar mesophase. Chem. Mater. 2008, 20, 1909–1915. [Google Scholar] [CrossRef]
- Sauer, S.; Saliba, S.; Tussetschläger, S.; Baro, A.; Frey, W.; Giesselmann, F.; Laschat, S.; Kantlehner, W. p-Alkoxybiphenyls with guanidinium head groups displaying smectic mesophases. Liq. Cryst. 2009, 36, 275–299. [Google Scholar] [CrossRef]
- Butschies, M.; Sauer, S.; Kessler, E.; Siehl, H.-U.; Claasen, B.; Fischer, P.; Frey, W.; Laschat, S. Influence of N-alkyl substituents and counterions on the structural and mesomorphic properties of guanidinium salts: Experiment and quantum chemical calculations. Chem. Phys. Chem. 2010, 11, 3752–3765. [Google Scholar] [PubMed]
- Yelamaggad, C.V.; Mathews, M.; Hiremath, U.S.; Shankar Rao, D.S.; Krishna Prasad, S. Self-assembly of chiral mesoionic heterocycles into smectic phases: A new class of polar liquid crystal. Tetrahedron Lett. 2005, 46, 2623–2626. [Google Scholar] [CrossRef]
- Causin, V.; Saielli, G. Effect of asymmetric substitution on the mesomorphic behaviour of low-melting viologen salts of bis(trifluoromethane)sulfonylamide. J. Mater. Chem. 2009, 19, 9153–9162. [Google Scholar] [CrossRef]
- Tanabe, K.; Yasuda, T.; Yoshio, M.; Kato, T. Viologen-based redox-active ionic liquid crystals forming columnar phases. Org. Lett. 2007, 9, 4271–4274. [Google Scholar] [CrossRef] [PubMed]
- Metallomesogens: Synthesis, Properties, and Applications, 1st ed.; Serrano, J.L. (Ed.) VCH: Weinheim, Germany, 1996.
- Galyametdinov, Y.G.; Haase, W.; Goderis, B.; Moors, D.; Driesen, K.; van Deun, R.; Binnemans, K. Magnetic alignment study of rare-earth-containing liquid crystals. J. Phys. Chem. B 2007, 111, 13881–13885. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, H.C.; Shiozaki, T.; Kamata, A.; Kishida, K.; Ohmori, T.; Kiriya, D.; Yamauchi, T.; Furukawa, H.; Kitagawa, S. A redox-active columnar metallomesogen and its cyclic voltammetric responses. J. Mater. Chem. 2007, 17, 4136–4138. [Google Scholar] [CrossRef]
- Cavero, E.; Uriel, S.; Romero, P.; Serrano, J.L.; Giménez, R. Tetrahedral zinc complexes with liquid crystalline and luminescent properties: Interplay between nonconventional molecular shapes and supramolecular mesomorphic order. J. Am. Chem. Soc. 2007, 129, 11608–11618. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, D.; Goc, J.; Ion, R.M. Photovoltaic and spectral properties of tetraphenyloporphyrin and metallotetraphenyloporphyrino dyes. J. Mol. Struct. 1998, 450, 239–246. [Google Scholar] [CrossRef]
- Vorländer, D. Verhalten der Salze organischer Säuren beim Schmelzen. Ber. Dtsch. Chem. Ges. 1910, 43, 3120–3135. [Google Scholar] [CrossRef]
- Garbovskiy, Y.; Koval’chuk, A.; Grydyakina, A.; Bugaychuk, S.; Mirnaya, T.; Klimusheva, G. Electrical conductivity of lyotropic and thermotropic ionic liquid crystals consisting of metal alkanoates. Liq. Cryst. 2007, 34, 599–603. [Google Scholar] [CrossRef]
- Casado Martínez, F.J.; Riesco Ramos, M.; Da Silva, I.; Labrador, A.; Redondo, M.I.; García Peréz, M.V.; López-Andrés, S.; Rodríguez Cheda, J.A. Thermal and structural study of the crystal phases and mesophases in the lithium and thallium(I) propanoates and pentanoates binary systems: Formation of mixed salts and stabilization of the ionic liquid crystal phase. J. Phys. Chem. B 2010, 114, 10075–10085. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bu, W.; Qi, W.; Wu, L. Self-assembled multibilayers of europium alkanoates: Structure, photophysics, and mesomorphic behavior. J. Phys. Chem. B 2005, 109, 21669–21676. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.B.; Cheng, C.S.; Zhang, B.Y.; He, X.Z. Synthesis and characterization of a novel liquid crystal-bearing ionic mesogen. Liq. Cryst. 2005, 32, 191–195. [Google Scholar] [CrossRef]
- Taubert, A.; Steiner, P.; Mantion, A. Ionic liquid crystal precursors for inorganic particles: Phase diagram and thermal properties of a CuCl nanoplatelet precursor. J. Phys. Chem. B 2005, 109, 15542–15547. [Google Scholar] [CrossRef]
- Dobbs, W.; Suisse, J.-M.; Douce, L.; Welter, R. Electrodeposition of silver particles and gold nanoparticles from ionic-liquid crystal precursors. Angew. Chem. 2006, 118, 4285–4288. [Google Scholar] [CrossRef]
- Getsis, A.; Balke, B.; Felser, C.; Mudring, A.V. Dysprosium-based ionic liquid crystals: Thermal, structural, photo- and magnetophysical properties. Cryst. Growth Des. 2009, 9, 4429–4437. [Google Scholar] [CrossRef]
- Mayoral, M.J.; Ovejero, P.; Campo, J.A.; Heras, J.V.; Pinilla, E.; Torres, M.R.; Cano, M. Ionic liquid crystals from β-diketonyl containing pyridinium cations and tetrachlorozincate anions. Inorg. Chem. Commun. 2009, 12, 214–218. [Google Scholar] [CrossRef]
- Lin, I.J.B.; Vasam, C.S. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety. J. Organomet. Chem. 2005, 690, 3498–3512. [Google Scholar] [CrossRef]
- Piguet, C.; Bünzli, J.C.G.; Donnio, B.; Guillon, D. Thermotropic lanthanidomesogens. Chem. Commun. 2006, 36, 3755–3768. [Google Scholar] [CrossRef]
- Terazzi, E.; Guénée, L.; Morgantini, P.Y.; Bernardinelli, G.; Donnio, B.; Guillon, D.; Piguet, C. Tuning the polarization along linear polyaromatic strands for rationally inducing mesomorphism in lanthanide nitrate complexes. Chem. Eur. J. 2007, 13, 1674–1691. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Terazzi, E.; Suárez, S.; Torelli, S.; Nozary, H.; Imbert, D.; Mamula, O.; Rivera, J.P.; Guillet, E.; Bénech, J.M.; Bernardinelli, G.; Scopelliti, R.; Donnio, B.; Guillon, D.; Bünzli, J.C.G.; Piguet, C. Introducing bulky functional lanthanide cores into thermotropic metallomesogens: A bottom-up approach. Adv. Funct. Mater. 2006, 16, 157–168. [Google Scholar] [CrossRef]
- Jensen, T.B.; Terazzi, E.; Buchwalder, K.L.; Guénée, L.; Nozary, H.; Schenk, K.; Heinrich, B.; Donnio, B.; Guillon, D.; Piguet, C. Dimerization of dendrimeric lanthanide complexes: Thermodynamic, thermal, and liquid-crystalline properties. Inorg. Chem. 2010, 49, 8601–8619. [Google Scholar] [CrossRef] [PubMed]
- Suárez, S.; Mamula, O.; Scopelliti, R.; Donnio, B.; Guillon, D.; Terazzi, E.; Piguet, C.; Bünzli, J.C.G. Lanthanide luminescent mesomorphic complexes with macrocycles derived from diaza-18-crown-6. New J. Chem. 2005, 29, 1323–1334. [Google Scholar] [CrossRef]
- Percec, V.; Johansson, G.; Rodenhouse, R. Molecular recognition directed phase transitions in side-chain liquid crystalline polymers containing crown ethers. Macromolecules 1992, 25, 2563–2565. [Google Scholar] [CrossRef]
- Coco, S.; Cordovilla, C.; Espinet, P.; Gallani, J.-L.; Guillon, D.; Donnio, B. Supramolecular aggregates in fluid phases: Mesomorphic ortho-palladated complexes with substituted crown ethers and their potassium adducts. Eur. J. Inorg. Chem. 2008, 2008, 1210–1218. [Google Scholar] [CrossRef]
- Steinke, N.; Frey, W.; Baro, A.; Laschat, S.; Drees, C.; Nimtz, M.; Hägele, C.; Giesselmann, F. Columnar and smectic liquid crystals based on crown ethers. Chem. Eur. J. 2006, 12, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Steinke, N.; Jahr, M.; Lehmann, M.; Baro, A.; Frey, W.; Tussetschläger, S.; Sauer, S.; Laschat, S. Crown ethers with lateral ortho-terphenyl units: Effect of ester groups and sodium salts on the mesomorphic properties. J. Mater. Chem. 2009, 19, 645–654. [Google Scholar] [CrossRef]
- Kaller, M.; Tussetschläger, S.; Fischer, P.; Deck, C.; Baro, A.; Giesselmann, F.; Laschat, S. Columnar mesophases controlled by counterions in potassium complexes of dibenzo[18]crown-6 derivatives. Chem. Eur. J. 2009, 15, 9530–9542. [Google Scholar] [CrossRef] [PubMed]
- Kaller, M.; Deck, C.; Meister, A.; Hause, G.; Baro, A.; Laschat, S. Counterion effects on the columnar mesophases of triphenylene-substituted [18]crown-6 ethers: Is flatter better? Chem. Eur. J. 2010, 16, 6326–6337. [Google Scholar] [CrossRef] [PubMed]
- Steinke, N.; Kaller, M.; Nimtz, M.; Baro, A.; Laschat, S. Columnar liquid crystals derived from crown ethers with two lateral ester-substituted ortho-terphenyl units: Unexpected destabilisation of the mesophase with potassium iodide. Liq. Cryst. 2010, 37, 1139–1149. [Google Scholar] [CrossRef]
- Kaharu, T.; Ishii, R.; Takahashi, S. Liquid-crystalline gold-isonitrile complexes. J. Chem. Soc. Chem. Commun. 1994, 1349–1350. [Google Scholar]
- Lee, Y.A.; McGarrach, J.E.; Lachicotte, R.J.; Eisenberg, R. Multiple emissions and brilliant white luminescence from gold(I) o,o’-di(alkyl)dithiophosphate dimers. J. Am. Chem. Soc. 2002, 124, 10662–10663. [Google Scholar] [CrossRef] [PubMed]
- Bayón, R.; Coco, S.; Espinet, P. Gold liquid crystals displaying luminescence in the mesophase and short F···F interactions in the solid state. Chem. Eur. J. 2005, 11, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Pucci, D.; Barberio, G.; Bellusci, A.; Crispini, A.; Donnio, B.; Giorgini, L.; Ghedini, M.; La Deda, M.; Szerb, E.I. Silver coordination complexes as room-temperature multifunctional materials. Chem. Eur. J. 2006, 12, 6738–6747. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, A.; Ghedini, M.; Giorgini, L.; Gozzo, F.; Szerb, E.I.; Crispini, A.; Pucci, D. Anion dependent mesomorphism in coordination networks based on 2,2’-bipiridine silver(I) complexes. Dalton Trans. 2009, 36, 7381–7389. [Google Scholar] [CrossRef] [PubMed]
- Torralba, M.C.; Campo, J.A.; Heras, J.V.; Bruce, D.W.; Cano, M. Mesomorphism of ionic allylpalladium(II) complexes containing pzR2py as ligands and [BF4]−, [PF6]− or [CF3SO3]− as counteranions. Dalton Trans. 2006, 32, 3918–3926. [Google Scholar] [CrossRef] [PubMed]
- Torralba, M.C.; Cano, M.; Campo, J.A.; Heras, J.V.; Pinilla, E.; Torres, M.R. Liquid crystal behaviour of ionic allylpalladium complexes containing 2-pyrazolylpyridine as bidentate N,N’-ligand. J. Organomet. Chem. 2006, 691, 765–778. [Google Scholar] [CrossRef]
- Cardinaels, T.; Ramaekers, J.; Guillon, D.; Donnio, B.; Binnemans, K. A propeller-like uranyl metallomesogen. J. Am. Chem. Soc. 2005, 127, 17602–17603. [Google Scholar] [CrossRef] [PubMed]
- Cardinaels, T.; Ramaekers, J.; Nockemann, P.; Driesen, K.; Van Hecke, K.; Van Meervelt, L.; Lei, S.; De Feyter, S.; Guillon, D.; Donnio, B.; Binnemans, K. Imidazo[4,5-f]-1,10-phenanthrolines: Versatile ligands for the design of metallomesogens. Chem. Mater. 2008, 20, 1278–1291. [Google Scholar] [CrossRef]
- Cardinaels, T.; Ramaekers, J.; Driesen, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Goderis, B.; Binnemans, K. Thermotropic ruthenium(II)-containing metallomesogens based on substituted 1,10-phenanthroline ligands. Inorg. Chem. 2009, 48, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.F.J.; Antonietti, M. Ionic self-assembly: Facile synthesis of supramolecular materials. Adv. Mater. 2003, 15, 673–683. [Google Scholar] [CrossRef]
- Camerel, F.; Faul, C.F.J. Combination of ionic self-assembly and hydrogen bonding as a tool for the synthesis of liquid-crystalline materials and organogelators from a simple building block. Chem. Commun. 2003, 1515, 1958–1959. [Google Scholar] [CrossRef]
- Kato, T. Self-assembly of phase-segregated liquid crystal structures. Science 2002, 295, 2414–2418. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. Hydrogen-bonded liquid crystals: Molecular self-assembly for dynamically functional material. Struct. Bonding 2000, 96, 95–146. [Google Scholar]
- Kato, T.; Mizoshita, N.; Kanie, K. Hydrogen-bonded liquid crystalline materials: Supramolecular polymeric assembly and the induction of dynamic function. Macromol. Rapid Commun. 2001, 22, 797–814. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef]
- Yazaki, S.; Kamikawa, Y.; Yoshio, M.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Ionic liquid crystals: Self-assembly of imidazolium salts containing an l-glutamic acid moiety. Chem. Lett. 2008, 37, 538–539. [Google Scholar] [CrossRef]
- Noguchi, T.; Kishikawa, K.; Kohmoto, S. Volume effect of alkyl chains on organization of ionic self-assemblies toward hexagonal columnar mesophases. Bull. Chem. Soc. Jpn. 2008, 81, 778–783. [Google Scholar] [CrossRef]
- Noguchi, T.; Kishikawa, K.; Kohmoto, S. Tailoring liquid-crystalline supramolecular structures by ionic interactions. Chem. Lett. 2008, 37, 12–13. [Google Scholar] [CrossRef]
- Noguchi, T.; Kishikawa, K.; Kohmoto, S. Tailoring of ionic supramolecular assemblies based on ammonium carboxylates toward liquid-crystalline micellar cubic mesophases. Liq. Cryst. 2008, 35, 1043–1050. [Google Scholar] [CrossRef]
- Zakrevskyy, Y.; Smarsly, B.; Stumpe, J.; Faul, C.F.J. Highly ordered monodomain ionic self-assembled liquid-crystalline materials. Phys. Rev. E. 2005, 71, 021701:1–021701:12. [Google Scholar] [CrossRef]
- Olivier, J.H.; Camerel, F.; Barberá, J.; Retailleau, P.; Ziessel, R. Ionic liquid crystals formed by self-assembly around an anionic anthracene core. Chem. Eur. J. 2009, 15, 8163–8174. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.-H.; Camerel, F.; Ulrich, G.; Barberá, J.; Ziessel, R. Luminescent ionic liquid crystals from self-assembled bodipy disulfonate and imidazolium frameworks. Chem. Eur. J. 2010, 16, 7134–7142. [Google Scholar] [CrossRef] [PubMed]
- Camerel, F.; Ulrich, G.; Barberá, J.; Ziessel, R. Ionic self-assembly of ammonium-based amphiphiles and negatively charged bodipy and porphyrin luminophores. Chem. Eur. J. 2007, 13, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Ringstrand, B.; Monobe, H.; Kaszynski, P. Anion-driven mesogenicity: Ionic liquid crystals based on [closo-1-CB9H10]− cluster. J. Mater. Chem. 2009, 19, 4805–4812. [Google Scholar] [CrossRef]
- Kohmoto, S.; Hara, Y.; Kishikawa, K. Hydrogen-bonded ionic liquid crystals: Pyridinylmethylimidazolium as a versatile building block. Tetrahedron Lett. 2010, 51, 1508–1511. [Google Scholar] [CrossRef]
- Macknight, W.J.; Ponomarenko, E.A.; Tirrell, D.A. Self-assembled polyelectrolyte-surfactant complexes in nonaqueous solvents and in the solid state. Acc. Chem. Res. 1998, 31, 781–788. [Google Scholar] [CrossRef]
- Zhou, S.; Chu, B. Assembled materials: Polyelectrolyte-surfactant complexes. Adv. Mater. 2000, 12, 545–556. [Google Scholar]
- Batra, D.; Seifert, S.; Firestone, M.A. The effect of cation structure on the mesophase architecture of self-assembled and polymerized imidazolium-based ionic liquids. Macromol. Chem. Phys. 2007, 208, 1416–1427. [Google Scholar] [CrossRef]
- Lee, S.; Becht, G.A.; Lee, B.; Burns, C.T.; Firestone, M.A. Electropolymerization of a bifunctional ionic liquid monomer yields an electroactive liquid-crystalline polymer. Adv. Funct. Mater. 2010, 20, 2063–2070. [Google Scholar] [CrossRef]
- Jazkewitsch, O.; Ritter, H. Polymerizable ionic liquid crystals. Macromol. Rapid Commun. 2009, 30, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Meng, F.B.; Tian, M.; Xiao, W.Q. Side-chain liquid-crystalline polysiloxanes containing ionic mesogens and cholesterol ester groups. React. Funct. Polym. 2006, 66, 551–558. [Google Scholar] [CrossRef]
- Meng, F.B.; Zhang, B.Y.; Jia, Y.G.; Yao, D.S. Effect of ionic aggregates of sulphonate groups on the liquid crystalline behaviours of liquid crystalline elastomers. Liq. Cryst. 2005, 32, 183–189. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, X.; Lu, Q. Photosensitive polymer from ionic self-assembly of azobenzene dye and poly(ionic liquid) and its alignment characteristic toward liquid crystal molecules. Macromolecules 2007, 40, 7944–7950. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, X.; Lu, Q.; Su, B. Photosensitive liquid-crystalline supramolecules self-assembled from ionic liquid crystal and polyelectrolyte for laser-induced optical anisotropy. Macromolecules 2008, 41, 3884–3892. [Google Scholar] [CrossRef]
- Ujiie, S.; Furukawa, H.; Yano, Y.; Mori, A. Oriented glass states formed by ionic liquid–liquid crystals. Thin Solid Films 2006, 509, 185–188. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Boiko, N.I.; Shibaev, V.P. Liquid-crystalline dendrimers. Vysokomol. Soedin. Ser. A B 2001, 43, 1601–1650. [Google Scholar]
- Martín-Rapún, R.; Marcos, M.; Omenat, A.; Barberá, J.; Romero, P.; Serrano, J.L. Ionic thermotropic liquid crystal dendrimers. J. Am. Chem. Soc. 2005, 127, 7397–7403. [Google Scholar] [CrossRef] [PubMed]
- Marcos, M.; Martín-Rapún, R.; Omenat, A.; Barberá, J.; Serrano, J.L. Ionic liquid crystal dendrimers with mono-, di- and trisubstituted benzoic acids. Chem. Mater. 2006, 18, 1206–1212. [Google Scholar] [CrossRef]
- Antharjanam, P.K.S.; Jaseer, M.; Ragi, K.N.; Prasad, E. Intrinsic luminescence properties of ionic liquid crystals based on PAMAM and PPI dendrimers. J. Photochem. Photobiol. A Chem. 2009, 203, 50–55. [Google Scholar] [CrossRef]
- Cook, A.G.; Baumeister, U.; Tschierske, C. Supramolecular dendrimers: Unusual mesophases of ionic liquid crystals derived from protonation of DAB dendrimers with facial amphiphilic carboxylic acids. J. Mater. Chem. 2005, 15, 1708–1721. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Z.; Gehringer, L.; Frey, H.; Stiriba, S.E. Supramolecular thermotropic liquid crystalline materials with nematic mesophase based on methylated hyperbranched polyethylenimine and mesogenic carboxylic acid. Macromol. Rapid Commun. 2006, 27, 69–75. [Google Scholar] [CrossRef]
- Marcos, M.; Alcalá, R.; Barberá, J.; Romero, P.; Sánchez, C.; Serrano, J.L. Photosensitive ionic nematic liquid crystalline complexes based on dendrimers and hyperbranched polymers and a cyanoazobenzene carboxylic acid. Chem. Mater. 2008, 20, 5209–5217. [Google Scholar] [CrossRef]
- Taubert, A. CuCl nanoplatelets from an ionic liquid-crystal precursor. Angew. Chem. 2004, 116, 5494–5496. [Google Scholar] [CrossRef]
- Taubert, A.; Li, Z. Inorganic materials from ionic liquids. Dalton Trans. 2007, 7, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A.; Palivan, C.; Casse, O.; Gozzo, F.; Schmitt, B. Ionic liquid-crystal precursors (ILCPs) for CuCl nanoplatelets: The origin of the exothermic peak in DSC curves. J. Phys. Chem. C 2007, 111, 4077–4082. [Google Scholar] [CrossRef]
- Taubert, A.; Arbell, I.; Mecke, A.; Graf, P. Photoreduction of a crystalline Au(III) complex: A solid-state approach to metallic nanostructures. Gold Bull. 2006, 39, 205–211. [Google Scholar] [CrossRef]
- Wang, Q.T.; Wang, X.B.; Lou, W.J.; Hao, J.C. Stable blue- and green-emitting zinc oxide from ionic liquid crystal precursors. ChemPhysChem 2009, 10, 3201–3203. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Mukai, T.; Kanie, K.; Yoshizawa, M.; Ohno, H.; Kato, T. Layered ionic liquids: Anisotropic ion conduction in new self-organized liquid-crystalline materials. Adv. Mater. 2002, 14, 351–354. [Google Scholar] [CrossRef]
- Shimura, H.; Yoshio, M.; Hoshino, K.; Mukai, T.; Ohno, H.; Kato, T. Noncovalent approach to one-dimensional ion conductors: Enhancement of ionic conductivities in nanostructured columnar liquid crystals. J. Am. Chem. Soc. 2008, 130, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. From nanostructured liquid crystals to polymer-based electrolytes. Angew. Chem. 2010, 122, 8019–8021. [Google Scholar] [CrossRef]
- Yazaki, S.; Funahashi, M.; Kagimoto, J.; Ohno, H.; Kato, T. Nanostructured liquid crystals combining ionic and electronic functions. J. Am. Chem. Soc. 2010, 132, 7702–7708. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Tohidi, M. Design and characterization of liquid crystal-graphite composite electrodes. J. Phys. Chem. C 2010, 114, 6132–6140. [Google Scholar] [CrossRef]
- Binnemans, K.; Görller-Walrand, C. Lanthanide-containing liquid crystals and surfactants. Chem. Rev. 2002, 102, 2303–2345. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G. Benefiting from the unique properties of lanthanide ions. Acc. Chem. Res. 2006, 39, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.Q.; Huang, C.H. Highly Efficient OLEDs with Phosphorescent Materials, 1st ed.; Yersin, H., Ed.; Wiley-VCH: Weinheim, Germany, 2008; Chapter 12; pp. 391–420. [Google Scholar]
- Puntus, L.N.; Schenk, K.J.; Bünzli, J.C.G. Intense near-infrared luminescence of a mesomorphic ionic liquid doped with lanthanide β-diketonate ternary complexes. Eur. J. Inorg. Chem. 2005, 23, 4739–4744. [Google Scholar] [CrossRef]
- Binnemans, K. Luminescence of metallomesogens in liquid crystal state. J. Mater. Chem. 2009, 19, 448–453. [Google Scholar] [CrossRef]
- Highly Efficient OLEDs with Phosphorescent Materials, 1st ed.; Yersin, H. (Ed.) Wiley-VCH: Weinheim, Germany, 2008; pp. 1–390.
- Evans, R.C.; Douglas, P.; Winscom, C.J. Coordination complexes exhibiting room-temperature phosphorescence: Evaluation of their suitability as triplet emitters in organic light emitting diodes. Coord. Chem. Rev. 2006, 250, 2093–2126. [Google Scholar] [CrossRef]
- Yersin, H.; Winkenzeller, W.J. Highly Efficient OLEDs with Phosphorescent Materials, 1st ed.; Yersin, H., Ed.; Wiley-VCH: Weinheim, Germany, 2008; Chapter 1; pp. 1–97. [Google Scholar]
- Yersin, H.; Donges, D. Low-lying electronic states and photophysical properties of organometallic Pd(II) and Pt(II) compounds. modern research trends presented in detailed case studies. Top. Curr. Chem. 2001, 214, 81–186. [Google Scholar]
- Szerb, E.I.; Talarico, A.M.; Aiello, I.; Crispini, A.; Godbert, N.; Pucci, D.; Pugliese, T.; Ghedini, M. Green to red switch driven by order in an ionic IrIII liquid-crystalline complex. Eur. J. Inorg. Chem. 2010, 2010, 3270–3277. [Google Scholar] [CrossRef]
- O'Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Zakeeruddin, S.M.; Grätzel, M. Solvent-free ionic liquid electrolytes for mesoscopic dye-sensitized solar cells. Adv. Funct. Mater. 2009, 19, 2187–2202. [Google Scholar] [CrossRef]
- Gorlov, M.; Kloo, L. Ionic liquid electrolytes for dye-sensitized solar cells. Dalton Trans. 2008, 2655–2666. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Ionic liquid crystal as a hole transport layer of dye-sensitized solar cells. Chem. Commun. 2005, 6, 740–742. [Google Scholar] [CrossRef]
- Kawano, R.; Nazeeruddin, Md.K.; Sato, A.; Grätzel, M.; Watanabe, M. Amphiphilic ruthenium dye as an ideal sensitizer in conversion of light to electricity using ionic liquid crystal electrolyte. Electrochem. Commun. 2007, 9, 1134–1138. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Masaki, N.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Dye-sensitized TiO2 solar cells using imidazolium-type ionic liquid crystal systems as effective electrolytes. J. Phys. Chem. B 2007, 111, 4763–4769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhai, J.; He, J.; Chen, X.; Chen, L.; Zhang, L.; Tian, Y.; Jiang, L.; Zhu, D. High-performance all-solid-state dye-sensitized solar cells utilizing imidazolium-type ionic crystal as charge transfer layer. Chem. Mater. 2008, 20, 6022–6028. [Google Scholar] [CrossRef]
- Dobbs, W.; Heinrich, B.; Bourgogne, C.; Donnio, B.; Terazzi, E.; Bonnet, M.E.; Stock, F.; Erbacher, P.; Bolcato-Bellemin, A.L.; Douce, L. Mesomorphic imidazolium salts: new vectors for efficient siRNA transfection. J. Am. Chem. Soc. 2009, 131, 13338–13346. [Google Scholar] [CrossRef] [PubMed]
- Desigaux, L.; Sainlos, M.; Lambert, O.; Chevre, R.; Letrou-Bonneval, E.; Vigneron, J.P.; Lehn, P.; Lehn, J.M.; Pitard, B. Self-assembled lamellar complexes of siRNA with lipidic aminoglycoside derivatives promote efficient siRNA delivery and interference. Proc. Natl. Akad. Sci.USA 2007, 104, 16534–16539. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).