Tailoring Iridium Valence States on ZSM-5 for Enhanced Catalytic Performance in CO Selective Catalytic Reduction of NO under Oxygen-Enriched Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. CO-SCR Performance Evaluation
2.3. Catalyst Characterization
3. Results and Discussion
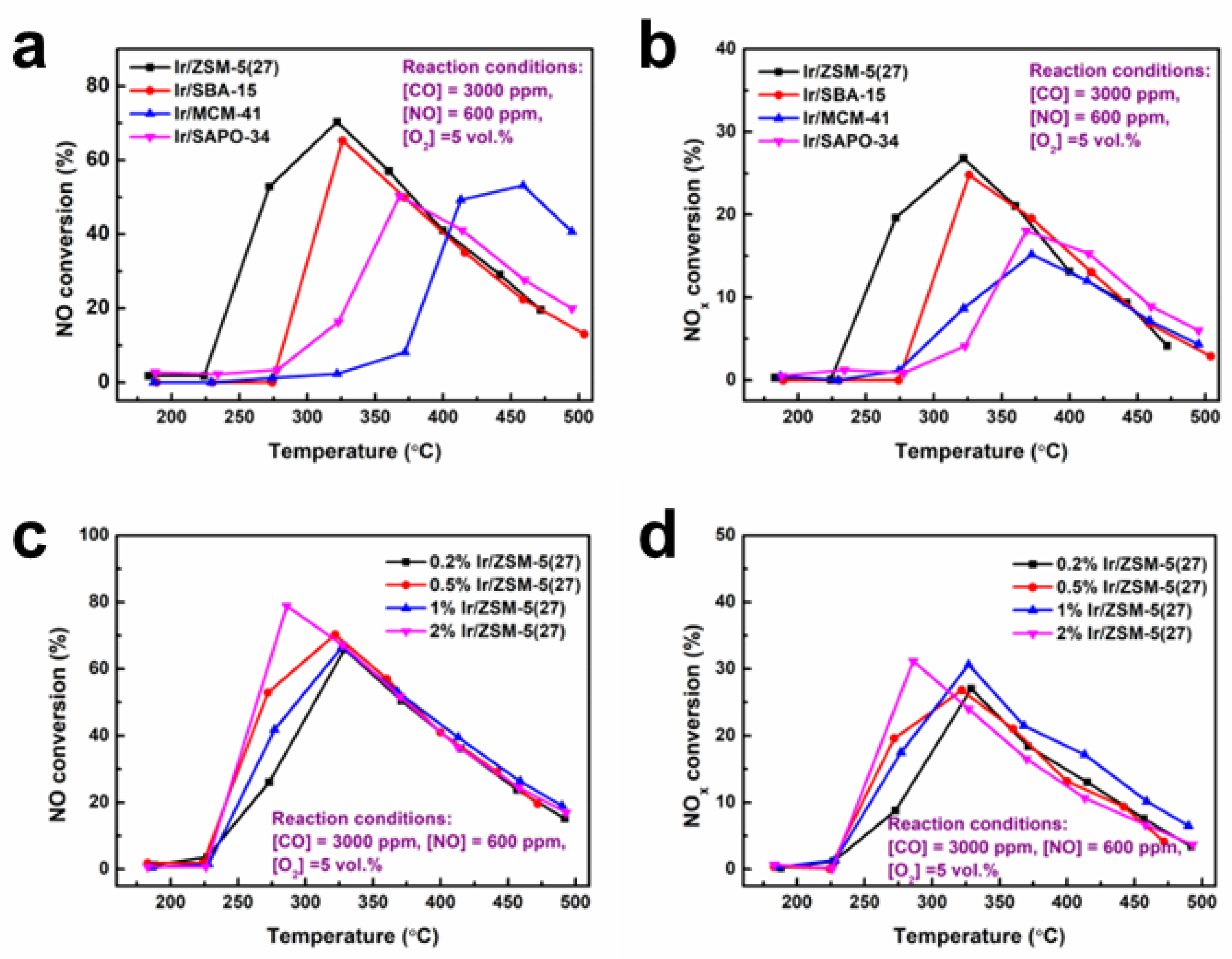
3.1. The Influence of Catalyst Carrier and Ir Loading Amount on Catalytic Performance
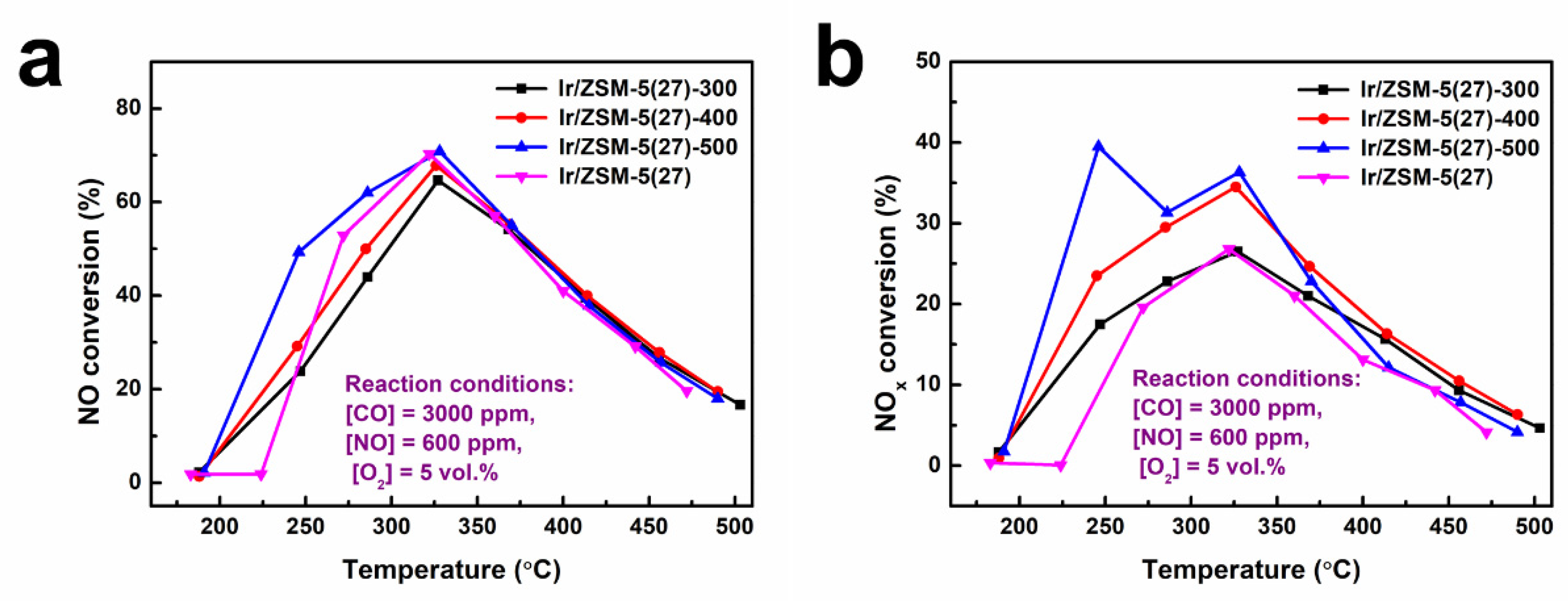
3.2. Optimization of Reduction Pretreatment
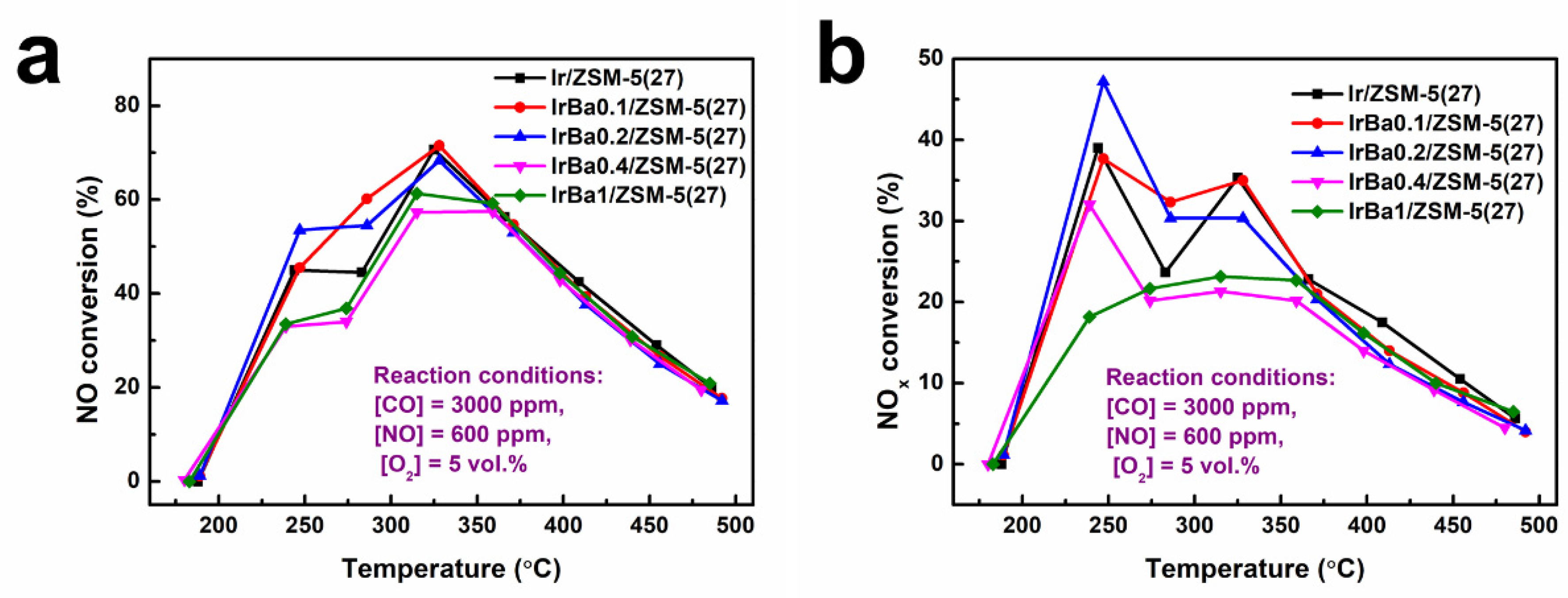
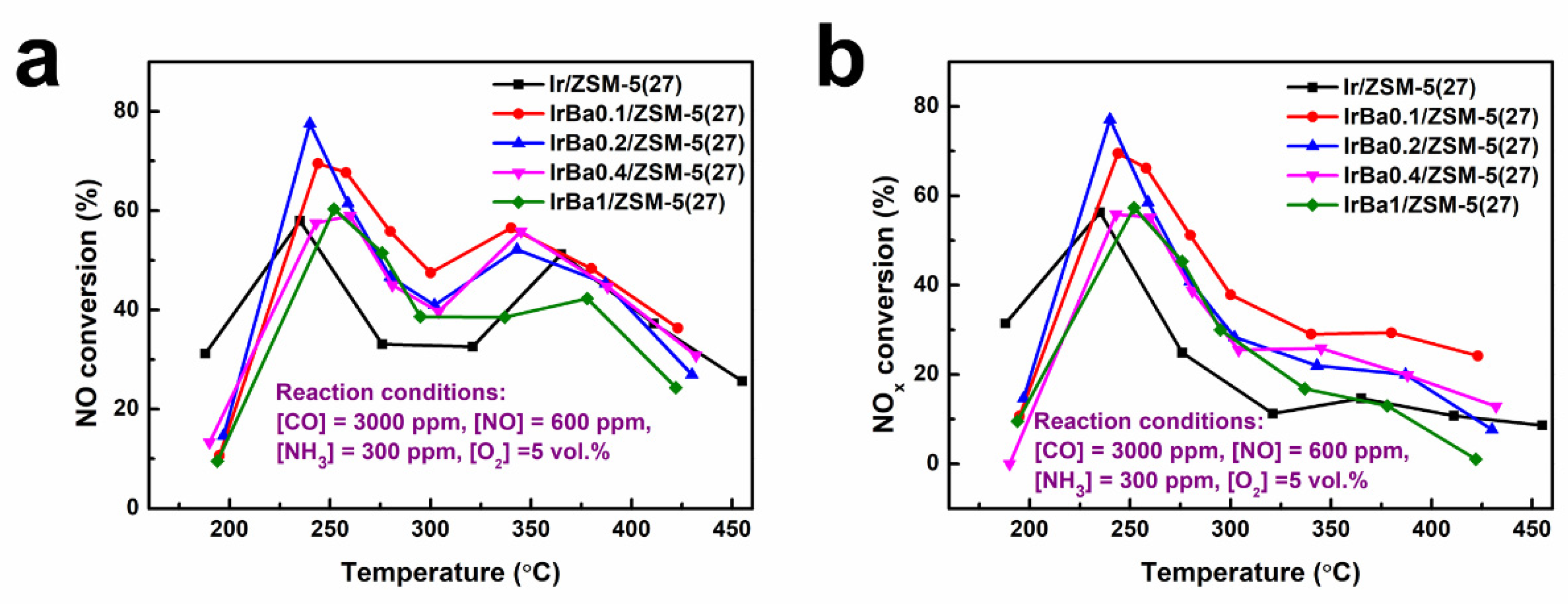
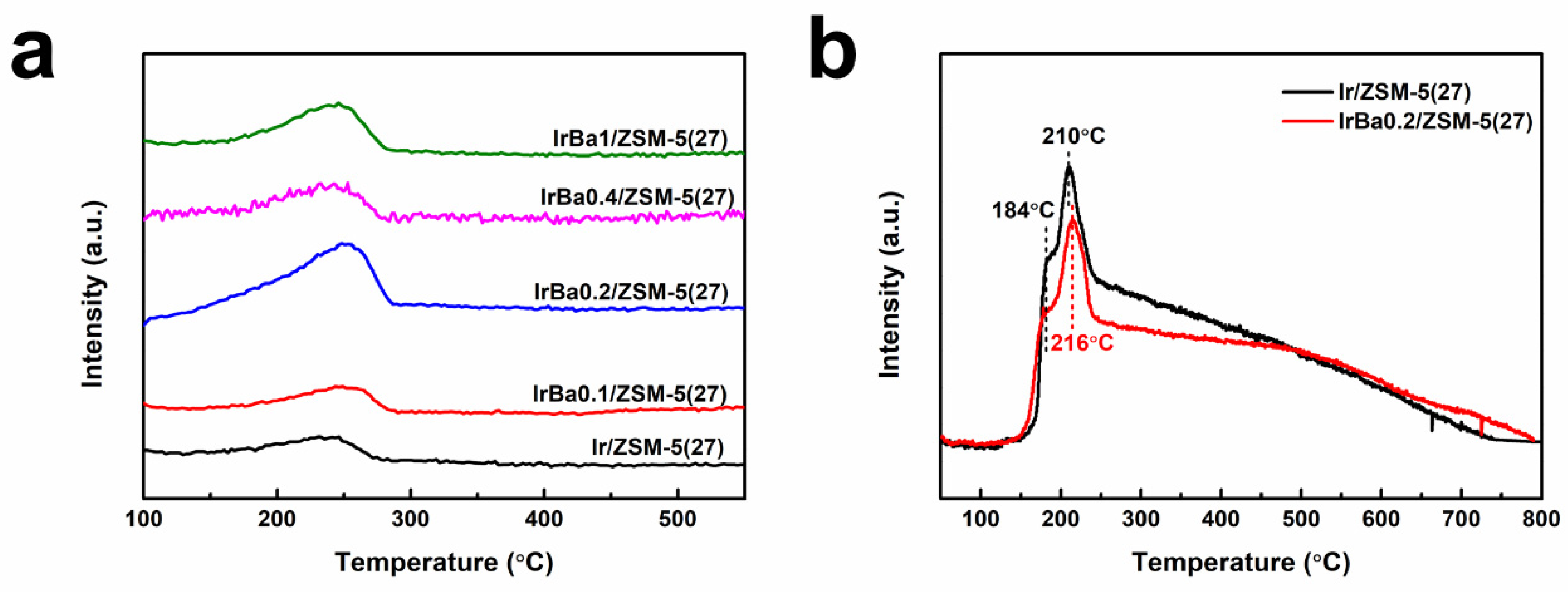
3.3. Influence of Ba Addition
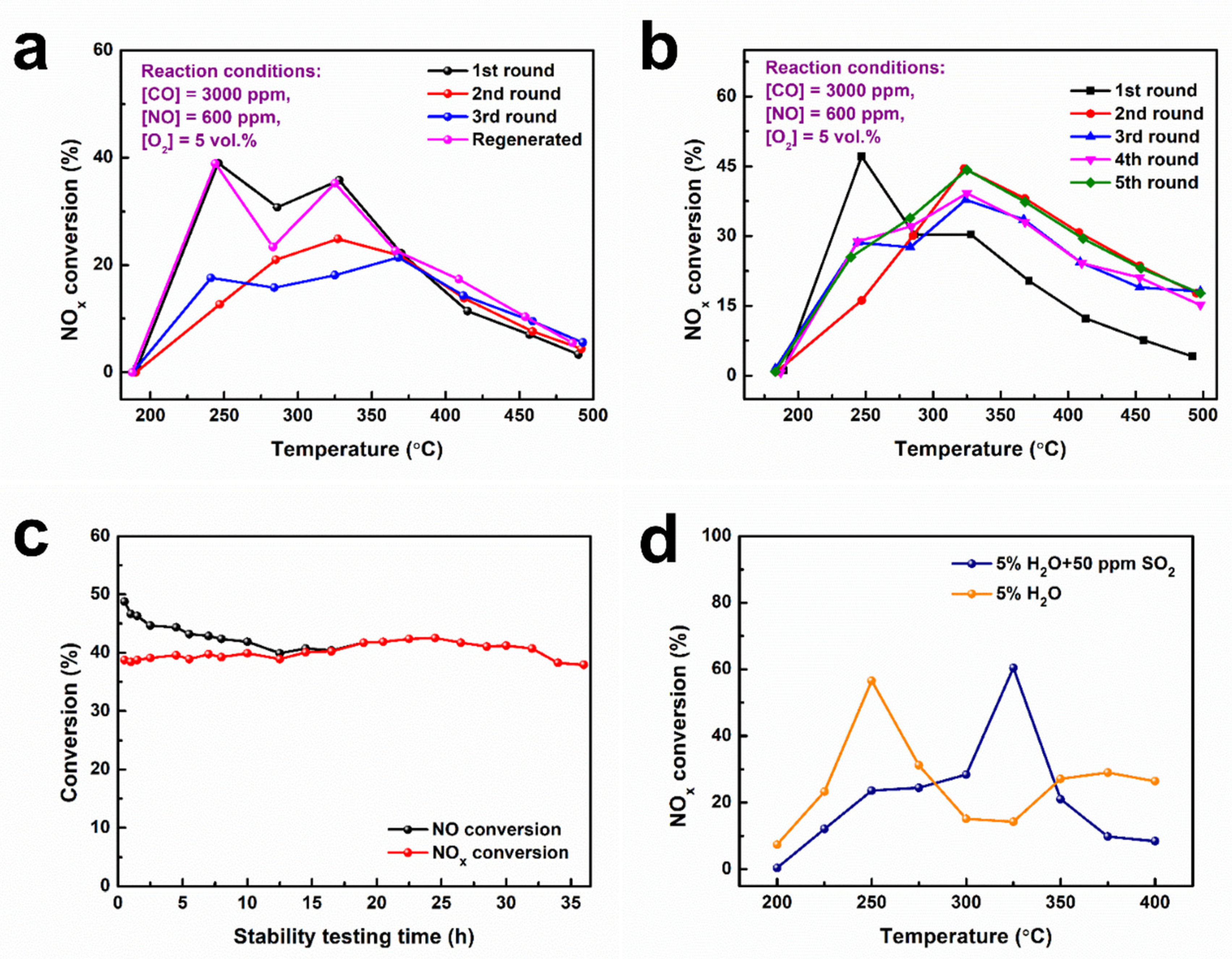
3.4. The Stability of Catalysts and the Influence of Other Components on CO-SCR Catalytic Performance
3.5. NH3-CO-SCR Performance on the Ba-Modified Catalyst
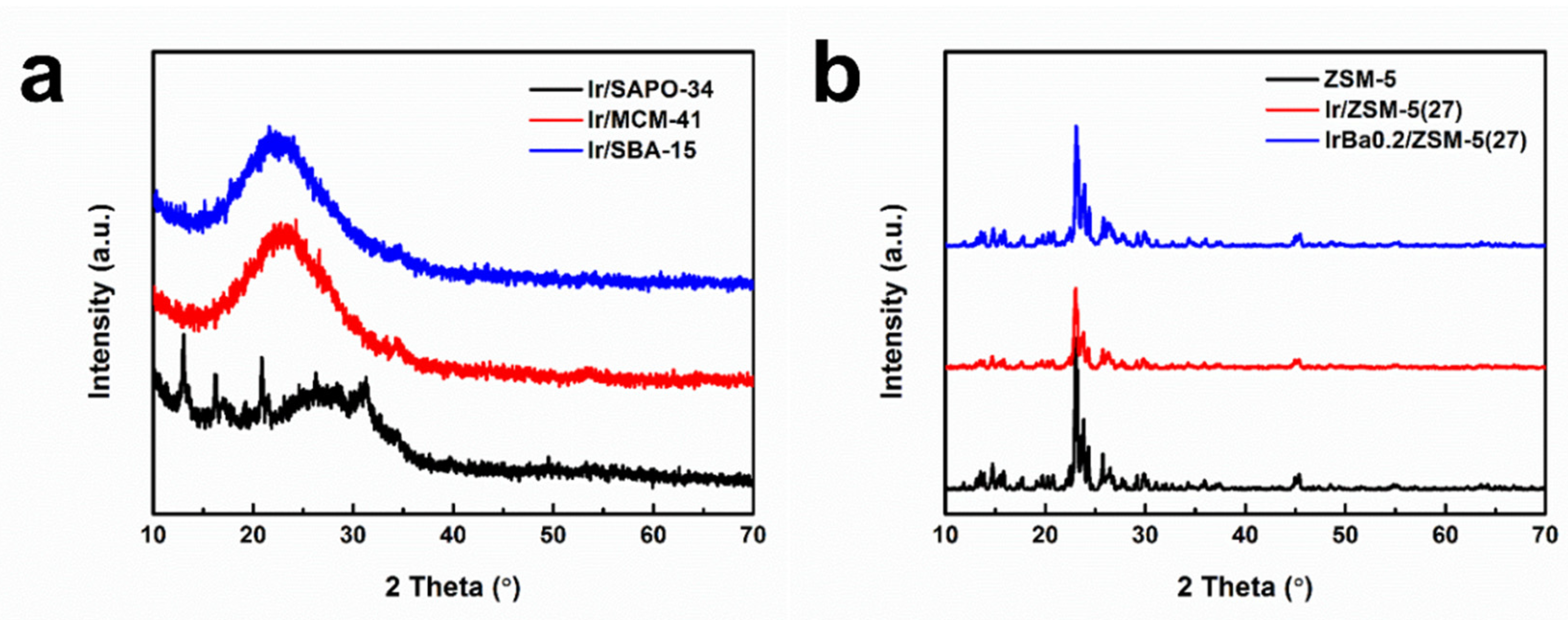
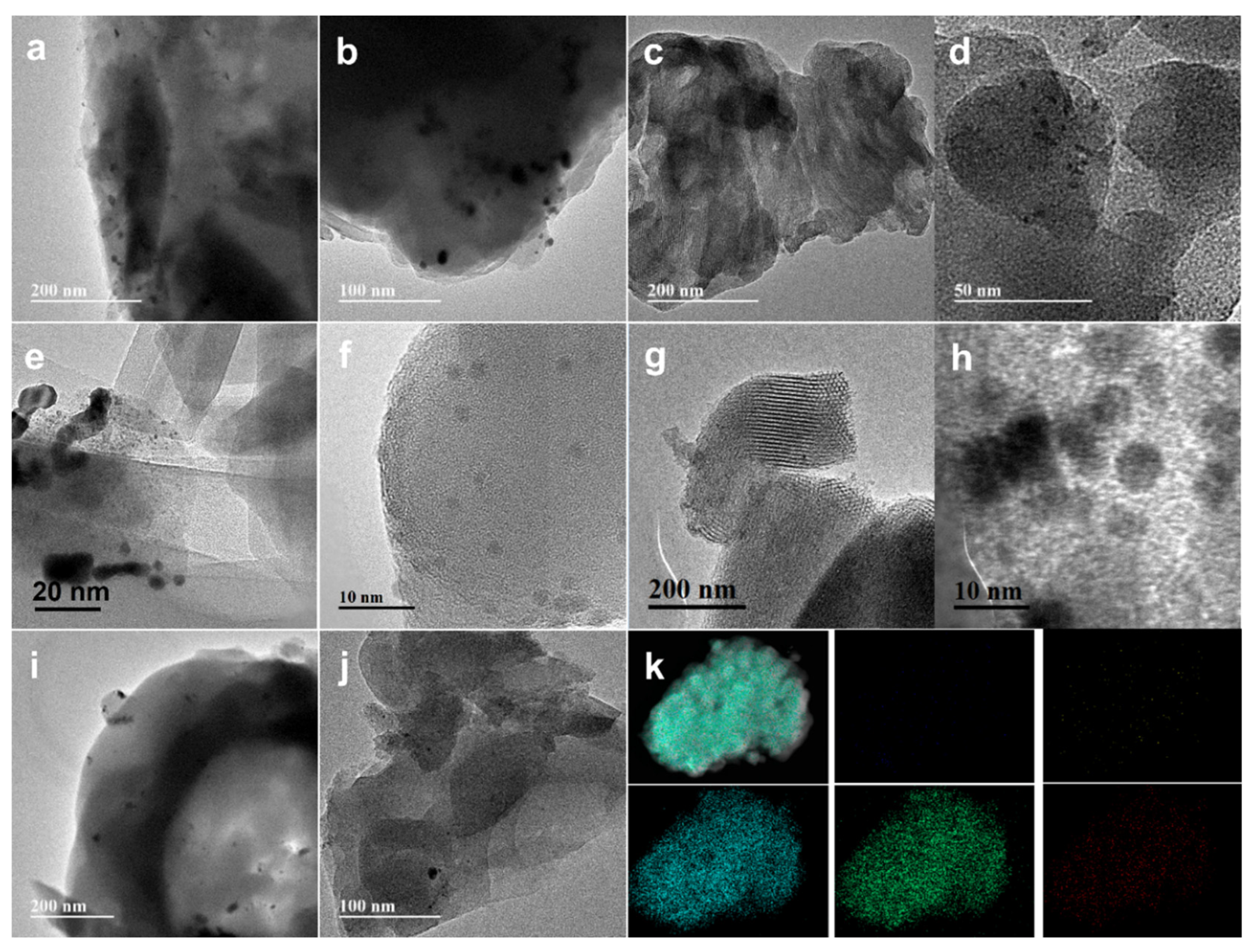
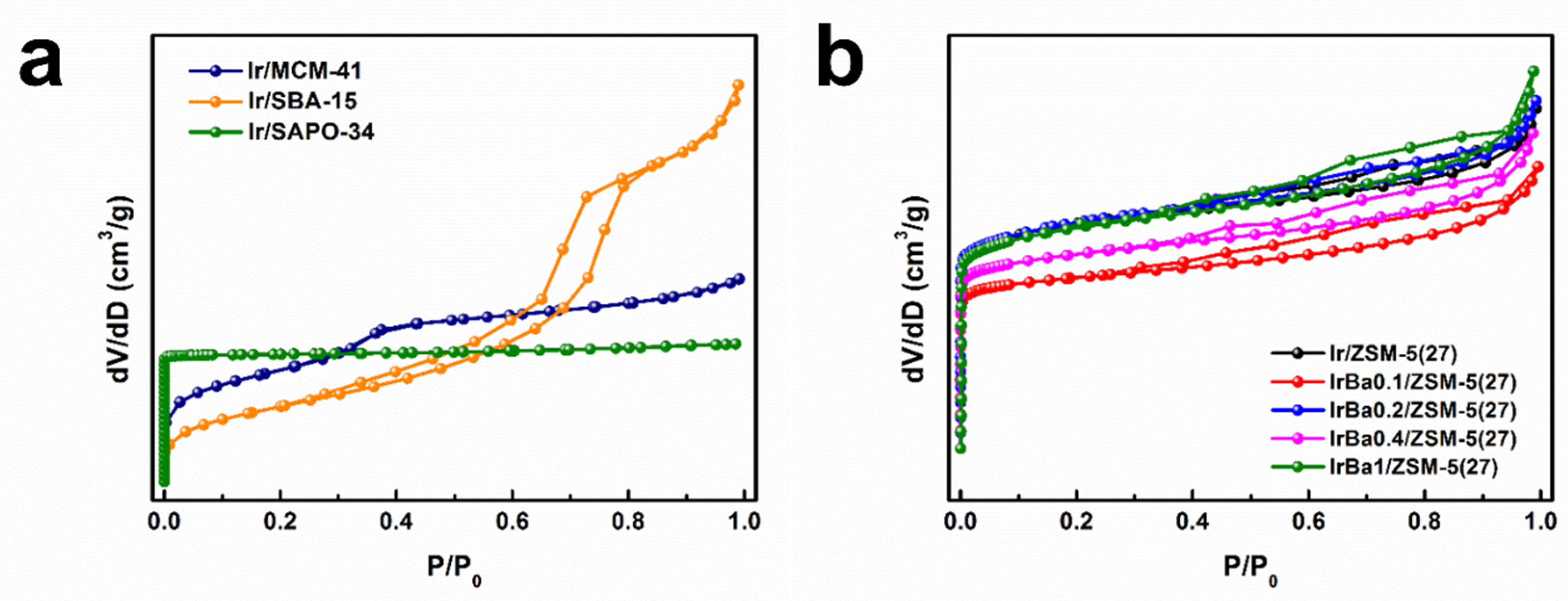
3.6. Physical Properties of as-Made Catalysts
3.7. Adsorption and Redox Properties of as-Made Catalysts
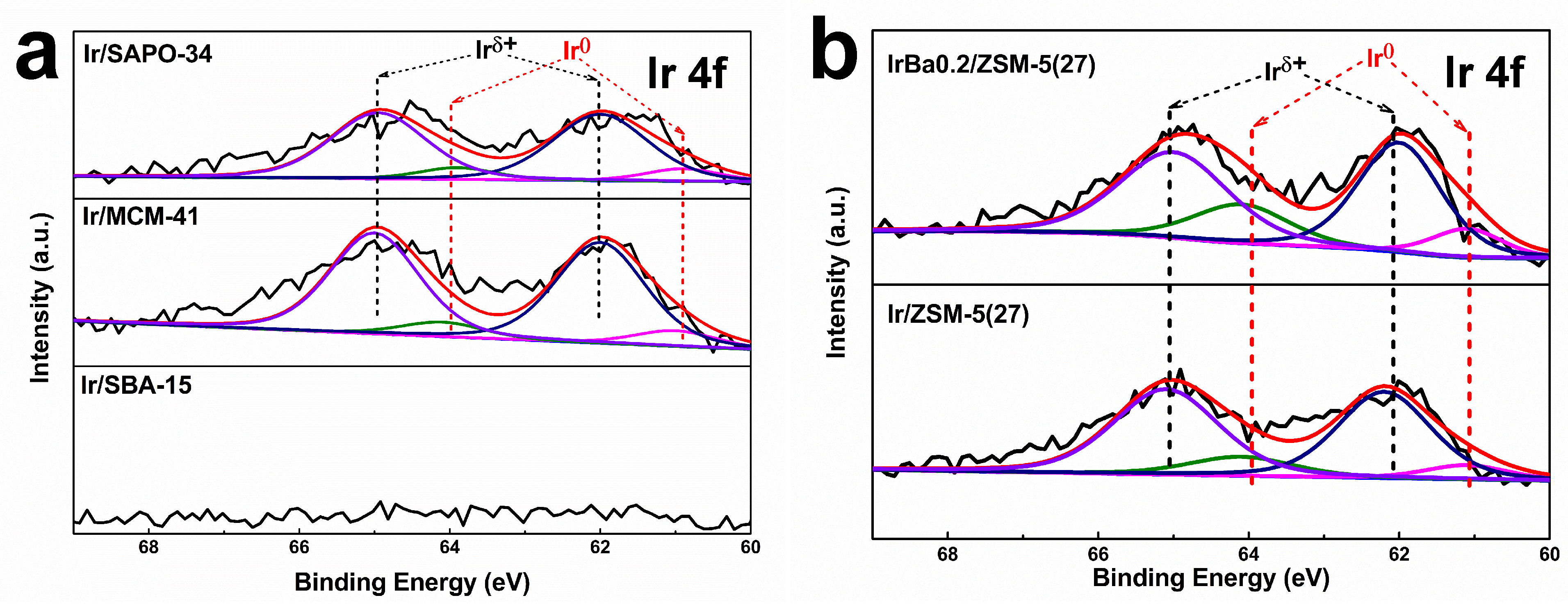
3.8. X-ray Photoelectron Spectroscopy (XPS) of as-Made Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Li, Y.; Lin, Y.; Zhu, T. A review of the catalysts used in the reduction of NO by CO for gas purification. Environ. Sci. Pollut. Res. 2020, 27, 6723–6748. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Luo, G.H.; Gholami, F.; Yang, F. Recent advances in selective catalytic reduction of NOx by carbon monoxide for flue gas cleaning process: A review. Catal. Rev.-Sci. Eng. 2020, 63, 68–119. [Google Scholar] [CrossRef]
- Liu, S.; Ji, Y.; Liu, B.; Xu, W.; Chen, W.; Yu, J.; Zhong, Z.; Xu, G.; Zhu, T.; Su, F. Co single atoms and CoOx nanoclusters anchored on Ce0.75Zr0.25O2 synergistically boosts the NO reduction by CO. Adv. Funct. Mater. 2023, 33, 2303297. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, S.; Zhu, H.; Xu, W.; Jiang, R.; Zhang, Y.; Yu, J.; Chen, W.; Jia, L.; Jiang, J.; et al. Isolating contiguous ir atoms and forming Ir-W intermetallics with negatively charged Ir for efficient NO reduction by CO. Adv. Mater. 2022, 34, 2205703. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Liu, L.; Boronat, M.; Arenal, R.; Concepcion, P.; Corma, A. Low-temperature catalytic NO reduction with CO by subnanometric Pt clusters. ACS Catal. 2019, 9, 11530–11541. [Google Scholar] [CrossRef] [PubMed]
- Bera, P.; Patil, K.C.; Jayaram, V.; Subbanna, G.N.; Hegde, M.S. Ionic dispersion of Pt and Pd on CeO2 by combustion method: Effect of metal-ceria interaction on catalytic activities for NO reduction and CO and hydrocarbon oxidation. J. Catal. 2000, 196, 293–301. [Google Scholar] [CrossRef]
- Kerkar, R.D.; Salker, A.V. Synergistic effect of modified Pd-based cobalt chromite and manganese oxide system towards NO-CO redox detoxification reaction. Environ. Sci. Pollut. Res. 2020, 27, 27061–27071. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, G.; Marquez, A.M.; Pedro Holgado, J.; Taylor, M.J.; Wheatley, A.E.H.; Mehta, J.P.; Fernandez Sanz, J.; Beaumont, S.K.; Lambert, R.M. Comprehensive experimental and theoretical study of the CO plus NO reaction catalyzed by Au/Ni nanoparticles. ACS Catal. 2019, 9, 4919–4929. [Google Scholar] [CrossRef]
- Oton, L.F.; Oliveira, A.C.; de Araujo, J.C.S.; Araujo, R.S.; de Sousa, F.F.; Saraiva, G.D.; Lang, R.; Otubo, L.; da Silva Duarte, G.C.; Campos, A. Selective catalytic reduction of NOx by CO (CO-SCR) over metal-supported nanoparticles dispersed on porous alumina. Adv. Powder Technol. 2020, 31, 464–476. [Google Scholar] [CrossRef]
- Song, J.H.; Park, D.C.; You, Y.-W.; Kim, Y.J.; Kim, S.M.; Heo, I.; Kim, D.H. Kinetic and DRIFTS studies of IrRu/Al2O3 catalysts for lean NOx reduction by CO at low temperature. Catal. Sci. Technol. 2020, 10, 8182–8195. [Google Scholar] [CrossRef]
- Song, J.H.; Park, D.C.; You, Y.-W.; Chang, T.S.; Heo, I.; Kim, D.H. Lean NOx reduction by CO at low temperature over bimetallic IrRu/Al2O3 catalysts with different Ir:Ru ratios. Catal. Sci. Technol. 2020, 10, 2120–2136. [Google Scholar] [CrossRef]
- Nanba, T.; Shinohara, S.; Masukawa, S.; Uchisawa, J.; Ohi, A.; Obuchi, A. Formation of active sites on Ir/WO3-SiO2 for selective catalytic reduction of NO by CO. Appl. Catal. B Environ. 2008, 84, 420–425. [Google Scholar] [CrossRef]
- Nanba, T.; Shinohara, S.; Uchisawa, J.; Masukawa, S.; Ohi, A.; Obuchi, A. Enhancement of activity of Ir catalysts for the selective catalytic reduction of NO by CO. Chem. Lett. 2006, 35, 450–451. [Google Scholar] [CrossRef]
- Gholami, Z.; Luo, G. Low-temperature selective catalytic reduction of NO by CO in the presence of O2 over Cu:Ce catalysts supported by multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2018, 57, 8871–8883. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Wang, F.; Xie, Z.; Hao, Z.; Liu, L.; Shen, B. Promotion effect of Ni doping on the oxygen resistance property of Fe/CeO2 catalyst for CO-SCR reaction: Activity test and mechanism investigation. J. Hazard. Mater. 2022, 431, 128622. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Komppa, V. Structural evolution of highly active Ir-based catalysts for the selective reduction of NO with reductants in oxidizing conditions. Top. Catal. 2001, 16, 217–223. [Google Scholar] [CrossRef]
- Nanba, T.; Wada, K.-i.; Masukawa, S.; Uchisawa, J.; Obuchi, A. Enhancement of activity of Ir catalysts for selective catalytic reduction of NO with CO by physical mixing with SiO2. Appl. Catal. A Gen. 2010, 380, 66–71. [Google Scholar] [CrossRef]
- Ogura, M.; Kawamura, A.; Matsukata, M.; Kikuchi, E. Catalytic activity of Ir for NO-CO reaction in the presence of SO2 and excess oxygen. Chem. Lett. 2000, 29, 146–147. [Google Scholar] [CrossRef]
- Sasaki, M.; Sultana, A.; Haneda, M.; Hamada, H. Practical evaluation of the catalytic performance of Ir/SiO2-based catalysts for selective reduction of NO with CO. Top. Catal. 2009, 52, 1803–1807. [Google Scholar] [CrossRef]
- Takahashi, A.; Fujitani, T.; Nakamura, I.; Katsuta, Y.; Haneda, M.; Hamada, H. Excellent promoting effect of Ba addition on the catalytic activity of Ir/WO3-SiO2 for the selective reduction of NO with CO. Chem. Lett. 2006, 35, 420–421. [Google Scholar] [CrossRef]
- Haneda, M.; Fujitani, T.; Hamada, H. Effect of iridium dispersion on the catalytic activity of Ir/SiO2 for the selective reduction of NO with CO in the presence of O2 and SO2. J. Mol. Catal. A Chem. 2006, 256, 143–148. [Google Scholar] [CrossRef]
- Yoshinari, T.; Sato, K.; Haneda, M.; Kintaichi, Y.; Hamada, H. Remarkable promoting effect of coexisting SO2 on the catalytic activity of Ir/SiO2 for NO reduction in the presence of oxygen. Catal. Commun. 2001, 2, 155–158. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.; Seo, Y.; Kim, J.-C.; Ryoo, R. n-Heptane hydroisomerization over Pt/MFI zeolite nanosheets: Effects of zeolite crystal thickness and platinum location. J. Catal. 2013, 301, 187–197. [Google Scholar] [CrossRef]
- Goel, S.; Zones, S.I.; Iglesia, E. Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement. J. Am. Chem. Soc. 2014, 136, 15280–15290. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Wu, Z.; Iglesia, E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation. J. Am. Chem. Soc. 2010, 132, 9129–9137. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; Di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, P.; Perez, Y.; Hernandez-Garrido, J.C.; Fajardo, M.; Calvino, J.J.; Corma, A. The promotional effect of Sn-beta zeolites on platinum for the selective hydrogenation of α,β-unsaturated aldehydes. Phys. Chem. Chem. Phys. 2013, 15, 12048–12055. [Google Scholar] [CrossRef]
- Haneda, M.; Kudo, H.; Nagao, Y.; Fujitani, T.; Hamada, H. Enhanced activity of Ba-doped Ir/SiO2 catalyst for NO reduction with CO in the presence of O2 and SO2. Catal. Commun. 2006, 7, 423–426. [Google Scholar] [CrossRef]
- Inomata, H.; Shimokawabe, M.; Kuwana, A.; Arai, M. Selective reduction of NO with CO in the presence of O2 with Ir/WO3 catalysts: Influence of preparation variables on the catalytic performance. Appl. Catal. B Environ. 2008, 84, 783–789. [Google Scholar] [CrossRef]
- Tamai, T.; Haneda, M.; Fujitani, T.; Hamada, H. Promotive effect of Nb2O5 on the catalytic activity of Ir/SiO2 for NO reduction with CO under oxygen-rich conditions. Catal. Commun. 2007, 8, 885–888. [Google Scholar] [CrossRef]
- Sasaki, M.; Mita, T.; Tsujimura, T.; Nanba, T.; Obuchi, A.; Haneda, M.; Hamada, H. Performance of Ba-doped Ir/WO3-SiO2 catalyst for selective catalytic reduction of NOx with CO in diesel exhaust. J. Jpn. Pet. Inst 2008, 51, 356–359. [Google Scholar] [CrossRef]
- Song, J.H.; Park, D.C.; You, Y.-W.; Kim, Y.J.; Lee, J.H.; Heo, I.; Kim, D.H. Promotive effects of Ba addition on lean NOx reduction by CO over IrRu/Al2O3 catalyst. Chem. Eng. J. 2023, 452, 139331. [Google Scholar] [CrossRef]
- Doi, Y.; Haneda, M. Catalytic performance of supported Ir catalysts for NO reduction with C3H6 and CO in slight lean conditions. Catal. Today 2018, 303, 8–12. [Google Scholar] [CrossRef]
- Haneda, M.; Hamada, H. Promoting effect of coexisting H2O on the activity of Ir/WO3/SiO2 catalyst for the selective reduction of NO with CO. Chem. Lett. 2008, 37, 830–831. [Google Scholar] [CrossRef]
- Haneda, M.; Hamada, H. Promotional role of H2O in the selective catalytic reduction of NO with CO over Ir/WO3/SiO2 catalyst. J. Catal. 2010, 273, 39–49. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Bai, Y.; Wu, X.; Wang, H.; Wu, Z. High performance iridium loaded on natural halloysite nanotubes for CO-SCR reaction. Fuel 2024, 357, 129938. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Dai, Q.G.; Wang, W.; Wang, X.Y.; Lu, G.Z. Sandwich-structured CeO2@ZSM-5 hybrid composites for catalytic oxidation of 1, 2-dichloroethane: An integrated solution to coking and chlorine poisoning deactivation. Appl. Catal. B Environ. 2017, 203, 31–42. [Google Scholar] [CrossRef]
- Pang, L.; Fan, C.; Shao, L.N.; Song, K.P.; Yi, J.X.; Cai, X.; Wang, J.; Kang, M.; Li, T. The Ce doping Cu/ZSM-5 as a new superior catalyst to remove NO from diesel engine exhaust. Chem. Eng. J. 2014, 253, 394–401. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Ke, R.; Hao, J. Improvement of catalytic activity and sulfur-resistance of Ag/TiO2-Al2O3 for NO reduction with propene under lean burn conditions. Appl. Catal. B Environ. 2008, 80, 202–213. [Google Scholar] [CrossRef]
- Torre-Abreu, C.; Ribeiro, M.F.; Henriques, C.; Delahay, G. NO TPD and H2-TPR studies for characterisation of CuMOR catalysts the role of Si/Al ratio, copper content and cocation. Appl. Catal. B Environ. 1997, 14, 261–272. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, I.; Kobayashi, Y.; Takahashi, A.; Haneda, M.; Hamada, H. Adsorption and reactions of NO on clean and CO-precovered Ir(111). J. Phys. Chem. B 2005, 109, 17603–17607. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Suzuki, K.; Toyoshima, R.; Monya, Y.; Yoshida, M.; Isegawa, K.; Amemiya, K.; Mase, K.; Mun, B.S.; Arman, M.A.; et al. Adsorption and reaction of CO and NO on Ir(111) under near ambient pressure conditions. Top. Catal. 2016, 59, 487–496. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, S.; Sun, Y.; Ouyang, W.; Zhou, Y.; Wang, H.; Wu, Z. Insight into the mechanism of selective catalytic reduction of NO by CO over a bimetallic IrRu/ZSM-5 catalyst in the absence/presence of O2 by isotopic C13O tracing methods. Environ. Sci. Technol. 2023, 57, 9105–9114. [Google Scholar] [CrossRef] [PubMed]
- Freakley, S.J.; Ruiz-Esquius, J.; Morgan, D.J. The X-ray photoelectron spectra of Ir, IrO2 and IrCl3 revisited. Surf. Interface Anal. 2017, 49, 794–799. [Google Scholar] [CrossRef]

| Catalysts | Reaction Conditions | Ir Loading Amount (%) | NO Conversion (%) | References |
|---|---|---|---|---|
| IrRu/Al2O3 | 50 ppm NO, 7000 ppm CO, 5% O2 | 1.9% | 88% | [12] |
| BaIrRu/Al2O3 | 50 ppm NO, 7000 ppm CO, 5% O2 | 1.9% | 90% | [34] |
| IrW-WO3/KIT-6 | 1000 ppm NO, 4000 ppm CO, 5% O2 | 1.0% | <60% | [5] |
| Ir/WO3 | 500 ppm NO, 3000 ppm CO, 5% O2, 2 ppm SO2, 1% H2O | 5.0% | 25% | [31] |
| Ir/Al2O3 | 500 ppm NO, 1000 ppm CO, 300 ppm C3H6, 0.5% O2, 5% H2O | 1.0% | nearly 80% | [35] |
| IrBa0.2/ZSM-5 | 600 ppm NO, 3000 ppm CO, 5% O2 | 0.5% | 68% | This work |
| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | ||
|---|---|---|---|---|
| Smicro | Stotal | Vmicro | Vtotal | |
| Ir/SAPO-34 | 658.2 | 679.3 | 0.26 | 0.29 |
| Ir/MCM-41 | - | 535.9 | - | 0.39 |
| Ir/SBA-15 | - | 372.2 | - | 0.91 |
| Ir/ZSM-5(27) | 362.1 | 415.9 | 0.15 | 0.25 |
| IrBa0.1/ZSM-5(27) | 197.4 | 251.8 | 0.10 | 0.20 |
| IrBa0.2/ZSM-5(27) | 291.0 | 370.4 | 0.14 | 0.27 |
| IrBa0.4/ZSM-5(27) | 234.8 | 301.6 | 0.12 | 0.24 |
| IrBa1.0/ZSM-5(27) | 247.1 | 323.9 | 0.12 | 0.27 |
| Samples | Ir0/(Ir0 + Irδ+) |
|---|---|
| Ir/SAPO-34 | 12.8% |
| Ir/MCM-41 | 12.1% |
| Ir/SBA-15 | - |
| Ir/ZSM-5(27) | 15.8% |
| IrBa0.2/ZSM-5(27) | 23.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Miao, C.; Ouyang, W.; Wang, L.; Wang, H.; Wu, Z. Tailoring Iridium Valence States on ZSM-5 for Enhanced Catalytic Performance in CO Selective Catalytic Reduction of NO under Oxygen-Enriched Environments. Materials 2024, 17, 1440. https://doi.org/10.3390/ma17061440
Bai Y, Miao C, Ouyang W, Wang L, Wang H, Wu Z. Tailoring Iridium Valence States on ZSM-5 for Enhanced Catalytic Performance in CO Selective Catalytic Reduction of NO under Oxygen-Enriched Environments. Materials. 2024; 17(6):1440. https://doi.org/10.3390/ma17061440
Chicago/Turabian StyleBai, Yarong, Chuhan Miao, Weilong Ouyang, Lang Wang, Haiqiang Wang, and Zhongbiao Wu. 2024. "Tailoring Iridium Valence States on ZSM-5 for Enhanced Catalytic Performance in CO Selective Catalytic Reduction of NO under Oxygen-Enriched Environments" Materials 17, no. 6: 1440. https://doi.org/10.3390/ma17061440





