Stimulation of Biological Structures on the Nanoscale Using Interfaces with Large Built-In Spontaneous Polarizations
Abstract
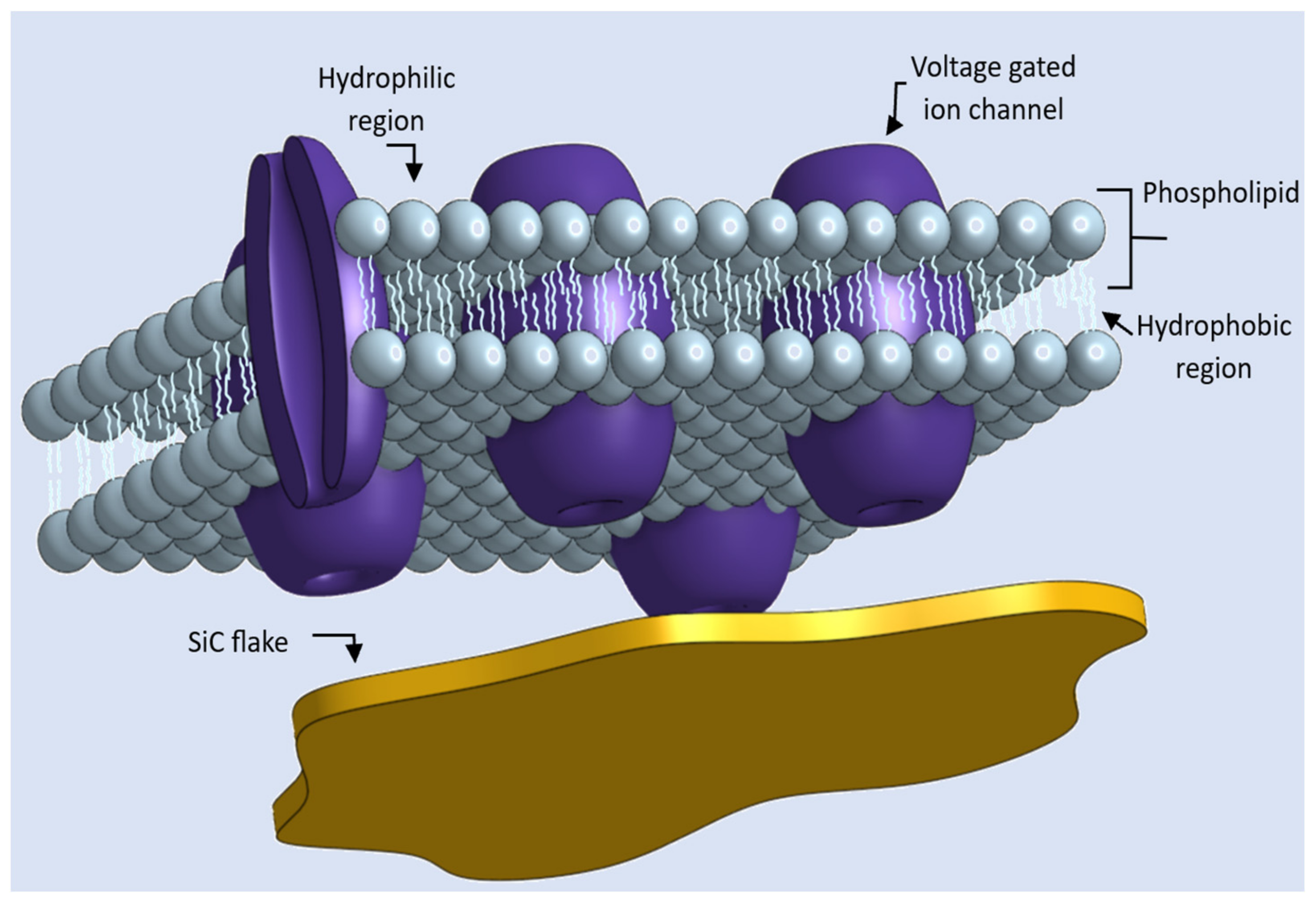
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, V.; Yi, J.; Isheim, D.; Rotenberg, M.; Meng, L.; Shi, F.; Chen, X.; Gao, X.; Prominski, A.; Jiang, Y.; et al. Laser writing of nitrogen-doped silicon carbide for biological modulation. Sci. Adv. 2020, 6, eaaz2743. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Lee, S.; Li, F. Direct synthesis of 2H–SiC nanowhiskers. Chem. Phys. Lett. 2003, 381, 628–633. [Google Scholar] [CrossRef]
- Nilsson, H.-E.; Hjelm, M. Monte Carlo simulation of electron transport in 2H-SiC using a three valley analytical conduction band model. J. Appl. Phys. 1999, 86, 6230–6233. [Google Scholar] [CrossRef]
- Chabi, S.; Chang, H.; Xia, Y.; Zhu, Y. From graphene to silicon carbide: Ultrathin silicon carbide flakes. Nanotechnology 2016, 27, 075602. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Hu, J.; Fu, W.; Ang, L.K.; Yang, H.Y. Graphene-Induced in Situ Growth of Monolayer and Bilayer 2D SiC Crystals Toward High-Temperature Electronics. ACS Appl. Mater. Interfaces 2019, 11, 39109–39115. [Google Scholar] [CrossRef]
- Benjamin, P. Laser Microfabrication and Testing of Silicon Carbide Diaphragms for MEMS Applications. Master’s Thesis, Iowa State University, Ames, IA, USA, 2009. [Google Scholar]
- He, R.; Zhou, N.; Zhang, K.; Zhang, X.; Zhang, L.; Wang, W.; Fang, D. Progress and challenges towards additive manufacturing of SiC ceramic. J. Adv. Ceram. 2021, 10, 637–674. [Google Scholar] [CrossRef]
- Khan, K.; Biswas, M.; Ahmadi, E. Growth of high quality (In,Ga)N films on O-face ZnO substrates by plasma-assisted molecular beam epitaxy. AIP Adv. 2020, 10, 075120. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, S.; Cho, D.-H.; Kang, B.; Kwon, H.; Kim, Y.; Park, S.O.; Jung, G.Y.; Shin, E.; Kim, W.-G.; et al. Direct exfoliation and dispersion of two-dimensional materials in pure water via temperature control. Nat. Commun. 2015, 6, 8294. [Google Scholar] [CrossRef] [PubMed]
- Ashutosh Sagara, R.; Feenstra, M.; Freitas, J.A., Jr. Growth of GaN on porous SiC by molecular beam epitaxy. In Porous SiC and GaN: Epitaxy, Catalysis, and Biotechnology Applications; Feenstra, R.M., Wood, C.E.C., Eds.; Wiley: West Sussex, UK, 2008. [Google Scholar]
- Ko, H.J.; Chen, Y.; Hong, S.K.; Yao, T. MBE growth of high-quality ZnO films on epi-GaN. J. Cryst. Growth 2000, 209, 816–821. [Google Scholar]
- Ko, H.-J.; Yao, T.; Chen, Y.; Hong, S.-K. Investigation of ZnO epilayers grown under various Zn/O ratios by plasma-assisted molecular-beam epitaxy. J. Appl. Phys. 2002, 92, 4354–4360. [Google Scholar] [CrossRef]
- Zareie, M.; Gholami, A.; Bahrami, M.; Rezaei, A.H.; Keshavarz, M.H. A simple method for preparation of micro-sized ZnO flakes. Mater. Lett. 2013, 91, 255–257. [Google Scholar] [CrossRef]
- Sholehah, A.; Faroz, D.F.; Huda, N.; Utari, L.; Septiani, N.L.W.; Yuliarto, B. Synthesis of ZnO Flakes on Flexible Substrate and Its Application on Ethylene Sensing at Room Temperature. Chemosensors 2020, 8, 2. [Google Scholar] [CrossRef]
- Li, Y.S.; Li, G.; Wang, S.X.; Gao, H.; Tan, Z.C. Preparation and characterization of nano-ZnO flakes prepared by reactive ion exchange method. J. Therm. Anal. Calorim. 2008, 95, 671–674. [Google Scholar] [CrossRef]
- Sayari, A. Characterization of Nanocrystalline ZnO Flakes Synthesized by a Simple Reaction Process. KONA Powder Part. J. 2013, 30, 119–124. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- White, J.A. Action Potential. In Encyclopedia of the Human Brain, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1–12. [Google Scholar] [CrossRef]
- Meshik, X.; Choi, M.; Baker, A.; Malchow, R.P.; Covnot, L.; Doan, S.; Mukherjee, S.; Farid, S.; Dutta, M.; Stroscio, M.A. Modulation of voltage-gated conductances of retinal horizontal cells by UV-excited TiO2 nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.; Hudspeth, A.J.; Mack, S. Principles of Neural Science, 6th ed.; McGraw Hill: New York, NY, USA, 2021; ISBN 10:1259642232/13:9781259642234. [Google Scholar]
- Stroscio, M.A.; Dutta, M.; Niwani, K.; Shi, P.; Ramadurai, D.; Rufo, S. Integrating and Tagging Biological Structures with Nanoscale Semiconductor Quantum-Dots Structures. In Biological Nanostructures and Applications of Nanostructures in Biology: Electrical, Mechanical & Optical Properties; Stroscio, M.A., Dutta, M., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2004. [Google Scholar]
- Alexson, D.; Chen, H.; Cho, M.; Dutta, M.; Li, Y.; Shi, P.; Raichura, A.; Ramadurai, D.; Parikh, S.; A Stroscio, M.; et al. Semiconductor nanostructures in biological applications. J. Phys. Condens. Matter 2005, 17, R637–R656. [Google Scholar] [CrossRef]
- Stroscio, M.; Dutta, M. Integrated Biological-Semiconductor Devices. Proc. IEEE 2005, 93, 1772–1783. [Google Scholar] [CrossRef]
- Zia, N.; Stroscio, M.A.; Dutta, M. The Use of Semiconductor Quantum Dots with Large, Built-In Spontaneous Polarizations for the Electric Potential Stimulation of Biological Structures on the Nanoscale. Nanomaterials 2023, 13, 3143. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Ion Channels and the Electrical Properties of Membranes. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26910/ (accessed on 27 November 2023).
- Cameron, M.A.; Al Abed, A.; Buskila, Y.; Dokos, S.; Lovell, N.H.; Morley, J.W. Differential effect of brief electrical stimulation on voltage-gated potassium channels. J. Neurophysiol. 2017, 117, 2014–2024. [Google Scholar] [CrossRef]
- Troy, W.; Dutta, M.; Stroscio, M. Spontaneous Polarization Calculations in Wurtzite II-Oxides, III-Nitrides, and SiC Polytypes through Net Dipole Moments and the Effects of Nanoscale Layering. Nanomaterials 2021, 11, 1956. [Google Scholar] [CrossRef]
- Troy, W.; Dutta, M.; Stroscio, M.A. Spontaneous Polarization in Nanoparticles. IEEE Electron. Device Lett. 2021, 42, 1838–1840. [Google Scholar] [CrossRef]
- Hunter, R.J. Foundations of Colloid Science: Characterization of colloidal dispersions. In Foundations of Colloid Science; Robert J. Hunter Oxford science publications; Hunter, R.J., Ed.; Oxford University Press: Oxford, UK, 1987; Volume 1, p. 24. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications New Paperback; Academic Press: London, UK, 1988; Available online: https://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=877203 (accessed on 27 November 2023).
- Bernardini, F.; Fiorentini, V.; Vanderbilt, D. Spontaneous polarization and piezoelectric constants of III-V nitrides. Phys. Rev. B 1997, 56, R10024–R10027. [Google Scholar] [CrossRef]
- Lähnemann, J.; Brandt, O.; Jahn, U.; Pfüller, C.; Roder, C.; Dogan, P.; Grosse, F.; Belabbes, A.; Bechstedt, F.; Trampert, A.; et al. Direct experimental determination of the spontaneous polarization of GaN. Phys. Rev. B 2012, 86, 081302. [Google Scholar] [CrossRef]
- Davydov, S.Y.; Troshin, A.V. Estimates of the spontaneous polarization in silicon carbide. Phys. Solid State 2007, 49, 759–761. [Google Scholar] [CrossRef]
- Choi, M.S.; Meshik, X.; Mukherjee, S.; Farid, S.; Doan, S.; Covnot, L.; Dutta, M.; Stroscio, M.A. Electrostatic force analysis, optical measurements, and structural characterization of zinc oxide colloidal quantum dots synthesized by sol-gel method. J. Appl. Phys. 2015, 118, 194304. [Google Scholar] [CrossRef]
- Meshik, X.; Farid, S.; Choi, M.; Lan, Y.; Mukherjee, S.; Datta, D.D.D.; Dutta, M.; Stroscio, M.A. Biomedical Applications of Quantum Dots, Nucleic Acid-Based Aptamers, and Nanostructures in Biosensors. Crit. Rev. Biomed. Eng. 2015, 43, 277–296. [Google Scholar] [CrossRef]
- Gao, N.; Gao, T.; Yang, X.; Dai, X.; Zhou, W.; Zhang, A.; Lieber, C.M. Specific detection of biomolecules in physiological solutions using graphene transistor biosensors. Proc. Natl. Acad. Sci. USA 2016, 113, 14633–14638. [Google Scholar] [CrossRef]
- Wennerström, H.; Estrada, E.V.; Danielsson, J.; Oliveberg, M. Colloidal stability of the living cell. Proc. Natl. Acad. Sci. USA 2020, 117, 10113–10121. [Google Scholar] [CrossRef]
- Kesler, V.; Murmann, B.; Soh, H.T. Going beyond the Debye Length: Overcoming Charge Screening Limitations in Next-Generation Bioelectronic Sensors. ACS Nano 2020, 14, 16194–16201. [Google Scholar] [CrossRef]
- Ma, H.; Wang, K.; Gao, Z.; Wang, H.; Wang, S.; Zhang, C.; Wang, G.; Bai, J. Current characteristic signals of aqueous solution transferring through microfluidic channel under non-continuous DC electric field. AIP Adv. 2014, 4, 107139. [Google Scholar] [CrossRef]
- Gil, B.; Keshavarz, M.; Wales, D.; Darzi, A.; Yeatman, E. Orthogonal Surface-Enhanced Raman Scattering/Field-Effect Transistor Detection of Breast and Colorectal Cancer-Derived Exosomes using Graphene as a Tag-Free Diagnostic Template. Adv. NanoBiomed Res. 2023, 3, 2300055. [Google Scholar] [CrossRef]





| ZnO P (High) C/m2 | ZnO P (Low) C/m2 | GaN P (High) C/m2 | GaN P (Low) C/m2 | 2H-SiC P (High) C/m2 | 2H-SiC P (Low) C/m2 | |
|---|---|---|---|---|---|---|
| 4 layers | 0.095 | −0.180 | 0.198 | −0.210 | 0.20 | −0.24 |
| 8 layers | 0.020 | −0.135 | 0.098 | −0.120 | 0.09 | −0.14 |
| 15 layers | −0.018 | −0.095 | 0.040 | −0.070 | 0.04 | −0.09 |
| 22 layers | −0.035 | −0.070 | 0.020 | −0.050 | 0.02 | −0.08 |
| Debye Length | 0.33 nm | 0.66 nm | 1 nm | 1.33 nm | 1.66 nm | 2 nm | 2.33 nm |
|---|---|---|---|---|---|---|---|
| Flake Thickness | |||||||
| 2.08 nm | −0.018 | −0.078 | −0.154 | −0.232 | −0.312 | −0.395 | −0.477 |
| 4.16 nm | −0.013 | −0.059 | −0.115 | −0.174 | −0.234 | −0.297 | −0.358 |
| 7.8 nm | −9.72 × 10−3 | −0.041 | −0.081 | −0.122 | −0.164 | −0.208 | −0.252 |
| 11.45 nm | −7.16 × 10−3 | −0.030 | −0.059 | −0.09 | −0.121 | −0.154 | −0.186 |
| 15 nm | −6.14 × 10−3 | −0.026 | −0.051 | −0.077 | −0.104 | −0.132 | −0.159 |
| 20 nm | −5.73 × 10−3 | −0.024 | −0.048 | −0.072 | −0.097 | −0.123 | −0.149 |
| 25 nm | −5.53 × 10−3 | −0.023 | −0.046 | −0.070 | −0.094 | −0.118 | −0.140 |
| 30 nm | −5.07 × 10−3 | −0.022 | −0.042 | −0.064 | −0.086 | −0.108 | −0.132 |
| Debye Length | 0.33 nm | 0.66 nm | 1 nm | 1.33 nm | 1.66 nm | 2 nm | 2.33 nm |
|---|---|---|---|---|---|---|---|
| Flake Thickness | |||||||
| 2.08 nm | −4.05 × 10−3 | −0.037 | −0.093 | −0.159 | −0.230 | −0.308 | −0.385 |
| 4.16 nm | −3.03 × 10−3 | −0.027 | −0.070 | −0.119 | −0.173 | −0.231 | −0.289 |
| 7.8 nm | −2.13 × 10−3 | −0.019 | −0.049 | −0.084 | −0.121 | −0.162 | −0.203 |
| 11.45 nm | −1.57 × 10−3 | −0.014 | −0.036 | −0.061 | −0.089 | −0.119 | −0.149 |
| 15 nm | −1.35 × 10−3 | −0.012 | −0.031 | −0.053 | −0.077 | −0.103 | −0.129 |
| 20 nm | −1.26 × 10−3 | −0.012 | −0.029 | −0.050 | −0.072 | −0.096 | −0.120 |
| 25 nm | −1.22 × 10−3 | −0.011 | −0.028 | −0.048 | −0.069 | −0.093 | −0.116 |
| 30 nm | −1.11 × 10−3 | −0.010 | −0.026 | −0.044 | −0.064 | −0.085 | −0.106 |
| Debye Length | 0.33 nm | 0.66 nm | 1 nm | 1.33 nm | 1.66 nm | 2 nm | 2.33 nm |
|---|---|---|---|---|---|---|---|
| Flake Thickness | |||||||
| 2.07 nm | −0.0215 | −0.091 | −0.179 | −0.270 | −0.364 | −0.461 | −0.557 |
| 4.14 nm | −0.0122 | −0.052 | −0.103 | −0.155 | −0.208 | −0.264 | −0.318 |
| 7.7 nm | −7.16 × 10−3 | −0.030 | −0.059 | −0.090 | −0.121 | −0.154 | −0.186 |
| 11.4 nm | −5.11 × 10−3 | −0.022 | −0.043 | −0.064 | −0.087 | −0.110 | −0.133 |
| Debye Length | 0.33 nm | 0.66 nm | 1 nm | 1.33 nm | 1.66 nm | 2 nm | 2.33 nm |
|---|---|---|---|---|---|---|---|
| Flake Thickness | |||||||
| 2.07 nm | −4.72 × 10−3 | −0.043 | −0.109 | −0.186 | −0.269 | −0.360 | −0.450 |
| 4.14 nm | −2.7 × 10−3 | −0.024 | −0.062 | −0.106 | −0.154 | −0.205 | −0.257 |
| 7.7 nm | −1.575 × 10−3 | −0.014 | −0.036 | −0.062 | −0.090 | −0.119 | −0.150 |
| 11.4 nm | −1.12 × 10−3 | −0.010 | −0.026 | −0.044 | −0.064 | −0.086 | −0.107 |
| Debye Length | 0.33 nm | 0.66 nm | 1 nm | 1.33 nm | 1.66 nm | 2 nm | 2.33 nm |
|---|---|---|---|---|---|---|---|
| Flake Thickness | |||||||
| 2.04 nm | −0.025 | −0.105 | −0.205 | −0.309 | −0.416 | −0.528 | −0.637 |
| 4.08 nm | −0.014 | −0.061 | −0.120 | −0.180 | −0.243 | −0.308 | −0.372 |
| 7.66 nm | −9.21 × 10−3 | −0.039 | −0.077 | −0.116 | −0.156 | −0.198 | −0.239 |
| 11.24 nm | −8.19 × 10−3 | −0.035 | −0.068 | −0.103 | −0.139 | −0.176 | −0.212 |
| Debye Length | 0.33 nm | 0.66 nm | 1 nm | 1.33 nm | 1.66 nm | 2 nm | 2.33 nm |
|---|---|---|---|---|---|---|---|
| Flake Thickness | |||||||
| 2.04 nm | −5.4 × 10−3 | −0.049 | −0.125 | −0.212 | −0.308 | −0.411 | −0.514 |
| 4.08 nm | −3.15 × 10−3 | −0.029 | −0.073 | −0.124 | −0.179 | −0.240 | −0.299 |
| 7.66 nm | −2.02 × 10−3 | −0.018 | −0.047 | −0.079 | −0.115 | −0.154 | −0.193 |
| 11.24 nm | −1.8 × 10−3 | −0.016 | −0.042 | −0.071 | −0.103 | −0.137 | −0.171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zia, N.; Stroscio, M.; Dutta, M. Stimulation of Biological Structures on the Nanoscale Using Interfaces with Large Built-In Spontaneous Polarizations. Materials 2024, 17, 2332. https://doi.org/10.3390/ma17102332
Zia N, Stroscio M, Dutta M. Stimulation of Biological Structures on the Nanoscale Using Interfaces with Large Built-In Spontaneous Polarizations. Materials. 2024; 17(10):2332. https://doi.org/10.3390/ma17102332
Chicago/Turabian StyleZia, Nida, Michael Stroscio, and Mitra Dutta. 2024. "Stimulation of Biological Structures on the Nanoscale Using Interfaces with Large Built-In Spontaneous Polarizations" Materials 17, no. 10: 2332. https://doi.org/10.3390/ma17102332





