The Combined Effects on Human Dental Pulp Stem Cells of Fast-Set or Premixed Hydraulic Calcium Silicate Cements and Secretome Regarding Biocompatibility and Osteogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Dental Pulp Stem Cells (hDPSCs)
2.2. Disks of Different MTAs and HCSCs for Experimentation
2.3. Categorization of Experimental Groups
2.4. Cell Viability Assay
2.5. Cell Migration Assay
2.6. Alkaline Phosphatase (ALP) Activity
2.7. Alizarin Red S (ARS) Staining Assay
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
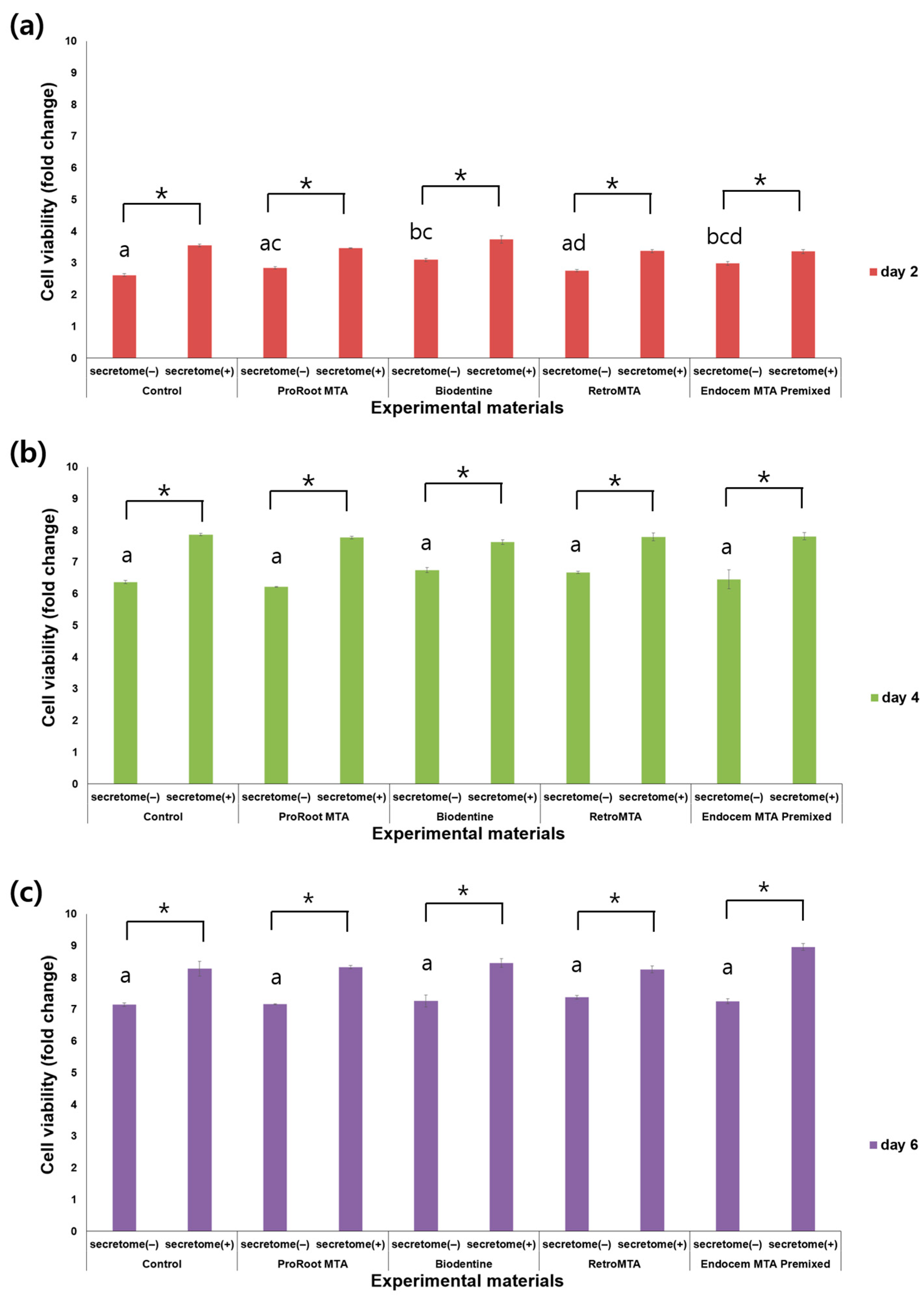
3.1. Cell Viability Assay
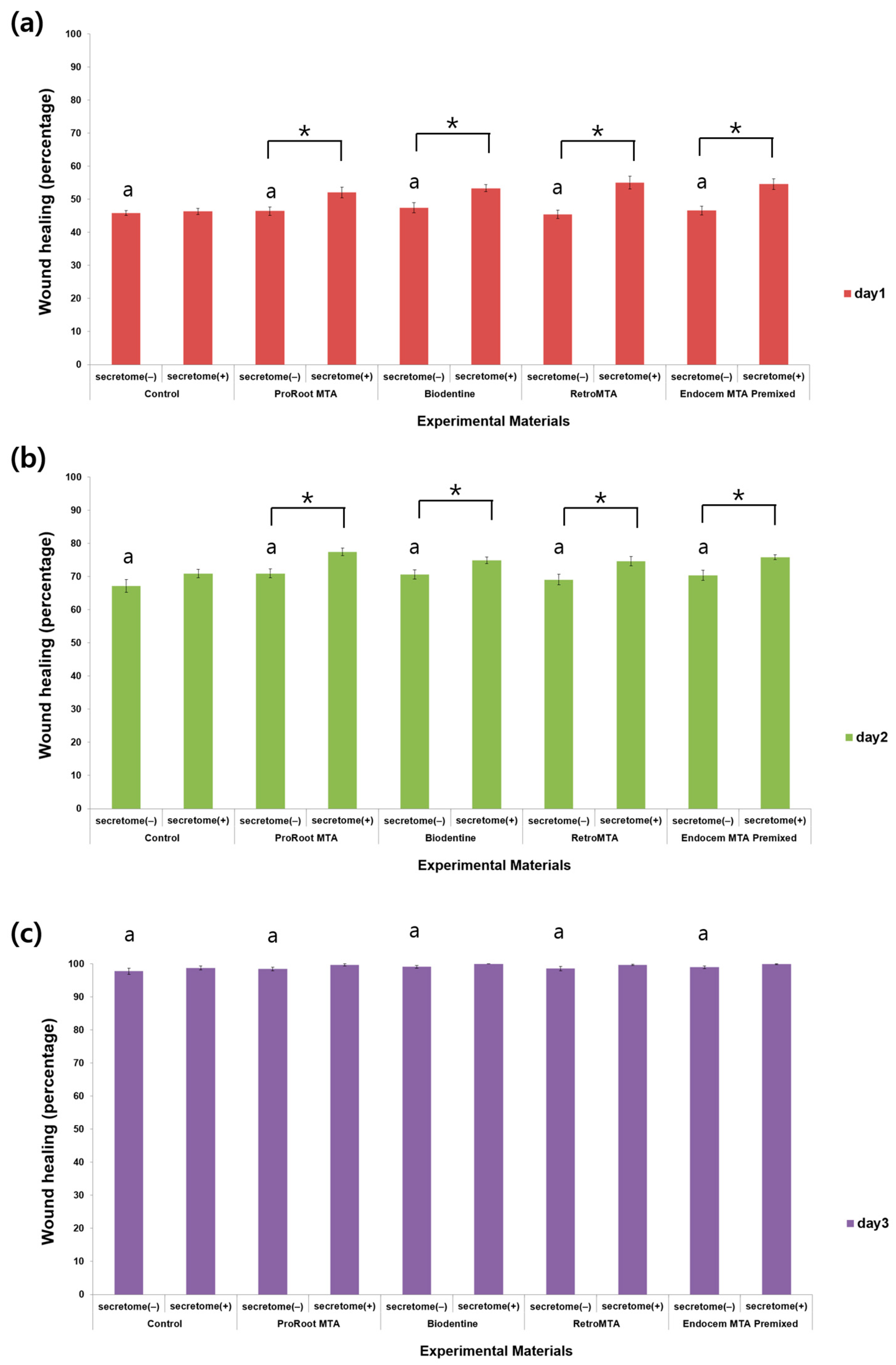
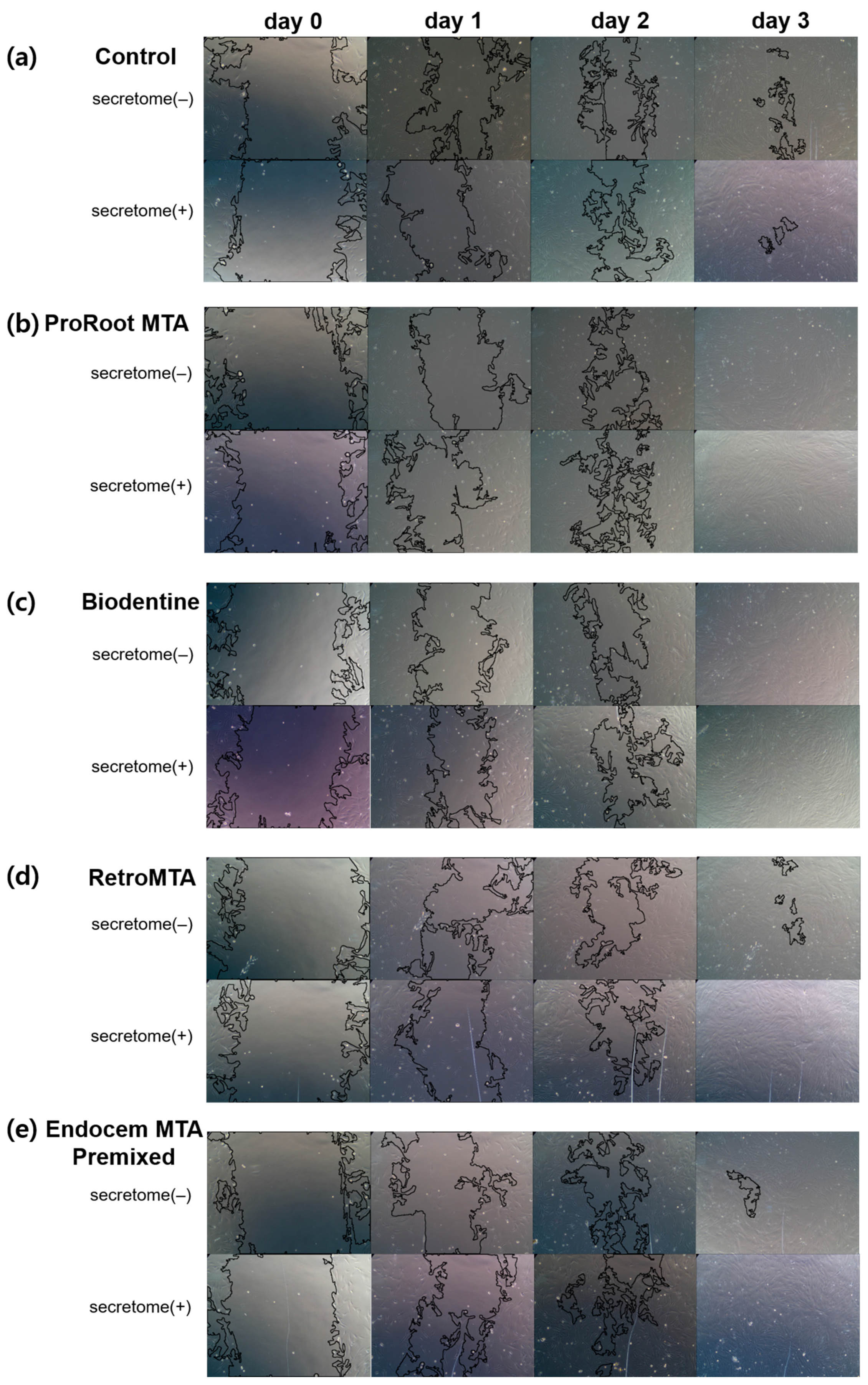
3.2. Cell Migration Assay
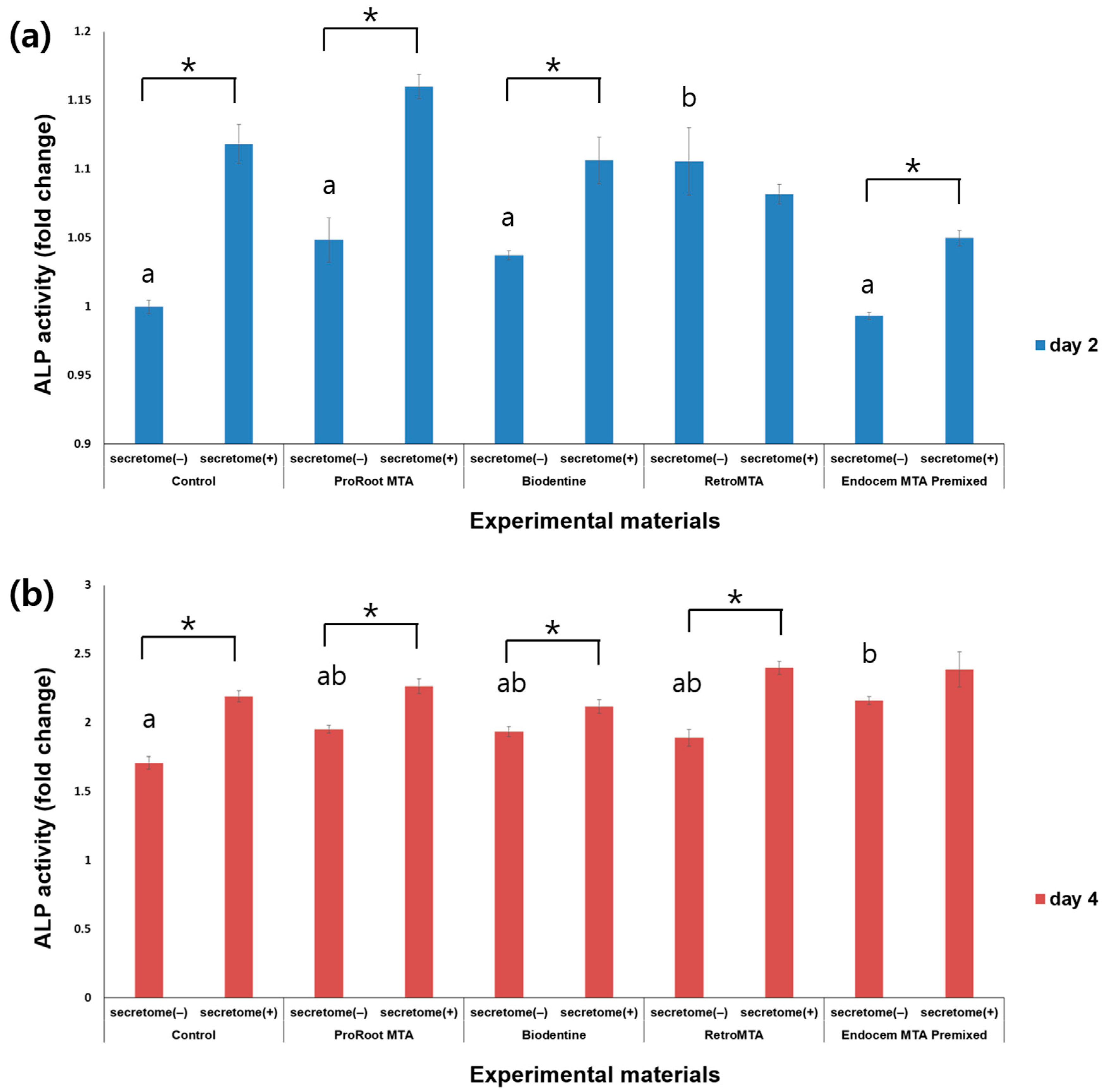
3.3. ALP Activity
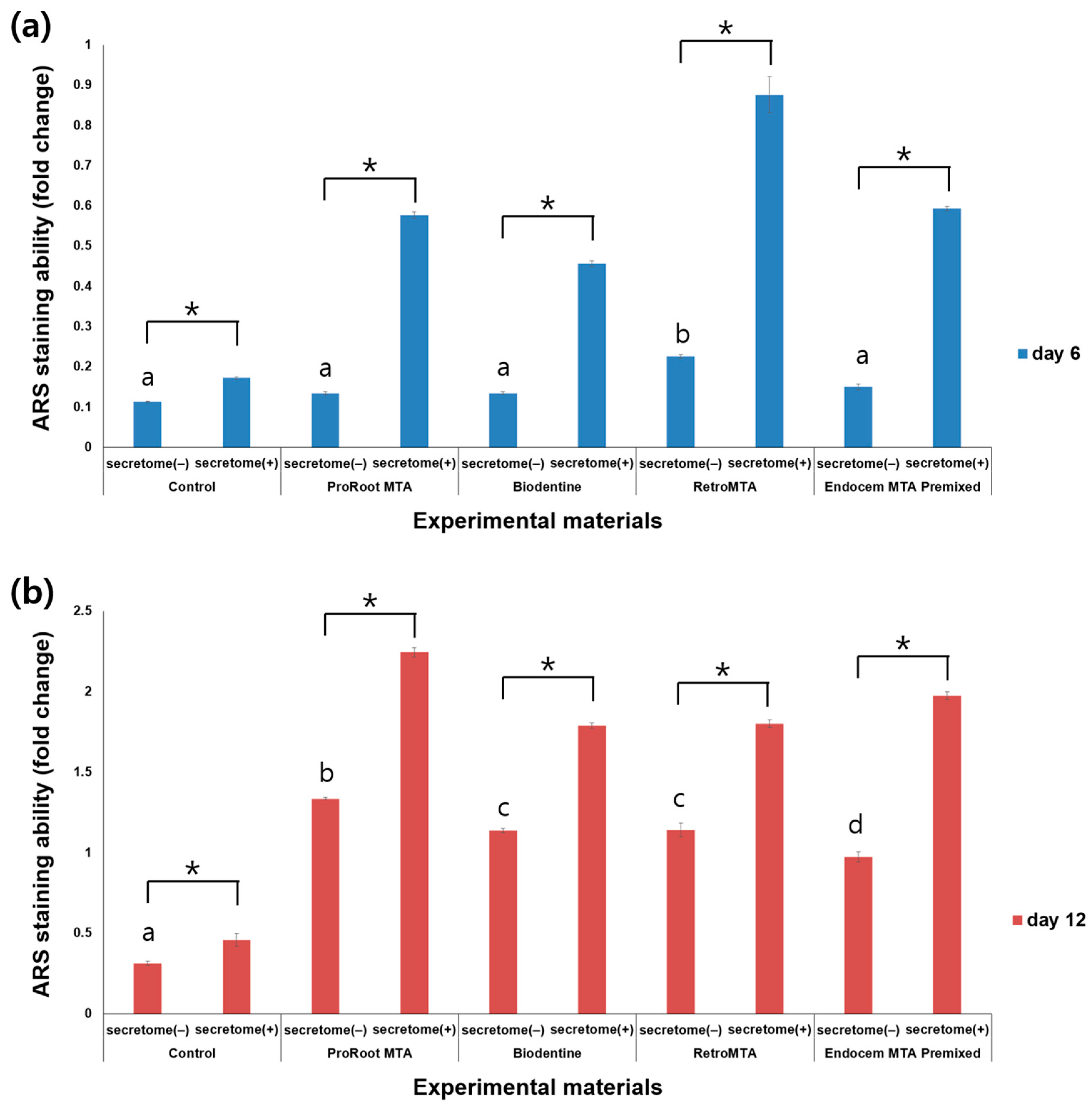
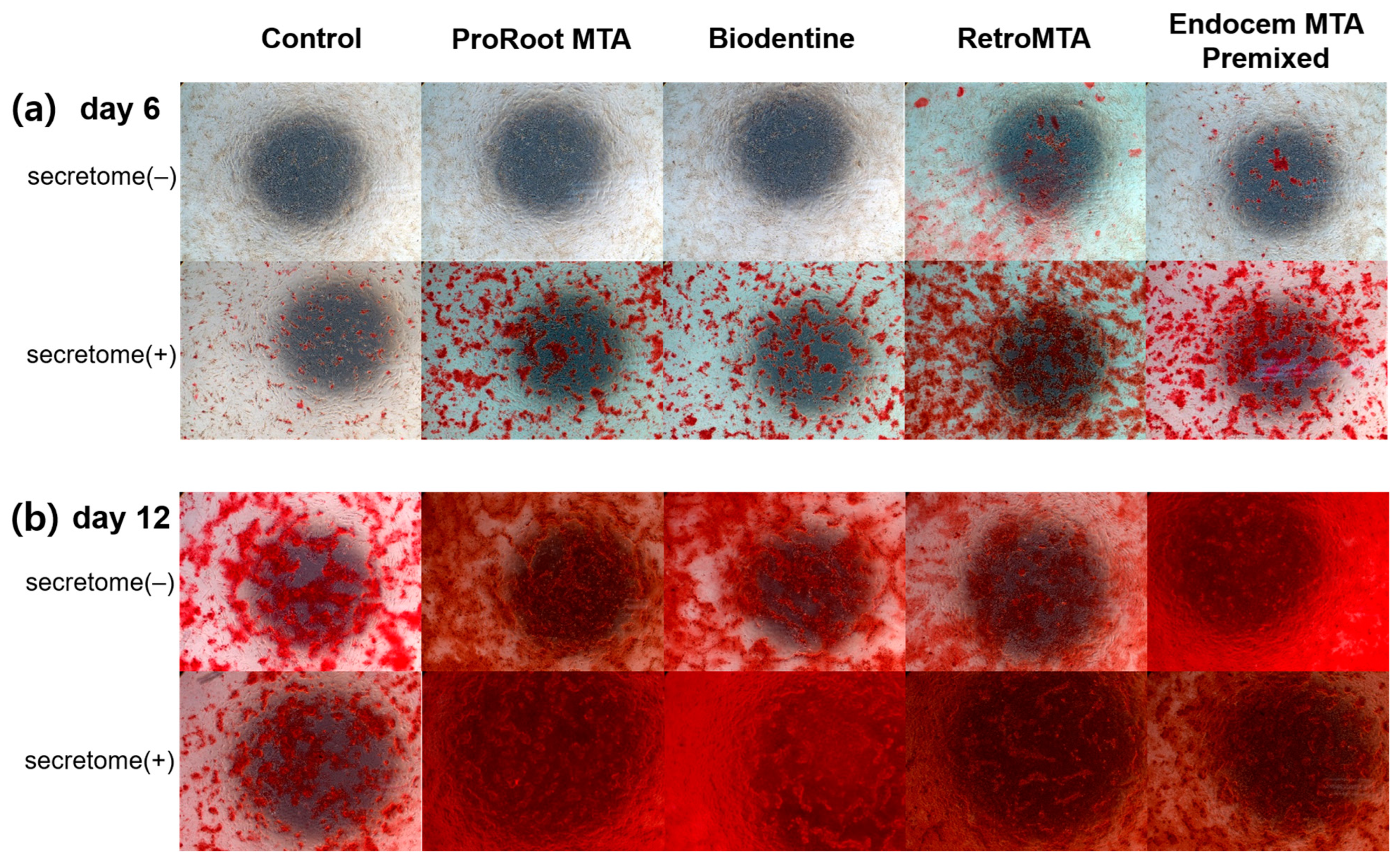
3.4. ARS Staining Assay
3.5. qRT-PCR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative Endodontics: A Comprehensive Review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review--Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.R.; Emara, R.; Taher, M.M.; Al-Allaf, F.A.; Almalki, M.; Almasri, M.A.; Siddiqui, S.S. Effects of Mineral Trioxide Aggregate, Calcium Hydroxide, Biodentine and Emdogain on Osteogenesis, Odontogenesis, Angiogenesis and Cell Viability of Dental Pulp Stem Cells. BMC Oral Health 2019, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Ber, B.S.; Hatton, J.F.; Stewart, G.P. Chemical Modification of ProRoot MTA to Improve Handling Characteristics and Decrease Setting Time. J. Endod. 2007, 33, 1231–1234. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Noula, G.; Lambrianidis, T. Ex Vivo Study of the Efficiency of Two Techniques for the Removal of Mineral Trioxide Aggregate Used as a Root Canal Filling Material. J. Endod. 2008, 34, 1239–1242. [Google Scholar] [CrossRef]
- Dominguez, M.S.; Witherspoon, D.E.; Gutmann, J.L.; Opperman, L.A. Histological and Scanning Electron Microscopy Assessment of Various Vital Pulp-Therapy Materials. J. Endod. 2003, 29, 324–333. [Google Scholar] [CrossRef]
- Roberts, H.W.; Toth, J.M.; Berzins, D.W.; Charlton, D.G. Mineral Trioxide Aggregate Material Use in Endodontic Treatment: A Review of the Literature. Dent. Mater. 2008, 24, 149–164. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Pitt Ford, T.R. Physical and Chemical Properties of a New Root-End Filling Material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium Silicate-Based Cements: Composition, Properties, and Clinical Applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef]
- Malkondu, Ö.; Karapinar Kazandağ, M.; Kazazoğlu, E.A. Review on Biodentine, a Contemporary Dentine Replacement and Repair Material. Biomed. Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Ma, J.; Shen, Y.; Stojicic, S.; Haapasalo, M. Biocompatibility of Two Novel Root Repair Materials. J. Endod. 2011, 37, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; Caicedo, R.; Ritwik, P.; Moiseyeva, R.; Kawashima, I. Physicochemical Basis of the Biologic Properties of Mineral Trioxide Aggregate. J. Endod. 2005, 31, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Kim, E.; Song, M.; Park, J.W.; Shin, S.J. Effects of Two Fast-Setting Calcium-Silicate Cements on Cell Viability and Angiogenic Factor Release in Human Pulp-Derived Cells. Odontology 2016, 104, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wongwatanasanti, N.; Jantarat, J.; Sritanaudomchai, H.; Hargreaves, K.M. Effect of Bioceramic Materials on Proliferation and Odontoblast Differentiation of Human Stem Cells from the Apical Papilla. J. Endod. 2018, 44, 1270–1275. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of Human Dental Pulp Capped with Biodentine and Mineral Trioxide Aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Chen, J.W.; Tsai, C.Y.; Aprecio, R.; Zhang, W.; Yochim, J.M.; Teng, N.; Torabinejad, M. Cytotoxicity and Antimicrobial Effects of a New Fast-Set MTA. Biomed. Res. Int. 2017, 2017, 2071247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, D.; Kim, S.Y. Biocompatibility and Osteogenic Potential of Calcium Silicate-Based Cement Combined with Enamel Matrix Derivative: Effects on Human Bone Marrow-Derived Stem Cells. Materials 2021, 14, 7750. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kye, M.; Ha, Y.J.; Kim, S.Y. Biocompatible Properties and Mineralization Potential of Premixed Calcium Silicate-Based Cements and Fast-Set Calcium Silicate-Based Cements on Human Bone Marrow-Derived Mesenchymal Stem Cells. Materials 2022, 15, 7595. [Google Scholar] [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int. J. Mol. Sci. 2021, 22, 12018. [Google Scholar] [CrossRef]
- El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Dental Stem Cell-Derived Secretome/Conditioned Medium: The Future for Regenerative Therapeutic Applications. Stem Cells Int. 2020, 2020, 7593402. [Google Scholar] [CrossRef]
- Çelik, B.N.; Mutluay, M.S.; Arikan, V.; Sari, Ş. The Evaluation of MTA and Biodentine as a Pulpotomy Materials for Carious Exposures in Primary Teeth. Clin. Oral Investig. 2019, 23, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, A.; Nikookhesal, M.; Erfanparast, L.; Løvschall, H.; Ranjkesh, B. Vital Pulp Therapy Following Pulpotomy in Immature First Permanent Molars with Deep Caries Using Novel Fast-Setting Calcium Silicate Cement: A Retrospective Clinical Study. J. Dent. 2022, 116, 103890. [Google Scholar] [CrossRef] [PubMed]
- Grawish, M.E.; Saeed, M.A.; Sultan, N.; Scheven, B.A. Therapeutic Applications of Dental Pulp Stem Cells in Regenerating Dental, Periodontal and Oral-Related Structures. World J. Meta Anal. 2021, 9, 176–192. [Google Scholar] [CrossRef]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S.; et al. Inhibition of SARS-CoV-2 (Previously 2019-nCoV) Infection by a Highly Potent Pan-Coronavirus Fusion Inhibitor Targeting Its Spike Protein That Harbors a High Capacity to Mediate Membrane Fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Gorduysus, M.; Avcu, N.; Gorduysus, O.; Pekel, A.; Baran, Y.; Avcu, F.; Ural, A.U. Cytotoxic Effects of Four Different Endodontic Materials in Human Periodontal Ligament Fibroblasts. J. Endod. 2007, 33, 1450–1454. [Google Scholar] [CrossRef]
- Wei, W.; Qi, Y.P.; Nikonov, S.Y.; Niu, L.N.; Messer, R.L.W.; Mao, J.; Primus, C.M.; Pashley, D.H.; Tay, F.R. Effects of an Experimental Calcium Aluminosilicate Cement on the Viability of Murine Odontoblast-Like Cells. J. Endod. 2012, 38, 936–942. [Google Scholar] [CrossRef]
- Luo, J.; Xu, J.; Cai, J.; Wang, L.; Sun, Q.; Yang, P. The In Vitro and In Vivo Osteogenic Capability of the Extraction Socket-Derived Early Healing Tissue. J. Periodontol. 2016, 87, 1057–1066. [Google Scholar] [CrossRef]
- Araújo, L.B.; Cosme-Silva, L.; Fernandes, A.P.; Oliveira, T.M.; Cavalcanti, B.D.N.; Gomes Filho, J.E.; Sakai, V.T. Effects of Mineral Trioxide Aggregate, BiodentineTM and Calcium Hydroxide on Viability, Proliferation, Migration and Differentiation of Stem Cells from Human Exfoliated Deciduous Teeth. J. Appl. Oral Sci. 2018, 26, e20160629. [Google Scholar] [CrossRef]
- Du, R.; Wu, T.; Liu, W.; Li, L.; Jiang, L.; Peng, W.; Chang, J.; Zhu, Y. Role of the Extracellular Signal-Regulated Kinase 1/2 Pathway in Driving Tricalcium Silicate-Induced Proliferation and Biomineralization of Human Dental Pulp Cells In Vitro. J. Endod. 2013, 39, 1023–1029. [Google Scholar] [CrossRef]
- Souza, L.C.; Yadlapati, M.; Dorn, S.O.; Silva, R.; Letra, A. Analysis of Radiopacity, pH and Cytotoxicity of a New Bioceramic Material. J. Appl. Oral Sci. 2015, 23, 383–389. [Google Scholar] [CrossRef]
- Kawamura, R.; Hayashi, Y.; Murakami, H.; Nakashima, M. EDTA Soluble Chemical Components and the Conditioned Medium from Mobilized Dental Pulp Stem Cells Contain an Inductive Microenvironment, Promoting Cell Proliferation, Migration, and Odontoblastic Differentiation. Stem Cell Res. Ther. 2016, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.R.; Tomson, P.L.; Berdal, A. Regenerative Endodontics: Regeneration or Repair? J. Endod. 2014, 40, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.; Rosa, T.P.; Herrera, D.R.; Jacinto, R.C.; Gomes, B.P.; Zaia, A.A. Evaluation of Cytotoxicity and Physicochemical Properties of Calcium Silicate-Based Endodontic Sealer MTA Fillapex. J. Endod. 2013, 39, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.W.; Mustafa, M.; Bårdsen, A.; Bletsa, A. Tricalcium Silicate Cements: Osteogenic and Angiogenic Responses of Human Bone Marrow Stem Cells. Eur. J. Oral Sci. 2019, 127, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, N.; Fan, Y.; Wang, Y.; Gu, Y.; Li, Z.; Pan, Y.; Romila, G.; Zhou, Z.; Yu, J. The Conditioned Medium of Calcined Tooth Powder Promotes the Osteogenic and Odontogenic Differentiation of Human Dental Pulp Stem Cells via MAPK Signaling Pathways. Stem Cells Int. 2019, 2019, 4793518. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, J.; Liu, H.; Niu, C.; Chen, D. Salidroside Promotes the Osteogenic and Odontogenic Differentiation of Human Dental Pulp Stem Cells Through the BMP Signaling Pathway. Exp. Ther. Med. 2022, 23, 55. [Google Scholar] [CrossRef]
- Camilleri, S.; McDonald, F. Runx2 and Dental Development. Eur. J. Oral Sci. 2006, 114, 361–373. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kanatani, N.; Rokutanda, S.; Yoshida, C.; Toyosawa, S.; Nakamura, R.; Takada, S.; Komori, T. Inhibition of the Terminal Differentiation of Odontoblasts and Their Transdifferentiation into Osteoblasts in Runx2 Transgenic Mice. Arch. Histol. Cytol. 2008, 71, 131–146. [Google Scholar] [CrossRef]
- Shang, W.; Xiong, S. Phenytoin Is Promoting the Differentiation of Dental Pulp Stem Cells into the Direction of Odontogenesis/Osteogenesis by Activating BMP4/Smad Pathway. Dis. Markers 2022, 2022, 7286645. [Google Scholar] [CrossRef]
- Jacob, A.; Zhang, Y.; George, A. Transcriptional Regulation of Dentin Matrix Protein 1 (DMP1) in Odontoblasts and Osteoblasts. Connect. Tissue Res. 2014, 55, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Haruyama, N.; Nishimura, F.; Kulkarni, A.B. Dentin Sialophosphoprotein and Dentin Matrix Protein-1: Two Highly Phosphorylated Proteins in Mineralized Tissues. Arch. Oral Biol. 2012, 57, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Lee, S.; Chen, D.; Kim, U.; Kim, Y.; Kim, S.; Kim, E. Effects of Different Calcium Silicate Cements on the Inflammatory Response and Odontogenic Differentiation of Lipopolysaccharide-Stimulated Human Dental Pulp Stem Cells. Materials 2019, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Daltoé, M.O.; Paula-Silva, F.W.; Faccioli, L.H.; Gatón-Hernández, P.M.; De Rossi, A.; Bezerra Silva, L.A. Expression of Mineralization Markers During Pulp Response to Biodentine and Mineral Trioxide Aggregate. J. Endod. 2016, 42, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, W.; Song, Z.; Tong, Z.; Li, S.; Ni, L. Mineral Trioxide Aggregate Promotes Odontoblastic Differentiation Via Mitogen-Activated Protein Kinase Pathway in Human Dental Pulp Stem Cells. Mol. Biol. Rep. 2012, 39, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The Mesenchymal Stem Cell Secretome: A New Paradigm Towards Cell-Free Therapeutic Mode in Regenerative Medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal Stem Cells Secretome: Current Trends and Future Challenges. Neural Regen. Res. 2020, 15, 75–77. [Google Scholar] [CrossRef]
- Gwam, C.; Mohammed, N.; Ma, X. Stem Cell Secretome, Regeneration, and Clinical Translation: A Narrative Review. Ann. Transl. Med. 2021, 9, 70. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Sousa, N.; Salgado, A.J. Mesenchymal Stem Cells Secretome: A New Paradigm for Central Nervous System Regeneration? Cell. Mol. Life Sci. 2013, 70, 3871–3882. [Google Scholar] [CrossRef]
- Sultan, N.; Amin, L.E.; Zaher, A.R.; Scheven, B.A.; Grawish, M.E. Dental Pulp Stem Cells: Novel Cell-Based and Cell-Free Therapy for Peripheral Nerve Repair. World J. Stomatol. 2019, 7, 1–19. [Google Scholar] [CrossRef]

| Material | Manufacturer | Composition | Powder Size | Batch Number |
|---|---|---|---|---|
| ProRoot MTA | Dentsply Tulsa Dental Specialties, Tulsa, OK, USA | Portland cement (tricalcium silicate, dicalcium silicate, and tricalcium aluminate) 75% Calcium sulfate dihydrate (gypsum) 5% Bismuth oxide 20% | 6.9 μm | 294002 |
| Biodentine | Septodont, Saint-Maur-dens-Fossés, France | Tricalcium silicate 80.1% Calcium carbonate 14.9% Zirconium Oxide 5% Calcium chloride and polycarboxylate as an aqueous liquid | 3.77 μm | B29557 |
| RetroMTA | BioMTA, Seoul, Republic of Korea | Calcium carbonate 60–80% Silicon dioxide 5–15% Aluminum oxide 5–10% Calcium zirconia complex 20–30% | 2.62 μm | RMCA04D03 |
| Endocem MTA Premixed | Maruchi, Wonju, Republic of Korea | Zirconium dioxide 45–55% Calcium silicate 20–25% Calcium aluminate 1–5% Calcium sulfate 1–5% Dimethyl sulfoxide 20–25% Thickening agent 1–5% | FD220323A | |
| secretome | Top Cell Bio Inc., Seoul, Republic of Korea |
| Gene | Primer Sequence |
|---|---|
| Runt-related transcription factor 2 (RUNX 2) | Forward: 5′-AAG TGC GGT GCA AAC TTT CT-3′ Reverse: 5′-TCT CGG TGG CTG CTA GTG A-3 |
| Osterix (OSX) | Forward: 5′-AGC CTC TGG CTA TGC AAA TGA-3′ Reverse: 5′-TGT AGA CAC TAG GCA GGC AGT CA-3 |
| Suppressor of Mothers against Decapentaplegic (SMAD1) | Forward: 5′-CCA CTG GAA TGC TGT TTT CC-3′ Reverse: 5′-GTA AGC TCA TAG ACT GTC TCA AAT CC-3′ |
| Dentin sialophosphoprotein (DSPP) | Forward: 5′-GGG AAT ATT GAG GGC TGG AA-3′ Reverse: 5′-TCA TTG TGA CCT GCA TCG CC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Y.-J.; Lee, D.; Kim, S.-Y. The Combined Effects on Human Dental Pulp Stem Cells of Fast-Set or Premixed Hydraulic Calcium Silicate Cements and Secretome Regarding Biocompatibility and Osteogenic Differentiation. Materials 2024, 17, 305. https://doi.org/10.3390/ma17020305
Ha Y-J, Lee D, Kim S-Y. The Combined Effects on Human Dental Pulp Stem Cells of Fast-Set or Premixed Hydraulic Calcium Silicate Cements and Secretome Regarding Biocompatibility and Osteogenic Differentiation. Materials. 2024; 17(2):305. https://doi.org/10.3390/ma17020305
Chicago/Turabian StyleHa, Yun-Jae, Donghee Lee, and Sin-Young Kim. 2024. "The Combined Effects on Human Dental Pulp Stem Cells of Fast-Set or Premixed Hydraulic Calcium Silicate Cements and Secretome Regarding Biocompatibility and Osteogenic Differentiation" Materials 17, no. 2: 305. https://doi.org/10.3390/ma17020305







