Effect of Mineral Admixtures on Physical, Mechanical, and Microstructural Properties of Flue Gas Desulfurization Gypsum-Based Self-Leveling Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
- (1)
- Proportion of desulfurization gypsum-based self-leveling mortar
- (2)
- Single addition experiment of mineral admixtures
- (3)
- Figure 2 depicts the preparation process of GSLM. Initially, desulfurization building gypsum, cement, mineral admixtures, and admixtures were proportionally weighed. Mineral admixtures and admixtures were added at different percentages of gypsum content, and all measured powders were introduced into the mixer for thorough blending. Proportionally weighed water was poured into the mixing pot, and the uniformly mixed powder was added to the water within a 5-second timeframe. Subsequently, the slurry was mixed for 1 min before being poured into the cement sand mold for molding.
2.3. Material Characterizations
3. Results and Discussion
3.1. Determination of the Basic Mix Ratio of Flue Gas Desulfurization Gypsum-Based Self-Leveling Mortar
3.2. Effect of Mineral Admixtures on Physical and Mechanical Properties of Flue Gas Desulfurization Gypsum-Based Self-Leveling Mortar
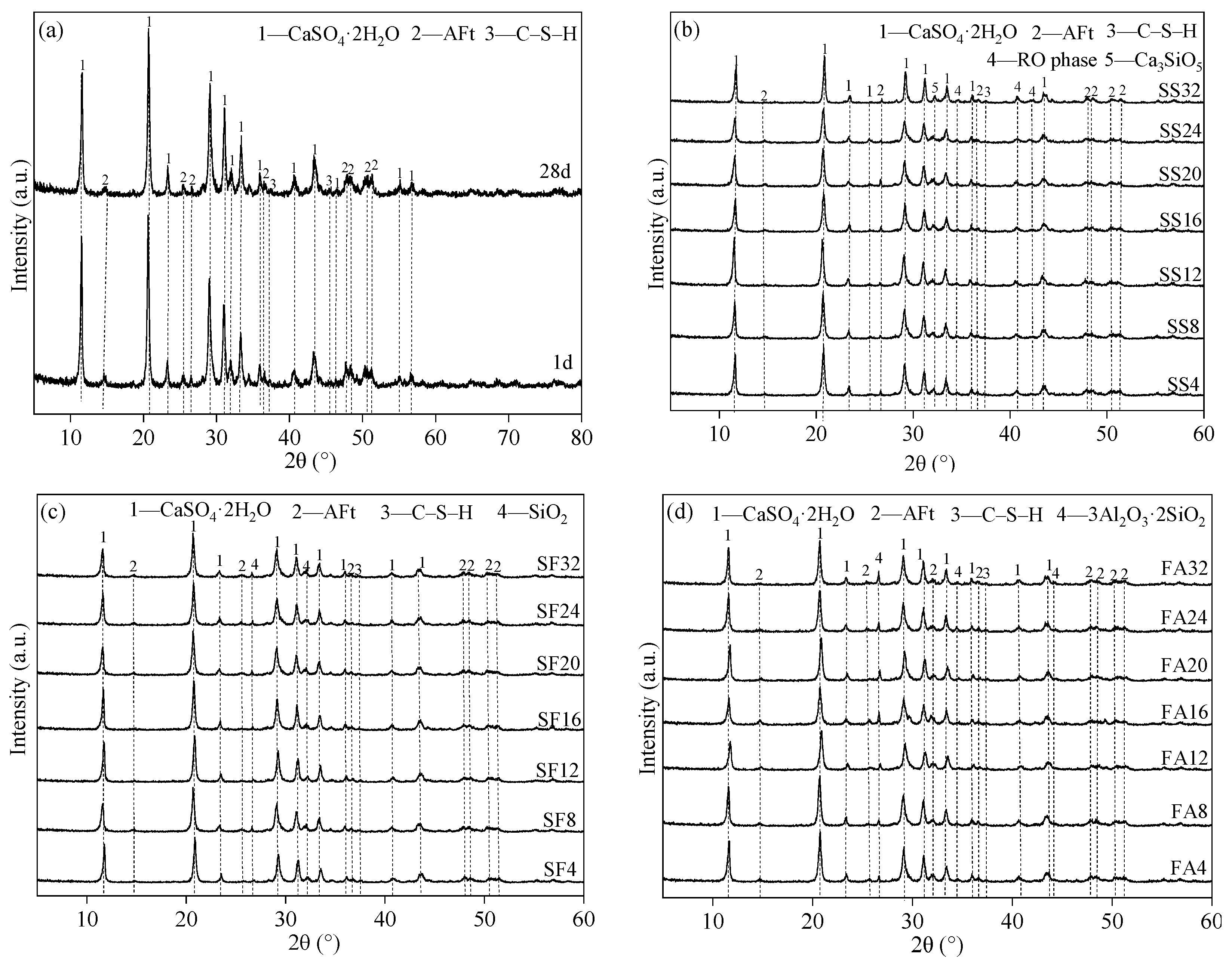
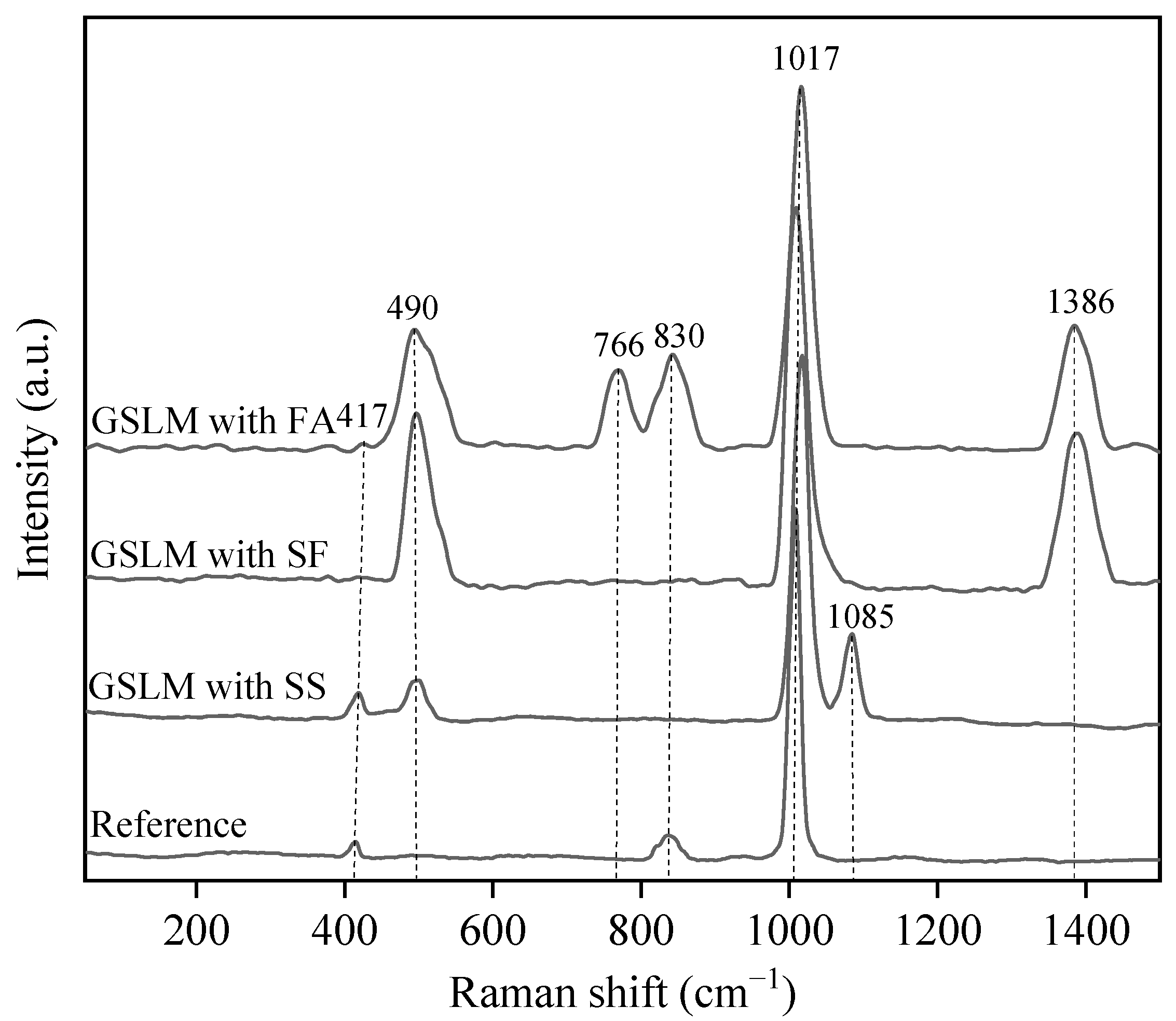
3.3. Effect of Mineral Admixtures on Microstructural Properties of Flue Gas Desulfurization Gypsum-Based Self-Leveling Mortar
4. Conclusions
- (1)
- The GSLM with SF exhibited the lowest initial fluidity, attributed to the finer particles, larger specific surface area, and higher water absorption capacity of SF particles. However, the smooth spherical nature of SF, with a glass bead effect, had minimal impact on the 30 min fluidity of GSLM.
- (2)
- The strength of GSLM demonstrated an initial increase followed by a decrease with rising SS, SF, and FA content. The mineral admixtures effectively filled internal pores in GSLM, but excessive content could compromise the structure between hydration products. Consequently, the optimal content for SS, SF, and FA in GSLMs is 16%, 20%, and 20%, respectively.
- (3)
- The hydration products of GSLMs with SS, SF, and FA mainly comprised CaSO4·2H2O, C–S–H gel and AFt. A small amount of SS exhibited continued hydration due to the stimulation of calcium hydroxide. The spherical nature of SF and FA, combined with a weak interface with hydration products, facilitated crack propagation from the interface under stress.
- (4)
- In the future, the effects of three-phase composition, particle size of desulfurization building gypsum, and multiple mineral admixtures on the performance of gypsum-based self-leveling mortar should be researched.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aakriti; Maiti, S.; Jain, N.; Malik, J. A comprehensive review of flue gas desulphurized gypsum: Production, properties, and applications. Constr. Build. Mater. 2023, 393, 131918. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and resource utilization of flue gas desulfurized gypsum in China—A review. Environ. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Liu, Y.; Dou, Z.; Zhang, T.-A. Summary of research progress on industrial flue gas desulfurization technology. Sep. Purif. Technol. 2022, 281, 119849. [Google Scholar] [CrossRef]
- Rivero, A.J.; Sathre, R.; Navarro, J.G. Life cycle energy and material flow implications of gypsum plasterboard recycling in the European Union, Resources. Conserv. Recycl. 2016, 108, 171–181. [Google Scholar] [CrossRef]
- Wang, T.; Wu, K.; Wu, M. Development of green binder systems based on flue gas desulfurization gypsum and fly ash incorporating slag or steel slag powders. Constr. Build. Mater. 2020, 265, 120275. [Google Scholar] [CrossRef]
- Geraldo, R.H.; Costa, A.R.; Kanai, J.; Silva, J.S.; Souza, J.D.; Andrade, H.M.; Goncalves, J.P.; Fontanini, P.S.; Camarini, G. Calcination parameters on phosphogypsum waste recycling. Constr. Build. Mater. 2020, 256, 119406. [Google Scholar] [CrossRef]
- Jian, S.; Yang, X.; Gao, W.; Li, B.; Gao, X.; Huang, W.; Tan, H.; Lei, Y. Study on performance and function mechanisms of whisker modified flue gas desulfurization (FGD) gypsum. Constr. Build. Mater. 2021, 301, 124341. [Google Scholar] [CrossRef]
- Lei, D.Y.; Guo, L.P.; Sun, W.; Liu, J.P.; Miao, C.W. Study on properties of untreated FGD gypsum-based high-strength building materials. Constr. Build. Mater. 2017, 153, 765–773. [Google Scholar] [CrossRef]
- Yang, L.; Jing, M.; Lu, L.; Zhu, X.; Zhao, P.; Chen, M.; Li, L.; Liu, J. Effects of modified materials prepared from wastes on the performance of flue gas desulfurization gypsum-based composite wall materials. Constr. Build. Mater. 2020, 257, 119519. [Google Scholar] [CrossRef]
- Xu, L.; Wu, K.; Li, N.; Zhou, X.; Wang, P. Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement. J. Clean. Prod. 2017, 161, 803–811. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of steel slag and gypsum for building materials and associated reaction mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]
- Bartley, P.C.; Erbrick, L.B.; Knotts, M.J.; Watts, D.B.; Torbert, H.A. Influence of Flue Gas Desulfurization Gypsum on Phosphorous Loss in Pine Bark Substrates. Agriculture 2023, 13, 283. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Liao, Q.; Wu, J.; Li, J.; Zhang, L. Effect of superabsorbent polymers on the drying and autogenous shrinkage properties of self-leveling mortar. Constr. Build. Mater. 2019, 201, 401–407. [Google Scholar] [CrossRef]
- Canbaz, M.; Topçu, İ.B.; Ateşin, Ö. Effect of admixture ratio and aggregate type on self-leveling screed properties. Constr. Build. Mater. 2016, 116, 32l–325. [Google Scholar] [CrossRef]
- Anjos, M.A.; Araujo, T.R.; Ferreira, R.L.; Farias, E.C.; Martinelli, A.E. Properties of self—leveling mortars incorporating a high-volume of sugar cane bagasse ash as partial Portland cement replacement. J. Build. Eng. 2020, 32, 101694. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Peng, J.; Cao, W.; Liu, J. Physical and mechanical properties of gypsum-based composites reinforced with PVA and PP fibers. Constr. Build. Mater. 2018, 163, 695–705. [Google Scholar] [CrossRef]
- Kondratieva, N.; Barre, M.; Goutenoire, F.; Sanytsky, M. Study of modified gypsum binder. Constr. Build. Mater. 2017, 149, 535–542. [Google Scholar] [CrossRef]
- Wu, H.C.; Xia, Y.M.; Hu, X.Y.; Liu, X. Improvement on mechanical strength and water absorption of gypsum modeling material with synthetic polymers. Ceram. Int. 2014, 40, 14899–14906. [Google Scholar] [CrossRef]
- Nguyen, H.; Adesanya, E.; Ohenoja, K.; Kriskova, L.; Pontikes, Y.; Kinnunen, P.; Illikainen, M. Byproduct-based ettringite binder–a synergy between ladle slag and gypsum. Constr. Build. Mater. 2019, 197, 143–151. [Google Scholar] [CrossRef]
- Liu, C.B.; Gao, J.M.; Tang, Y.B.; Chen, X. Preparation and characterization of gypsum–based materials used for 3D robocasting. J. Mater. Sci. 2018, 53, 16415–16422. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Liu, J.; Miao, Y.; Duan, S. Study on application of gypsum-based self-leveling mortar in floor heating backfill. Mater. Sci. Eng. 2020, 780, 042010. [Google Scholar] [CrossRef]
- Qi, H.; Ma, B.; Tan, H.; Su, Y.; Lu, W.; Jin, Z. Influence of fluoride ion on the performance of PCE in hemihydrate gypsum pastes. J. Build. Eng. 2022, 46, 103582. [Google Scholar] [CrossRef]
- Jin, Z.; Ma, B.; Su, Y.; Lu, W.; Qi, H.; Hu, P. Effect of calcium sulphoaluminate cement on mechanical strength and waterproof properties of beta-hemihydrate phosphogypsum. Constr. Build. Mater. 2020, 242, 118198. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Q.; Luo, T. Reuse of phosphogypsum as hemihydrate gypsum: The negative effect and content control of H3PO4. Resources. Conserv. Recycl. 2021, 174, 105830. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Yan, Y. Utilization of original phosphogypsum as raw material for the preparation of self-leveling mortar. J. Clean. Prod. 2016, 127, 204e213. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Memon, S.A.; Dong, Z.; Li, D.; Cui, H. Effect of calcium sulfate type and dosage on properties of calcium aluminate cement-based self-leveling mortar. Constr. Build. Mater. 2018, 167, 253–262. [Google Scholar] [CrossRef]
- Xiao, X.; Li, J.; Meng, Q.; Hou, X.; Liu, Y.; Wang, X.; Wang, W.; Lu, S.; Li, Y.; Mao, Y.; et al. Reuse of by-product gypsum with solid wastes-derived sulfoaluminate cement modification for the preparation of self-leveling mortar and influence mechanism of H3PO4. Constr. Build. Mater. 2024, 411, 134298. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Liu, J.; Miao, Y. Study on the Properties of Gypsum-based Self-leveling Mortar using Molybdenum Tailings. Earth Environ. Sci. 2019, 330, 042002. [Google Scholar] [CrossRef]
- Silva, D.B.P.; Lima, N.B.; Estolano, A.; Nascimento, H.; Vilemen, P.; Padron-Hernández, E.; Carneiro, A.; Lima, N.; Povoas, Y. Producing a gypsum-based self-leveling mortar for subfloor modified by polycarboxylate admixture (PCE). Constr. Build. Mater. 2023, 364, 130007. [Google Scholar] [CrossRef]
- Tan, H.; Deng, X.; Gu, B.; Ma, B.; Luo, S.; Zhi, Z.; Guo, Y.; Zou, F. Effect of borax and sodium tripolyphosphate on fluidity of gypsum paste plasticized by polycarboxylate superplasticizer. Constr. Build. Mater. 2018, 176, 394–402. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Hou, Y.; Wu, Z. Optimization of mechanical properties and water absorption behavior of building gypsum by ternary matrix mixture. Constr. Build. Mater. 2022, 350, 128910. [Google Scholar] [CrossRef]
- Gou, M.; Zhao, M.; Zhou, L.; Zhao, J.; Hou, W.; Ma, W.; Hou, Z. Hydration and mechanical properties of FGD gypsum-cement-mineral powder composites. J. Build. Eng. 2023, 69, 106288. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Kong, D.; Peng, D.; Zheng, T. Research on optimizing performance of desulfurization-gypsum-based composite cementitious materials based on response surface method. Constr. Build. Mater. 2022, 341, 127874. [Google Scholar] [CrossRef]
- GB/T 20491-2017; Steel Slag Powder Used for Cement and Concrete. China Standards Publishing House: Beijing, China, 2017.
- GB/T 27690-2023; Silica Fume for Cement Mortar and Concrete. China Standards Publishing House: Beijing, China, 2023.
- GB/T 1596-2017; Fly Ash Used for Cement and Concrete. China Standards Publishing House: Beijing, China, 2017.
- JC/T 1023-2021; Gypsum Based Self-Leveling Compound for Floor. China Standards Publishing House: Beijing, China, 2021.
- Wu, Q.; Ma, H.; Chen, Q.; Huang, Z.; Zhang, C.; Yang, T. Preparation of waterproof block by silicate clinker modified FGD gypsum. Constr. Build. Mater. 2019, 214, 318–325. [Google Scholar] [CrossRef]
- Wan, Y.; Hui, X.; He, X.; Li, J.; Xue, J.; Feng, D.; Liu, X.; Wang, S. Performance of green binder developed from flue gas desulfurization gypsum incorporating Portland cement and large-volume fly ash. Constr. Build. Mater. 2022, 348, 128679. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Lesovik, V.; Chernysheva, N.; Fediuk, R.; Amran, M.; Murali, G.; de Azevedo, A.R.G. Optimization of fresh properties and durability of the green gypsum-cement paste. Constr. Build. Mater. 2021, 287, 123035. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, R. A novel gypsum-based self-leveling mortar produced by phosphorus building gypsum. Constr. Build. Mater. 2019, 226, 11–20. [Google Scholar] [CrossRef]
- Chen, M.X.; Li, L.B.; Zheng, Y.; Zhao, P.; Lu, L.; Cheng, X. Rheological and mechanical properties of admixtures modified 3D printing sulphoaluminate cementitious materials. Constr. Build. Mater. 2018, 189, 601–611. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, W.; Li, Y.; Ba, H.; Li, N.; Ju, Y.; Zhao, B.; Jia, G.; Hu, W. The mechanism of hydration reaction of granulated blast furnace slag-steel slag-refining slag-desulfurization gypsum-based clinker-free cementitious materials. J. Build. Eng. 2021, 44, 103289. [Google Scholar] [CrossRef]
- Lu, T.H.; Cchen, Y.L.; Shih, P.H.; Chang, J.E. Use of basic oxygen furnace slag finnes in the production of cementitious mortars and the effects on mortar expansion. Constr. Build. Mater. 2018, 167, 768–774. [Google Scholar] [CrossRef]
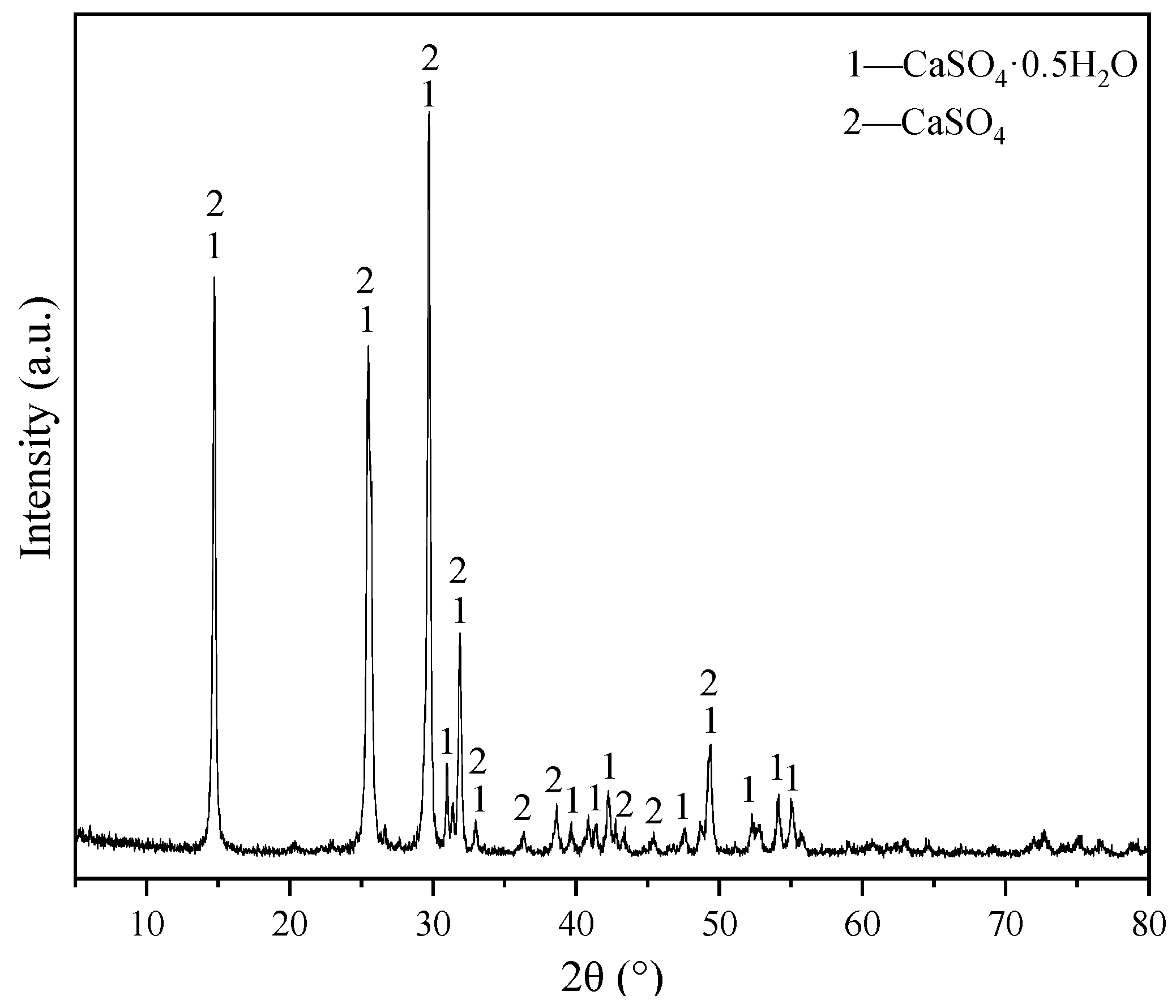

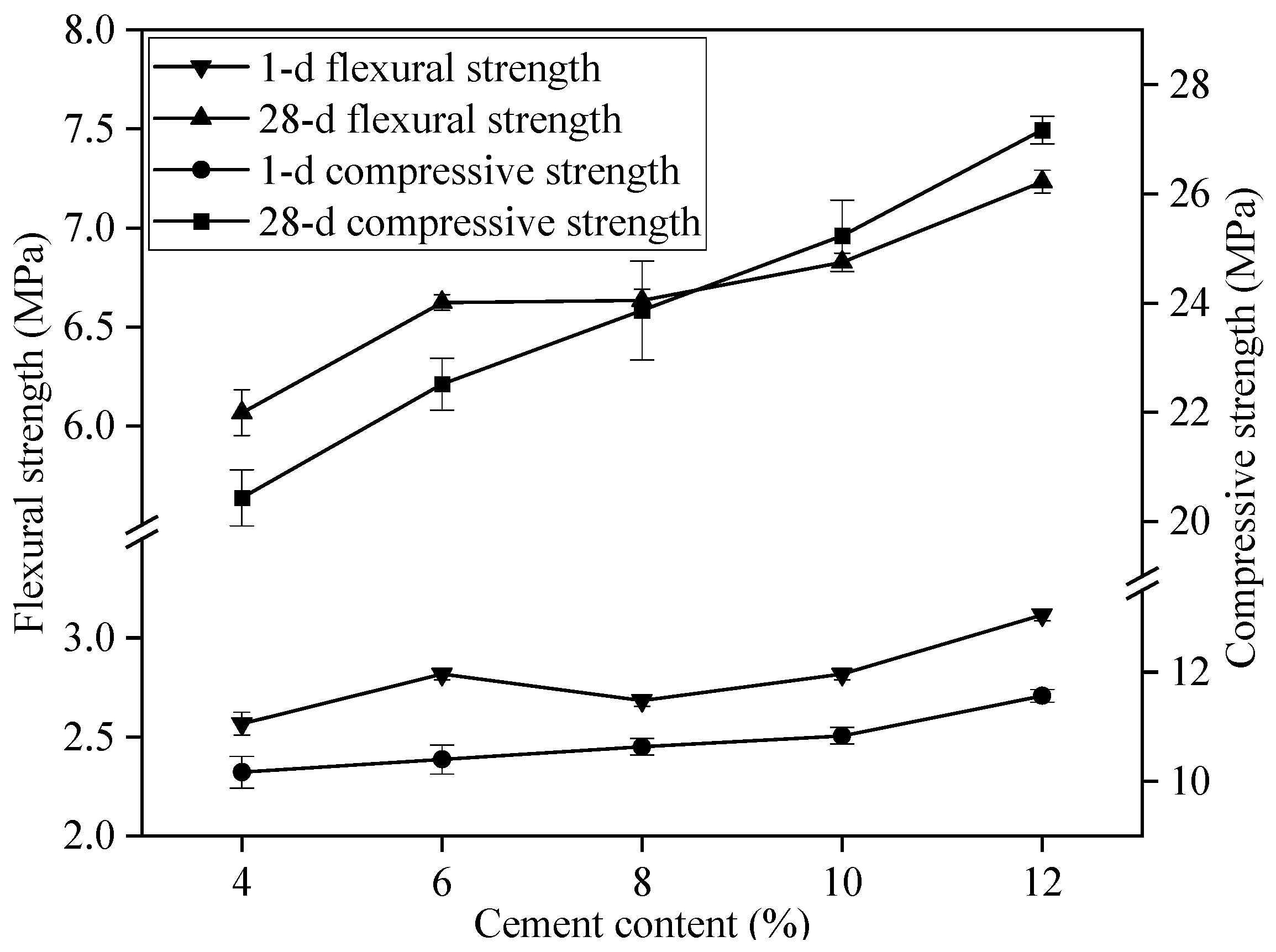


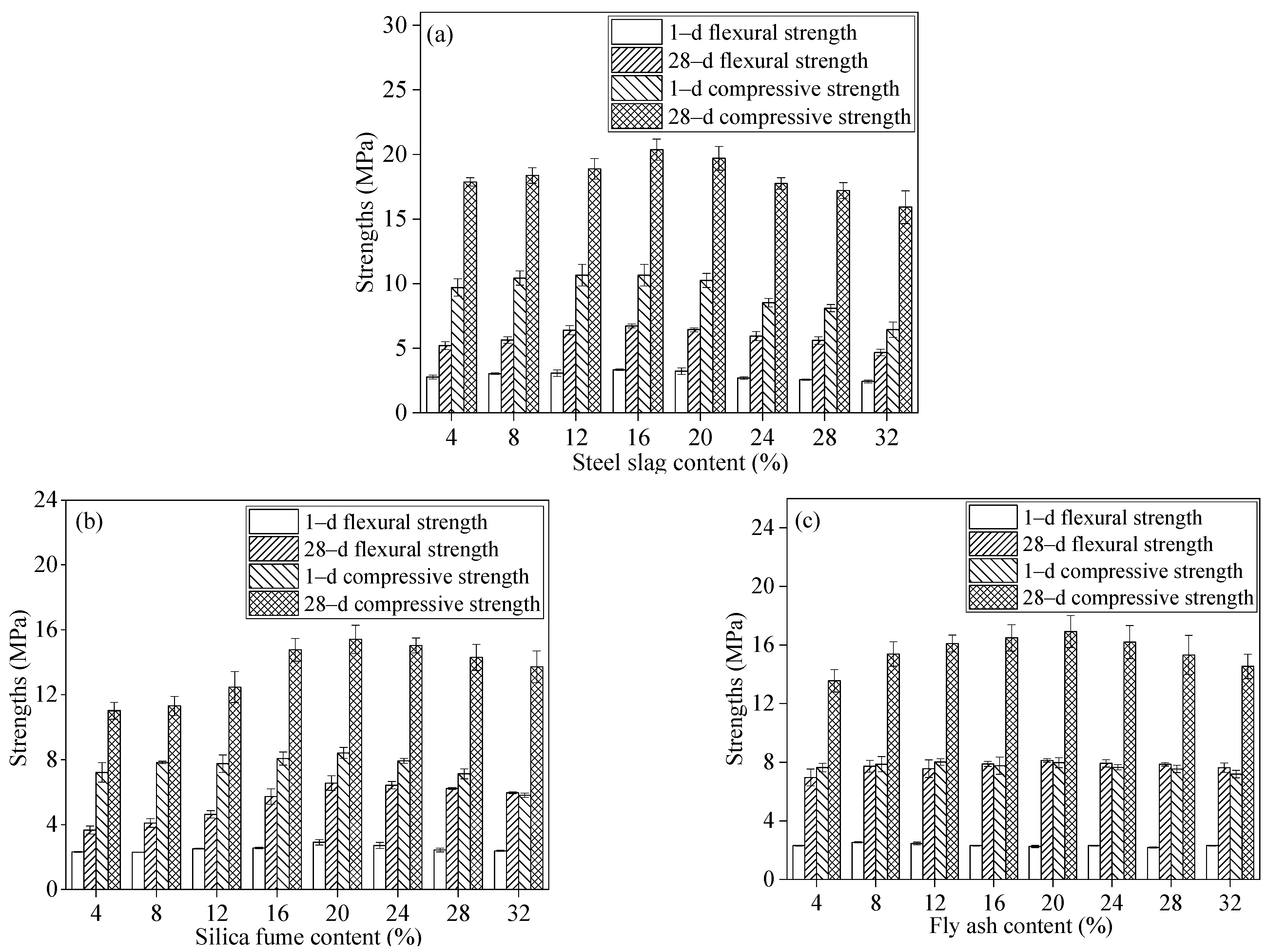
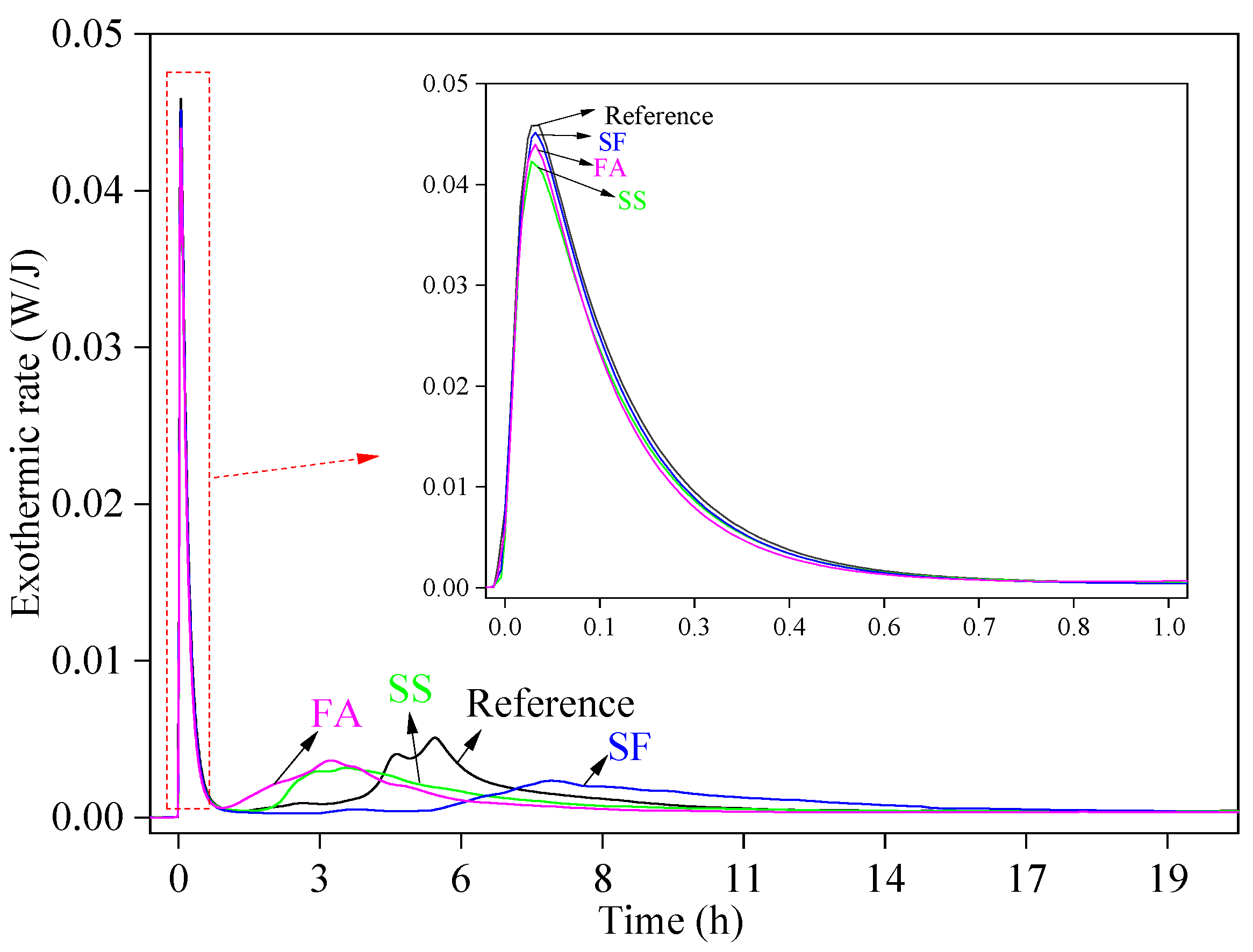

| Project | Numerical Value |
|---|---|
| Anhydrite (%) | 15.98 |
| Hemihydrate gypsum (%) | 84.02 |
| Dihydrate gypsum (%) | 0.00 |
| Setting time (min) | 3 |
| 2-h compressive strength (MPa) | 4.06 |
| 2-h flexural strength (MPa) | 2.26 |
| Requirement of normal consistency (g/100 g) | 85 |
| Compositions | Na2O | MgO | Al2O3 | SiO2 | SO3 | CaO | TiO2 | K2O | MnO | Fe2O3 | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gypsum | - | 0.81 | 1.07 | 1.97 | 54.10 | 40.60 | - | 0.20 | - | 0.44 | 0.81 |
| Cement (OPC) | - | 0.64 | 4.21 | 18.59 | 4.22 | 65.95 | 0.23 | - | - | 2.95 | 3.21 |
| Steel slag | 0.27 | 7.78 | 4.39 | 15.30 | 0.38 | 39.20 | 1.39 | - | 4.93 | 22.70 | 3.66 |
| Silica fume | - | 1.34 | 0.12 | 45.43 | 0.05 | 52.63 | - | 0.03 | 0.02 | 0.22 | 0.16 |
| Fly ash | 1.07 | 1.19 | 24.30 | 50.60 | 1.52 | 8.50 | 1.27 | 2.16 | - | 8.60 | 0.79 |
| Project | Numerical Value | Requirements in JC/T 1023-2021 |
|---|---|---|
| Desulfurization building gypsum (%) | 94 | - |
| Portland cement (%) | 6 | - |
| Polycarboxylate superplasticizer (%) | 0.638 | - |
| Polymeric amino acid retarder (%) | 0.638 | - |
| Defoamer (%) | 0.085 | - |
| HPMC (%) | 0.085 | - |
| Water-to-cement ratio | 0.54 | - |
| Initial fluidity (mm) | 150 | - |
| 30 min fluidity (mm) | 145 | ≥140 |
| 24-h flexural strength (MPa) strength/MPa | 2.8 | ≥2.0 |
| 24-h compressive strength (MPa) | 10.5 | ≥5.0 |
| 28-day flexural strength (MPa) strength/MPa | 6.6 | ≥6.0 |
| 28-day compressive strength (MPa) | 22.5 | ≥20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, Y.; Zhao, W.; Chen, C. Effect of Mineral Admixtures on Physical, Mechanical, and Microstructural Properties of Flue Gas Desulfurization Gypsum-Based Self-Leveling Mortar. Materials 2024, 17, 2227. https://doi.org/10.3390/ma17102227
Wang S, Chen Y, Zhao W, Chen C. Effect of Mineral Admixtures on Physical, Mechanical, and Microstructural Properties of Flue Gas Desulfurization Gypsum-Based Self-Leveling Mortar. Materials. 2024; 17(10):2227. https://doi.org/10.3390/ma17102227
Chicago/Turabian StyleWang, Shiyu, Yanxin Chen, Wei Zhao, and Chang Chen. 2024. "Effect of Mineral Admixtures on Physical, Mechanical, and Microstructural Properties of Flue Gas Desulfurization Gypsum-Based Self-Leveling Mortar" Materials 17, no. 10: 2227. https://doi.org/10.3390/ma17102227






