Sideritis raeseri—Modified Coatings on Ti-6Al-4V as a Carrier for Controlled Delivery Systems of Active Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Brushite
2.3. X-ray Diffraction Analysis
2.4. Preparation of Sideritis raeseri Extract
2.5. Preparation of Coatings
2.6. Fourier-Transform Infrared Spectroscopy Analysis
2.7. Incubation In Vitro
2.7.1. pH-Metric and Conductometric Analysis
2.7.2. Determination of Sorption Capacity of Biomaterials
2.8. Determination of Total Polyphenol Content
2.9. Determination of Antioxidation Potential via DPPH Method
2.10. Determination of Release Kinetics of Polyphenols
2.11. Morphology Analysis
3. Results
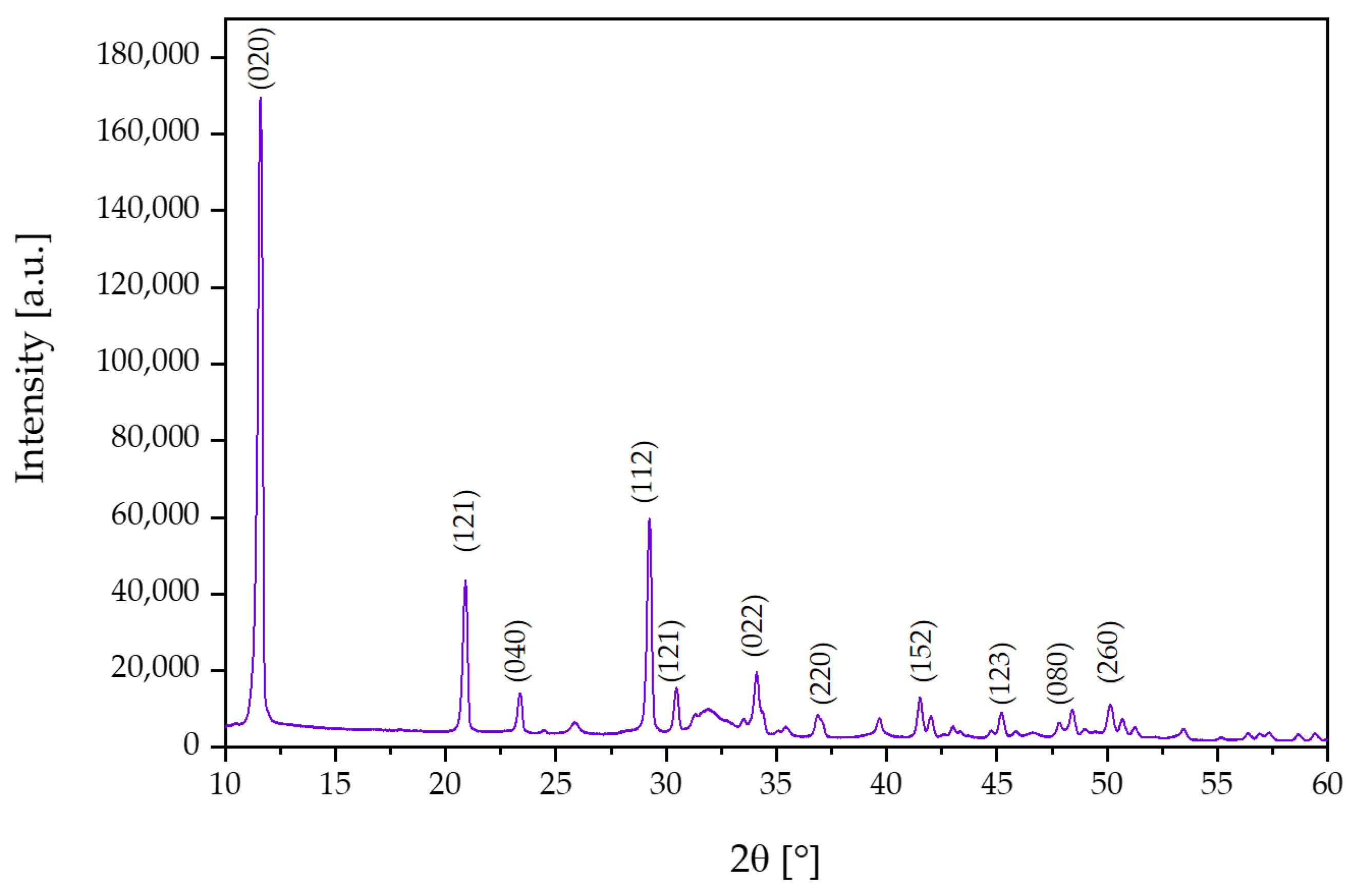
3.1. X-ray Diffraction Analysis
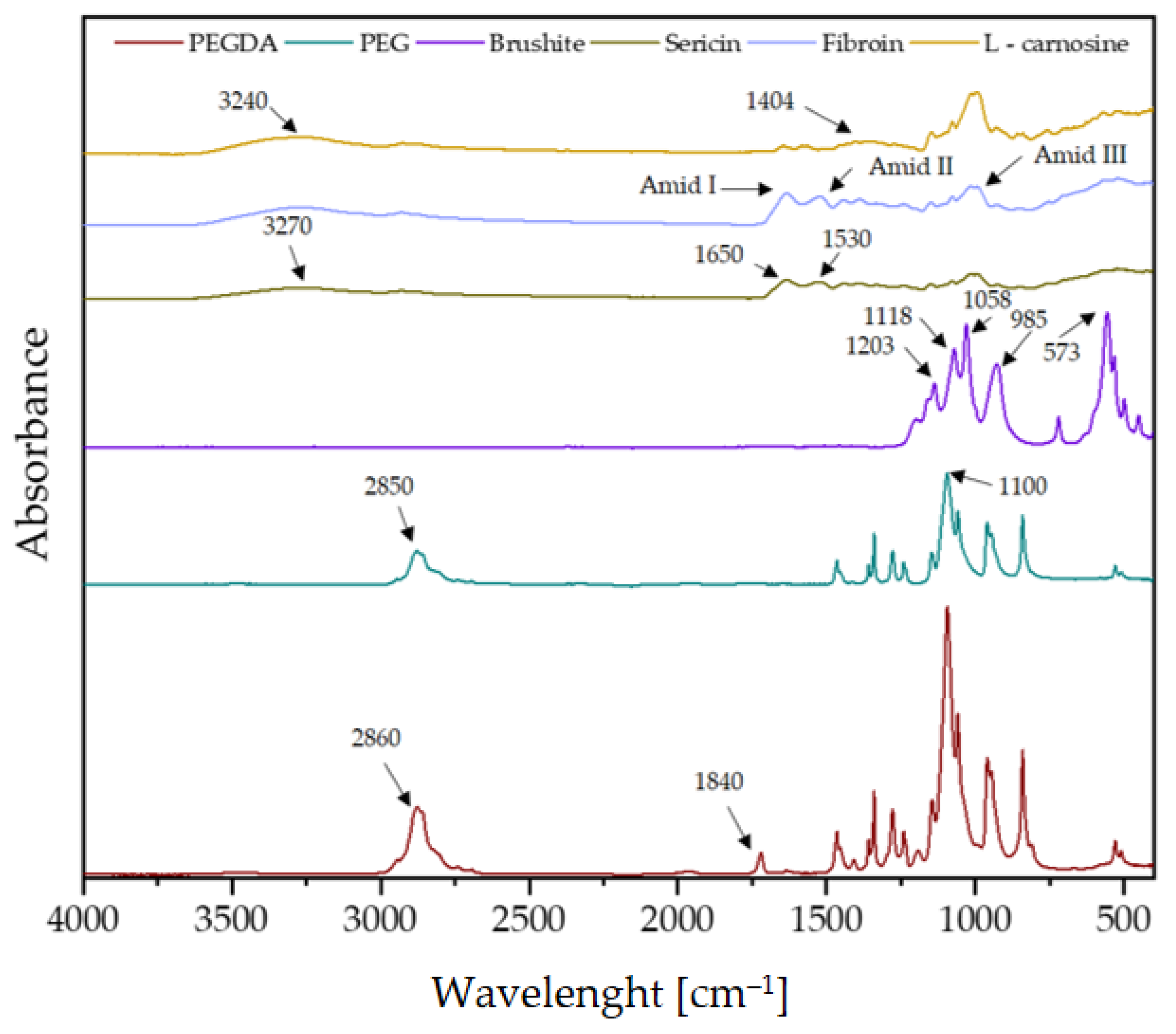
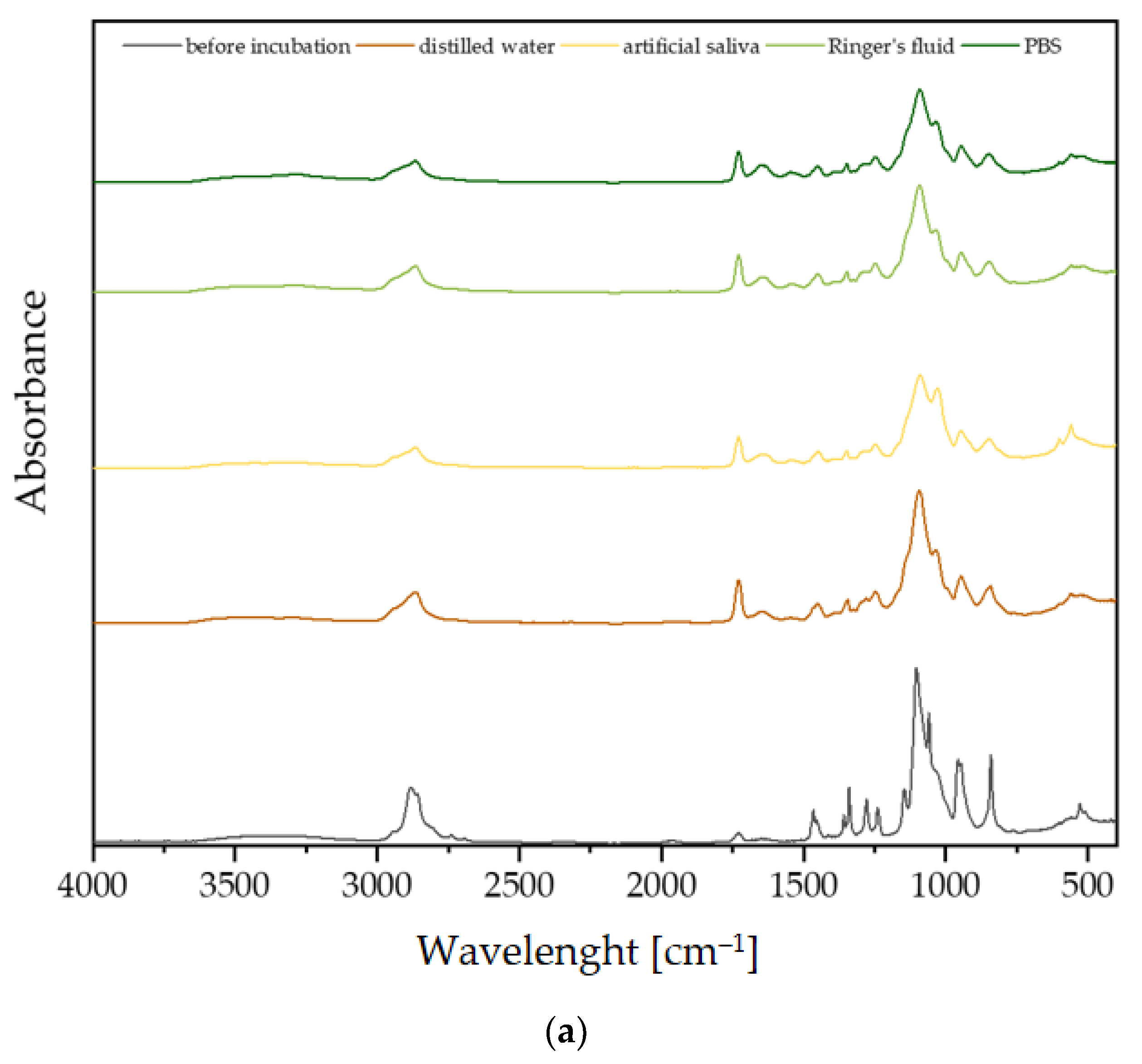
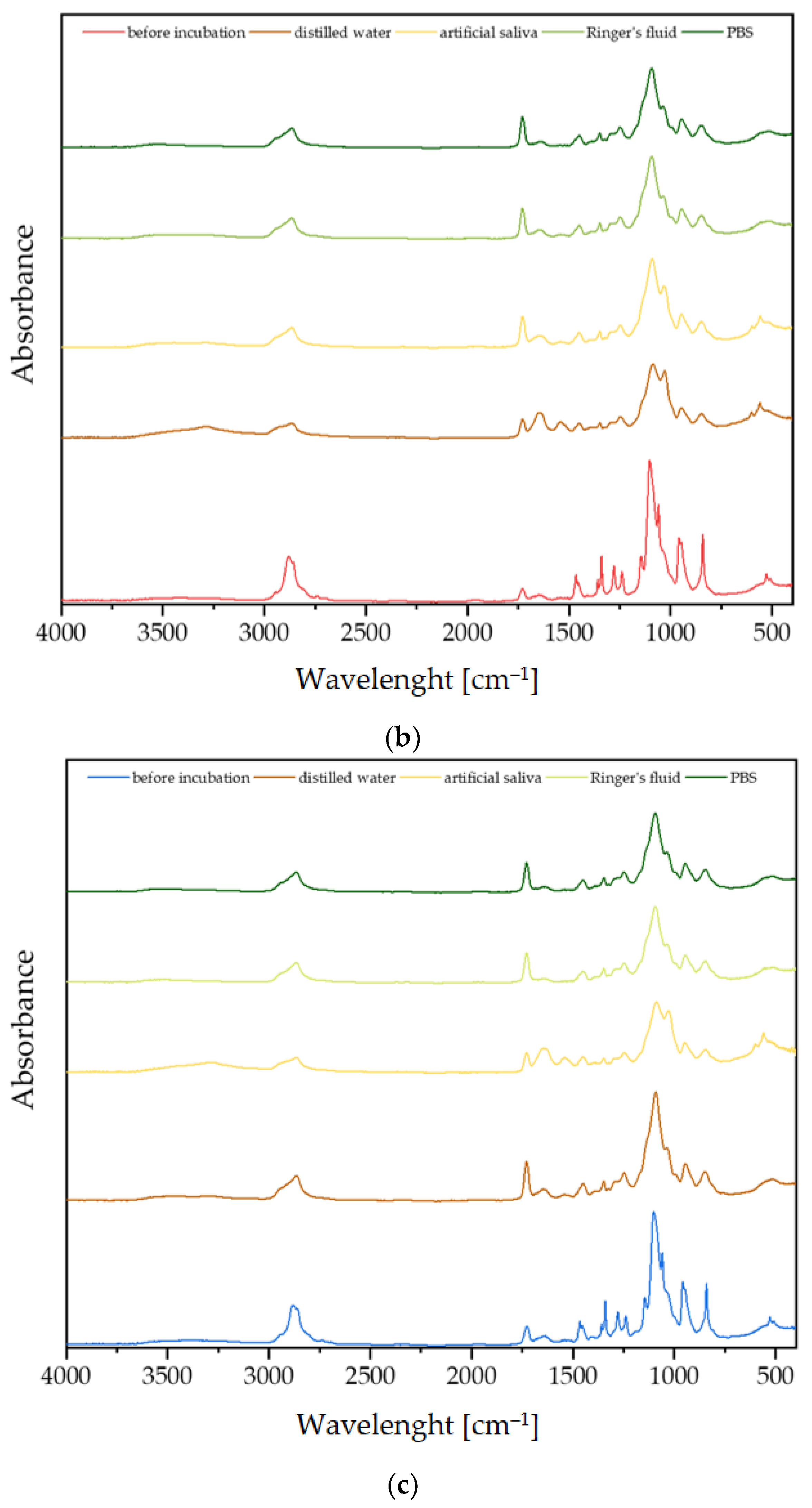
3.2. Fourier-Transform Infrared Spectroscopy Analysis
3.3. Incubation In Vitro
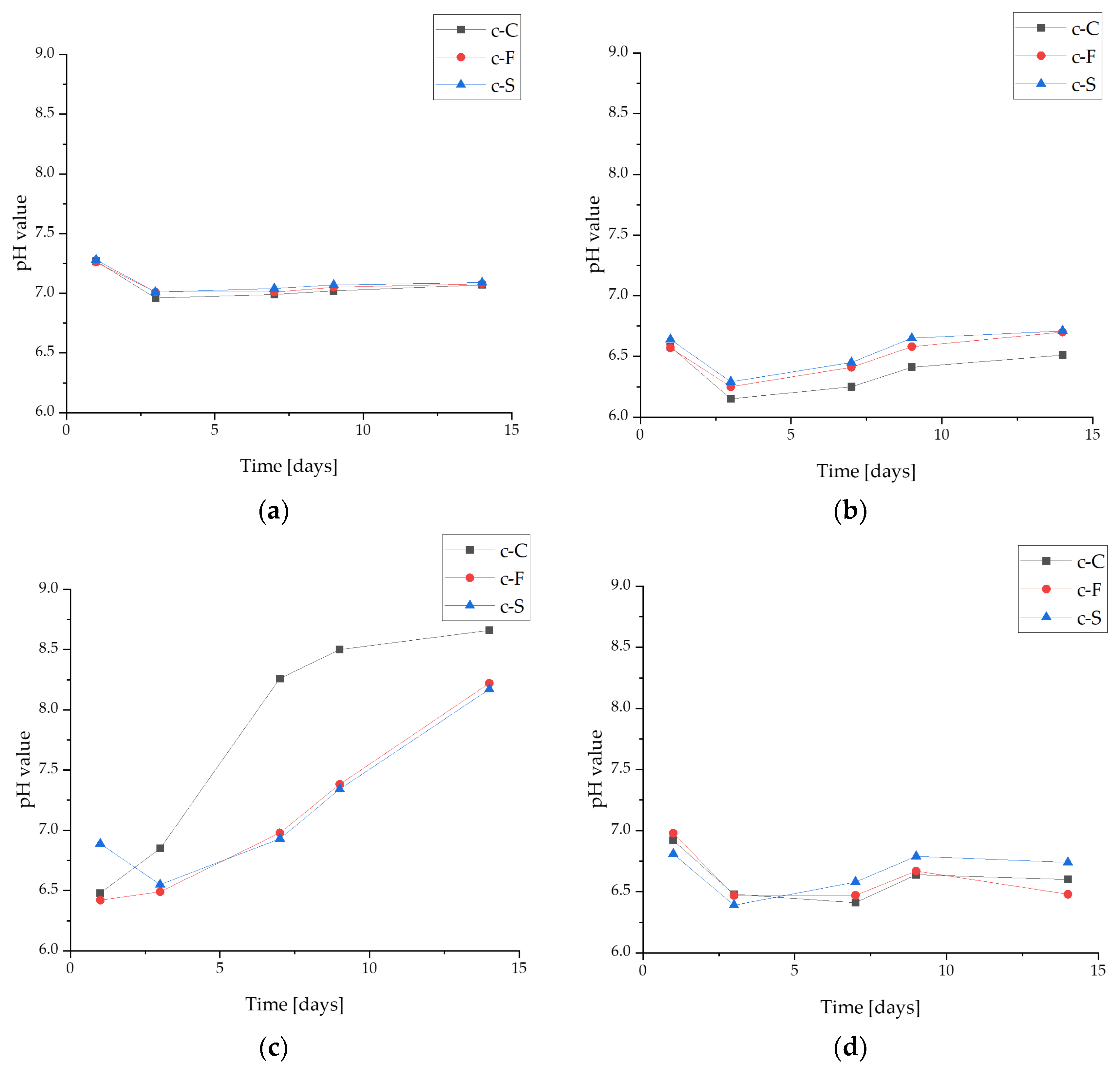
3.3.1. pH-Metric Analysis
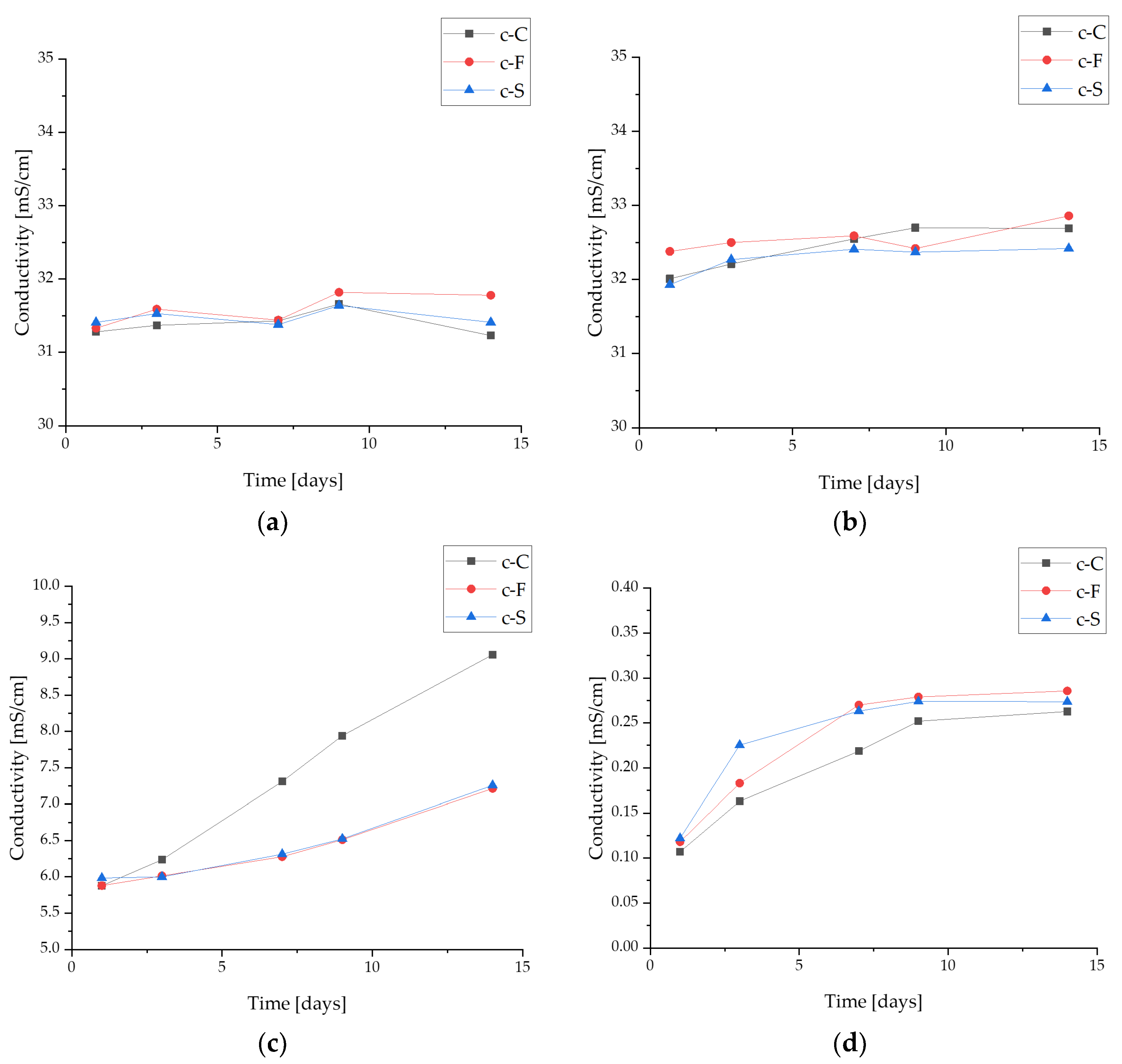
3.3.2. Conductivity Analysis
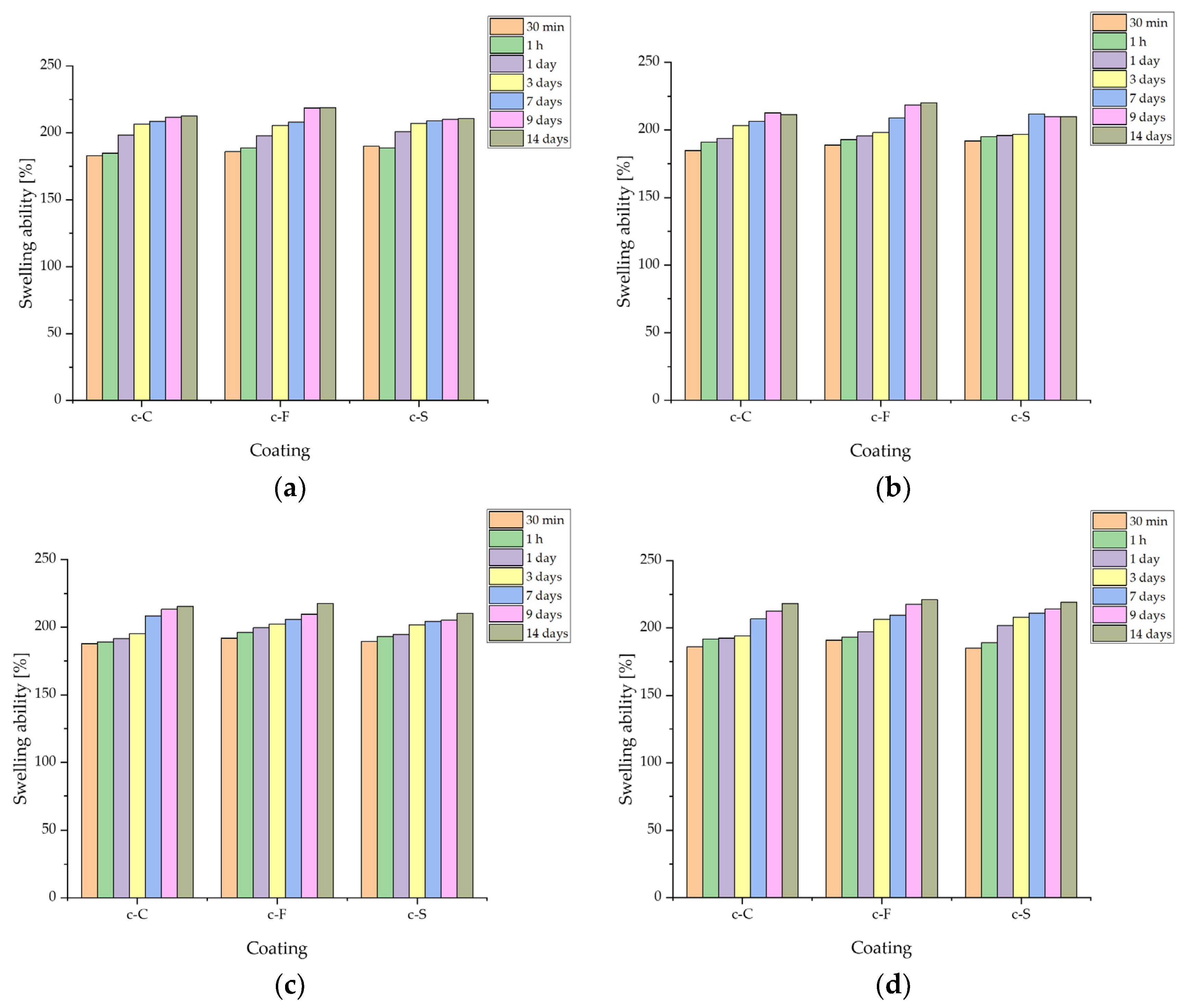
3.3.3. Determination of Sorption Capacity of Biomaterials
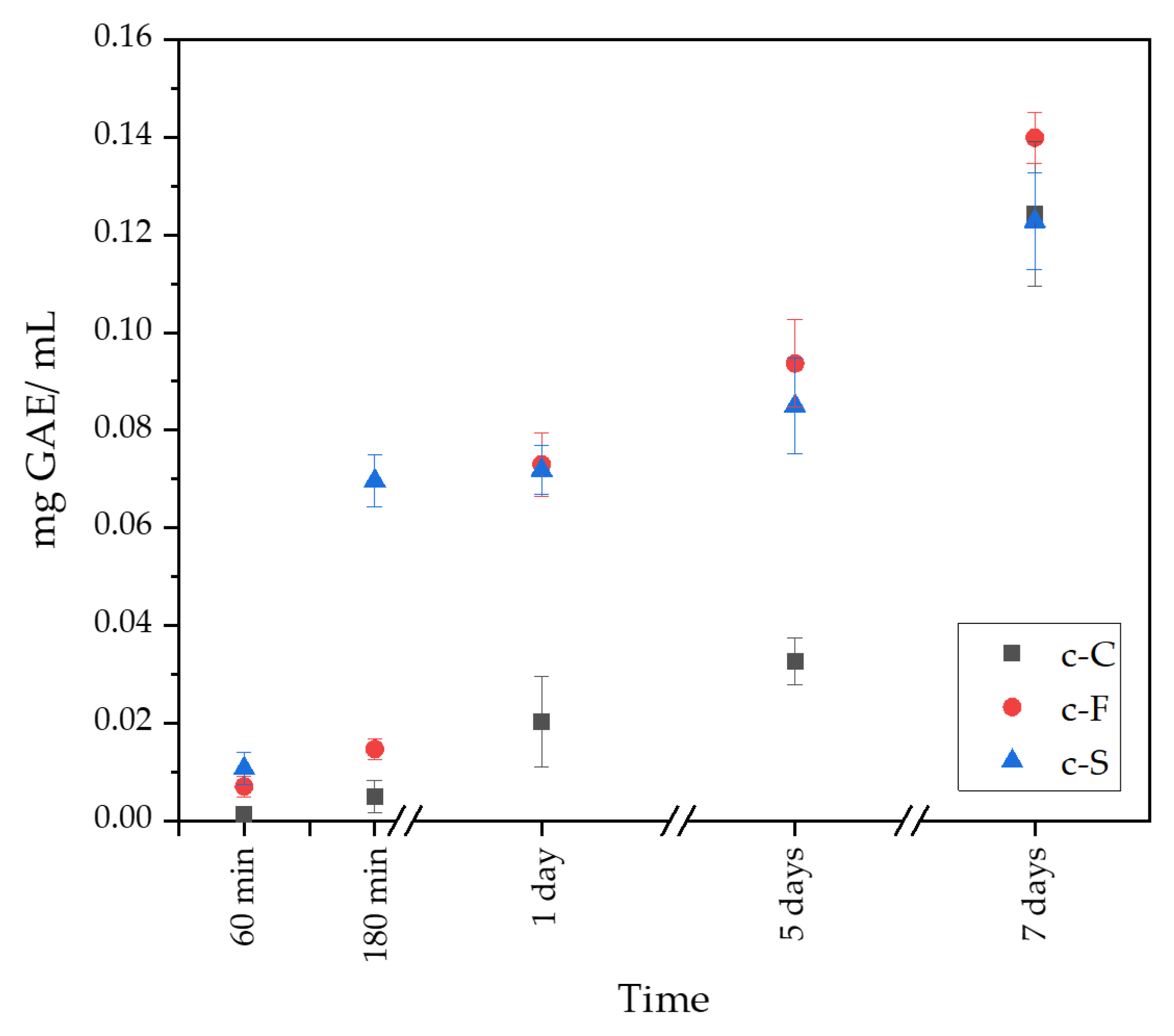
3.4. Determination of Total Polyphenol Content
3.5. Determination of Antioxidation Potential via DPPH Method
3.6. Determination of Release Kinetics of Polyphenols
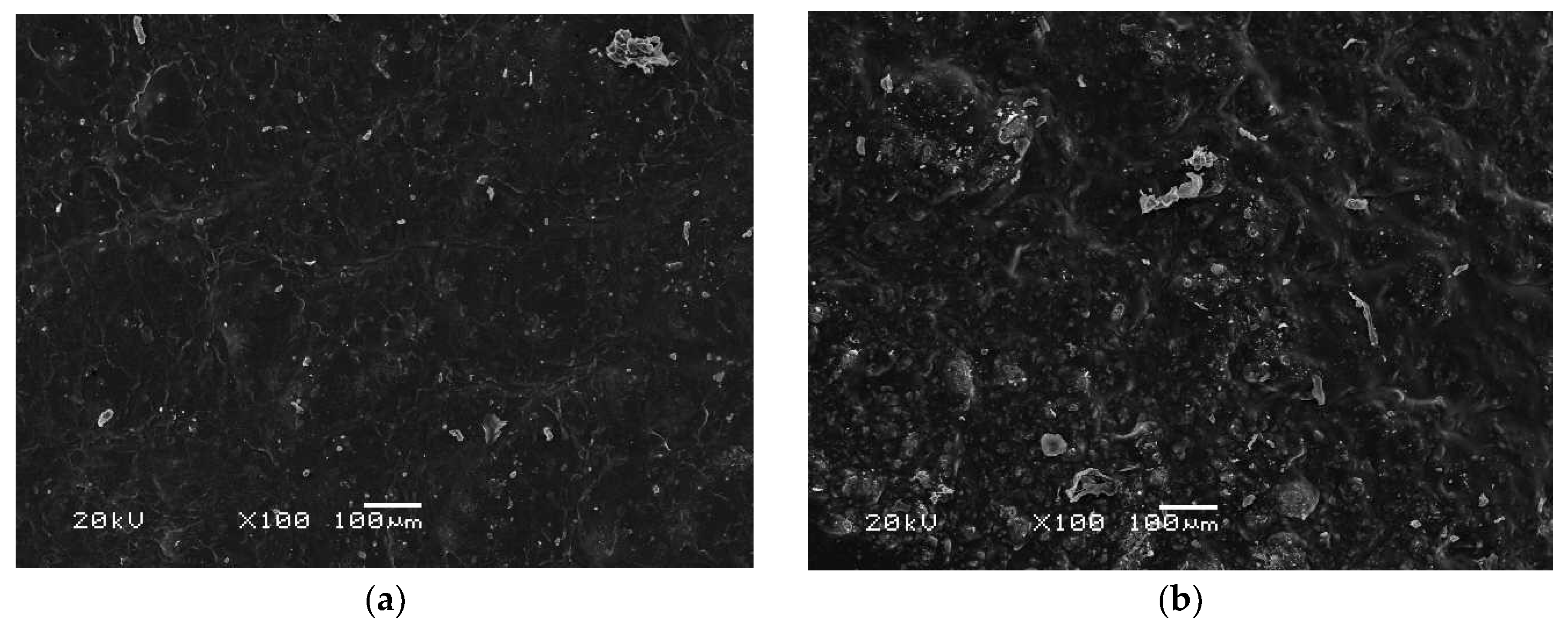
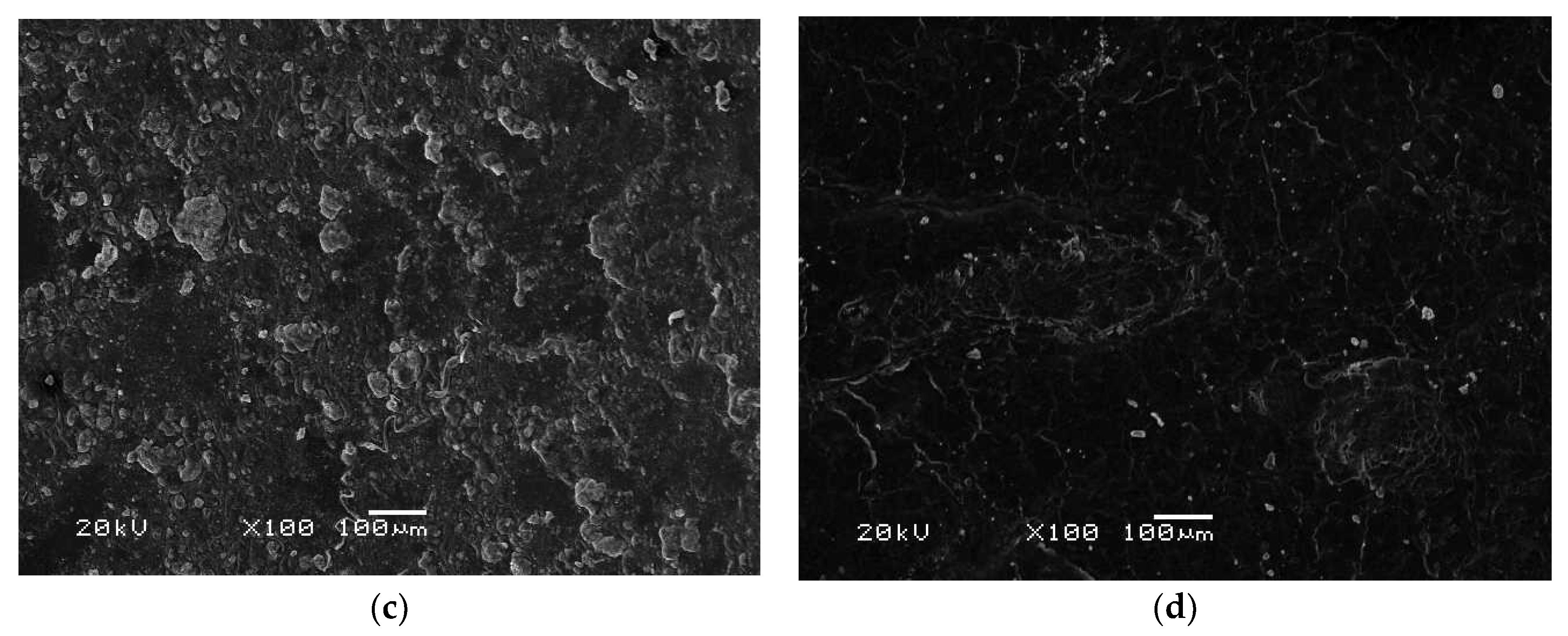
3.7. Morphology Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D Printed Porous Ti6Al4V Implants with Different Pore Sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Siti Nur Hazwani, M.R.; Lim, L.X.; Lockman, Z.; Zuhailawati, H. Fabrication of Titanium-Based Alloys with Bioactive Surface Oxide Layer as Biomedical Implants: Opportunity and Challenges. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2022, 32, 1–44. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M.; Berkem, A.S. Bioactive Coatings on Ti6Al4V Alloy Formed by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2016, 301, 85–93. [Google Scholar] [CrossRef]
- Haydar, H.J.; Al-Deen, J.; Abidali, A.K.; Mahmoud, A.A. Improved Performance of Ti6Al4V Alloy in Biomedical Applications—Review. J. Phys. Conf. Ser. 2021, 1973, 012146. [Google Scholar] [CrossRef]
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the Methodologies Used in the Synthesis Gold Nanoparticles by Chemical Reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Boanini, E.; Silingardi, F.; Gazzano, M.; Bigi, A. Synthesis and Hydrolysis of Brushite (DCPD): The Role of Ionic Substitution. Cryst. Growth Des. 2021, 21, 1689–1697. [Google Scholar] [CrossRef]
- Pina, S.; Vieira, S.I.; Rego, P.; Torres, P.M.C.; da Cruz e Silva, O.A.B.; da Cruz e Silva, E.F.; Ferreira, J.M.F. Biological Responses of Brushite-Forming Zn-and ZnSr-Substituted β-Tricalcium Phosphate Bone Cements. Eur. Cells Mater. 2010, 20, 162–177. [Google Scholar] [CrossRef]
- Türk, S.; Altınsoy; ÇelebiEfe, G.; Ipek, M.; Özacar, M.; Bindal, C. Biomimetric Coating of Monophasic Brushite on Ti6Al4V in New M-5xSBF. Surf. Coat. Technol. 2018, 351, 1–10. [Google Scholar] [CrossRef]
- Giocondi, J.L.; El-Dasher, B.S.; Nancollas, G.H.; Orme, C.A. Molecular Mechanisms of Crystallization Impacting Calcium Phosphate Cements. Trans. R. Soc. A 2010, 368, 1937–1961. [Google Scholar] [CrossRef]
- Kolagenowych, S. Fizykochemiczna Charakterystyka Mineralizacji Struktur Kolagenowych. Ph.D. Thesis, Uniwersytet Jagielloński, Kraków, Poland, 2018. [Google Scholar]
- Ebnesajjad, S. Handbook of Biopolymers and Biodegradable Plastics; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Preedy, V.R. Diabetes: Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Patel, V.; Rajendram, R. The Liver: Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Julien, E.; Coulon-Bublex, M.; Garel, A.; Royer, C.; Chavancy, G.; Prudhomme, J.C.; Couble, P. Comprehensive Molecular Insect Science; Pergamon: Bergama, Turkey, 2005. [Google Scholar]
- Bilska-Wilkosz, A.; Iciek, M.; Kowalczyk-Pachel, D. Biochemia z Elementami Chemii Biologicznej Dla Inżynierów; Politechnika Krakowska: Kraków, Poland, 2015. [Google Scholar]
- Manoukian, O.; Sardashti, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S. Encyclopedia Od Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1. [Google Scholar]
- Silva, S.S.; Fernandes, E.; Pina, S.; Silva-Correia, J.; Vieira, S.; Oliveira, J.; Reis, R. Comprehensive Biomaterials II, Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2. [Google Scholar]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk Sericin-Based Materials for Biomedical Applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef]
- Ahsan, F.; Ansari, T.M.; Usmani, S.; Bagga, P. An Insight on Silk Protein Sericin: From Processing to Biomedical Application. Drug Res. 2018, 68, 317–327. [Google Scholar] [CrossRef]
- Gobbo, V.A. Bioaterials Fuctionalisation with Polyphenols and Characterisation. Master’s Thesis, Politecnico di Torino, Torino, Italy, 2020. [Google Scholar]
- Anli, R.E.; Vural, N. Antioxidant Phenolic Substances of Turkish Red Wines from Different Wine Regions. Molecules 2009, 14, 289–297. [Google Scholar] [CrossRef]
- Kosiorek, A.; Oszmiański, J.; Golański, J. Podstawy Do Zastosowania Polifenoli Roślinnych Jako Nutraceutyków o Właściwościach Przeciwpyłkowych. Postępy Fitoter. 2013, 2, 108–117. [Google Scholar]
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a Versatile Component in Tissue Engineering. Acta Biomater. 2021, 119, 57–74. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as Antiobesity Agents: A Review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, Metabolism and Bioavailability of Flavonoids: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Drinić, Z.; Pljevljakušić, D.; Janković, T.; Zdunić, G.; Bigović, D.; Šavikin, K. Hydro-Distillation and Microwave-Assisted Distillation of Sideritis raeseri: Comparison of the Composition of the Essential Oil, Hydrolat and Residual Water Extract. Sustain. Chem. Pharm. 2021, 24, 100538. [Google Scholar] [CrossRef]
- Gabrieli, C.N.; Kefalas, P.G.; Kokkalou, E.L. Antioxidant Activity of Flavonoids from Sideritis raeseri. J. Ethnopharmacol. 2005, 96, 423–428. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Di Fabio, G.; D’Alonzo, D.; Guaragna, A.; Scapagnini, G.; Zarrelli, A. Traditional Uses, Chemical Composition and Biological Activities of Sideritis raeseri Boiss. & Heldr. J. Sci. Food Agric. 2017, 97, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Alanazi, A.; Aldhafeeri, K.A.; Alamer, O.; Alshaaer, M. Brushite: Synthesis, Properties, and Biomedical Applications. In Crystallization and Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Slota, D.; Gląb, M.; Tyliszczak, B.; Dogulas, T.E.L.; Rudnicka, K.; Miernik, K.; Urbaniak, M.M.; Rusek-Wala, P.; Sobczak-upiec, A. Composites Based on Hydroxyapatite and Whey Protein Isolate for Applications in Bone Regeneration. Materials 2021, 14, 2317. [Google Scholar] [CrossRef] [PubMed]
- Słota, D.; Florkiewicz, W.; Piętak, K.; Pluta, K.; Sadlik, J.; Miernik, K.; Sobczak-Kupiec, A. Preparation of PVP and Betaine Biomaterials Enriched with Hydroxyapatite and Its Evaluation as a Drug Carrier for Controlled Release of Clindamycin. Ceram. Int. 2022, 48, 35467–35473. [Google Scholar] [CrossRef]
- Słota, D.; Florkiewicz, W.; Piętak, K.; Szwed, A.; Włodarczyk, M.; Siwińska, M.; Rudnicka, K.; Sobczak-Kupiec, A. Preparation, Characterization, and Biocompatibility Assessment of Polymer-Ceramic Composites Loaded with Salvia Officinalis Extract. Materials 2021, 14, 6000. [Google Scholar] [CrossRef] [PubMed]
- Gilewska, G. Przydatność Różnych Technik Obrazowania Struktur Biologicznych Wykorzystujących Elektronowy Mikroskop Skaningowy. Pr. Inst. Elektrotechniki 2010, 244, 161–169. [Google Scholar]
- Moseke, C.; Bayer, C.; Vorndran, E.; Barralet, J.E.; Groll, J.; Gbureck, U. Low Temperature Fabrication of Spherical Brushite Granules by Cement Paste Emulsion. J. Mater. Sci. Mater. Med. 2012, 23, 2631–2637. [Google Scholar] [CrossRef] [PubMed]
- Binitha, M.P.; Pradyumnan, P.P. Dielectric Property Studies of Biologically Compatible Brushite Single Crystals Used as Bone Graft Substitute. J. Biomater. Nanobiotechnol. 2013, 04, 119–122. [Google Scholar] [CrossRef]
- Joseph, K.C.; Jethva, H.O.; Jogiya, B.; Chauhan, C.K.; Vaidya, A.D.B.; Joshi, M.J. In Vitro Growth Inhibition Study of Urinary Type Brushite Crystals in the Presence of Healthy Adult Urine and Tartaric Acid. IOSR J. Pharm. Biol. Sci. 2017, 12, 47–53. [Google Scholar]
- Sadlik, J.; Kosińska, E.; Słota, D.; Niziołek, K.; Tomala, A.; Włodarczyk, M.; Piątek, P.; Skibiński, J.; Jampilek, J.; Sobczak-Kupiec, A. Bioactive Hydrogel Based on Collagen and Hyaluronic Acid Enriched with Freeze-Dried Sheep Placenta for Wound Healing Support. Int. J. Mol. Sci. 2024, 25, 1687. [Google Scholar] [CrossRef]
- Trpkovska, M.; Šoptrajanov, B.; Malkov, P. FTIR Reinvestigation of the Spectra of Synthetic Brushite and Its Partially Deuterated Analogues. J. Mol. Struct. 1999, 480–481, 661–666. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wyeth, P. Using FTIR Spectroscopy to Detect Sericin on Historic Silk. Sci. China Chem. 2010, 53, 626–631. [Google Scholar] [CrossRef]
- Koperska, M.A.; Pawcenis, D.; Bagniuk, J.; Zaitz, M.M.; Missori, M.; Łojewski, T.; Łojewska, J. Degradation Markers of Fibroin in Silk through Infrared Spectroscopy. Polym. Degrad. Stab. 2014, 105, 185–196. [Google Scholar] [CrossRef]
- Durmus, Z.; Kavas, H.; Baykal, A.; Sozeri, H.; Alpsoy, L.; Elik, S.Ü.; Toprak, M.S. Synthesis and Characterization of L-Carnosine Coated Iron Oxide Nanoparticles. J. Alloys Compd. 2011, 509, 2555–2561. [Google Scholar] [CrossRef]
- Farid, R.M.; Gaafar, P.M.E.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Chemotherapeutic Potential of L-Carnosine from Stimuli-Responsive Magnetic Nanoparticles against Breast Cancer Model. Nanomedicine 2020, 15, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Saidi, M.; Dabbaghi, A.; Rahmani, S. Swelling and Drug Delivery Kinetics of Click-Synthesized Hydrogels Based on Various Combinations of PEG and Star-Shaped PCL: Influence of Network Parameters on Swelling and Release Behavior. Polym. Bull. 2020, 77, 3989–4010. [Google Scholar] [CrossRef]
- Dominguez-López, I.; Pérez, M.; Lamuela-Raventós, R.M. Total (Poly)Phenol Analysis by the Folin-Ciocalteu Assay as an Anti-Inflammatory Biomarker in Biological Samples. Crit. Rev. Food Sci. Nutr. 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.; Polat Köse, L.; Eşsiz, S.; Gülçin, İ. Synthesis and Biological Evaluation of Some 1-Naphthol Derivatives as Antioxidants, Acetylcholinesterase, and Carbonic Anhydrase Inhibitors. Arch. Pharm. 2021, 354, 2100113. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Yanchev, N.; Petkova, N.; Ivanov, I.; Delev, D. Total Polyphenolic Content and Antioxidant Activity of Different Extracts from Sideritis Scardica. Trop. J. Nat. Prod. Res. 2022, 6, 528–533. [Google Scholar] [CrossRef]
- Koleva, I.I.; Linssen, J.P.H.; Van Beek, T.A.; Evstatieva, L.N.; Kortenska, V.; Handjieva, N. Antioxidant Activity Screening of Extracts from Sideritis Species (Labiatae) Grown in Bulgaria. J. Sci. Food Agric. 2003, 83, 809–819. [Google Scholar] [CrossRef]
| Sample | 15% PEG in S. raeseri Extract [mL] | L-Carnosine 2% [mL] | Fibroin 2% [mL] | Sericin 2% [mL] | Brushite [g] |
|---|---|---|---|---|---|
| c-C | 5 | 5 | - | - | 0.175 |
| c-F | - | 5 | - | ||
| c-S | - | - | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niziołek, K.; Słota, D.; Sadlik, J.; Kosińska, E.; Korzeń, K.; Jampilek, J.; Sobczak-Kupiec, A. Sideritis raeseri—Modified Coatings on Ti-6Al-4V as a Carrier for Controlled Delivery Systems of Active Substances. Materials 2024, 17, 2250. https://doi.org/10.3390/ma17102250
Niziołek K, Słota D, Sadlik J, Kosińska E, Korzeń K, Jampilek J, Sobczak-Kupiec A. Sideritis raeseri—Modified Coatings on Ti-6Al-4V as a Carrier for Controlled Delivery Systems of Active Substances. Materials. 2024; 17(10):2250. https://doi.org/10.3390/ma17102250
Chicago/Turabian StyleNiziołek, Karina, Dagmara Słota, Julia Sadlik, Edyta Kosińska, Klaudia Korzeń, Josef Jampilek, and Agnieszka Sobczak-Kupiec. 2024. "Sideritis raeseri—Modified Coatings on Ti-6Al-4V as a Carrier for Controlled Delivery Systems of Active Substances" Materials 17, no. 10: 2250. https://doi.org/10.3390/ma17102250






