Role of Woody Biomass Ash Material in Immobilization of Cadmium, Lead and Zinc in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Description
2.2. Soil Properties
2.3. Biomass Ash (BA) Properties
2.4. List of Chemical Analyses
2.4.1. Biomass Ash (BA)
2.4.2. Soil
2.5. Methodology of Chemical Analyses
2.6. Statistical Analysis
3. Results
3.1. Contents of the Total Forms of Heavy Metals
3.2. Contents of the Available Forms of Heavy Metals
3.3. Correlations—Total Forms vs. Available Forms of Heavy Metals
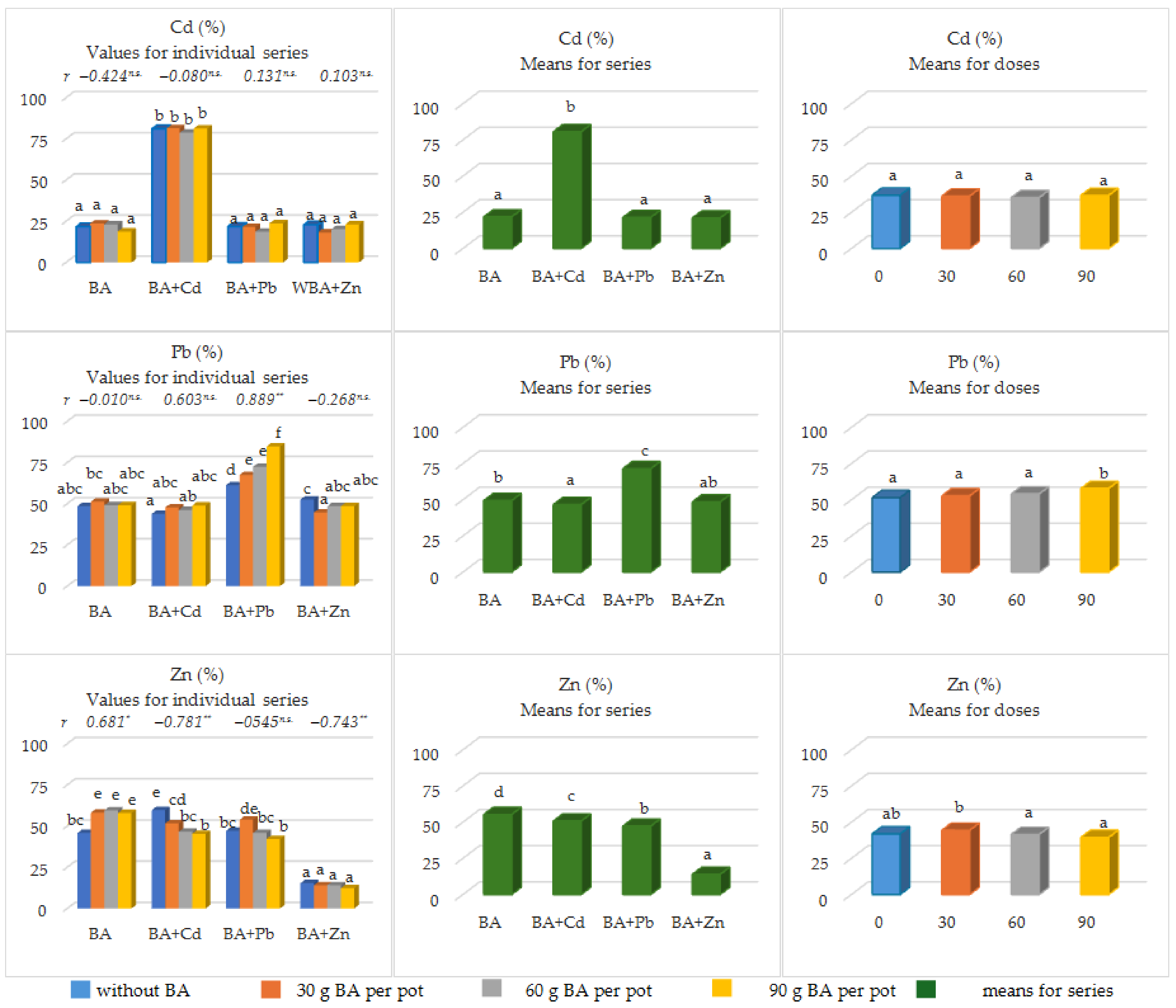
3.4. The Share of the Content of Available Forms of Heavy Metals in Their Total Forms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pazalja, M.; Salihović, M.; Sulejmanović, J.; Smajović, A.; Begić, S.; Špirtović-Halilović, S.; Sher, F. Heavy metals content in ashes of wood pellets and the health risk assessment related to their presence in the environment. Sci. Rep. 2021, 11, 17952. [Google Scholar] [CrossRef] [PubMed]
- Diatta, J.; Kowalski, M. Ashes from Combustion of Plant Biomass (Phytoashes)—Recyckling and Agro-Chemical Potential. In Proceedings of the Materials of XXIV International Conference on Ashes from the Energy Industry, Zakopane, Poland, 26–28 September 2017; pp. 1–15. Available online: http://unia-ups.pl/wp-content/uploads/2016/03/Diatta.pdf (accessed on 10 February 2024).
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of ashes from biomass combustion. Energies 2022, 15, 9653. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Hassan, M.; Liaquat, R.; Dawood, U.F. Challenges and opportunities in biomass ash management and its utilization in novel applications. Renew. Sustain. Energy Rev. 2021, 150, 111451. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Szczepaniak, M. Influence of biomass incineration temperature on the content of selected heavy metals in the ash used for fertilizing purposes. Appl. Sci. 2019, 9, 1790. [Google Scholar] [CrossRef]
- Smołka-Danielowska, D.; Jabłońska, M. Chemical and mineral composition of ashes from wood biomass combustion in domestic wood-fired furnaces. Int. J. Environ. Sci. Technol. 2022, 19, 5359–5372. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass—A brief review. J. Hazard. Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Rolka, E.; Żołnowski, A.C.; Wyszkowski, M.; Zych, W.; Skorwider-Namiotko, A. Wood Biomass ash (WBA) from the heat production process as a mineral amendment for improving selected soil properties. Energies 2023, 16, 5110. [Google Scholar] [CrossRef]
- Terelak, H.; Piotrowska, M.; Motowicka-Terelak, T.; Stuczyński, T.; Budzyńska, K. The Content of Heavy Metals and Sulphur in Soils of Agricultural Land of Poland and the Degree of Their Pollution with These Elements. Probl. Notebook. Progress Agric. Sci. 1995, 418, 45–60. Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-article-70433682-9b9d-21.463f-8d0c-171dd982101d (accessed on 12 February 2024).
- Ahmed, N.; Shah, A.R.; Danish, S.; Fahad, S.; Ali, M.A.; Zarei, T.; Vranová, V.; Datta, R. Immobilization of Cd in soil by biochar and new emerging chemically produced carbon. J. King Saud Univ. Agric. Sci. 2021, 33, 101472. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Wang, X.; Hussain, B.; Yaseen, M.; Aziz, M.Z.; Yang, X. Immobilization of cadmium and lead in contaminated paddy field using inorganic and organic additives. Sci. Rep. 2018, 8, 17839. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska, J.; Sienkiewicz, S.; Żarczyński, P.; Krzebietke, S. Environmental application of ash from incinerated biomass. Agronomy 2020, 10, 482. [Google Scholar] [CrossRef]
- Ministry of the Environment. Regulation of the Minister of the Environment of September 1, 2016 on the Method of Assessing Pollution of the Earth’s Surface. J. Laws Repub. Policy 2016, 1395, 1–86. [Google Scholar]
- Terelak, H.; Piotrowska, M.; Motowicka-Terelak, T.; Stuczyński, T.; Budzyńska, K.; Pietruch, C.; Sroczyński, W. Chemical properties of soils and the content of heavy metals and sulfur in soils and plants. In Expertise Prepared for the Ministry of Agriculture and Food; IUNG Publishing House: Puławy, Poland, 1994; pp. 1–96. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; World Soil Resources Report. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources; Reports No. 106; FAO: Rome, Italy, 2015; p. 192. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 18 November 2023).
- MPEC Olsztyn. Available online: https://sway.cloud.microsoft/MCrZLaAJFsDYw0bg?ref=email (accessed on 15 March 2024).
- Ministry of Climate. Regulation of the Minister of Climate of 3 January 2020 on the waste catalog. J. Laws Repub. Policy 2020, 10, 1–48. [Google Scholar]
- Karczewska, A.; Kabała, C. Methodology of Laboratory Analyzes of Soils and Plants; University of Life Sciences: Wrocław, Poland, 2008. [Google Scholar]
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plants Properties, 1st ed.; Institute of Environmental Protection: Warsaw, Poland, 1991. [Google Scholar]
- US Environmental Protection Agency. Method 3051. Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; US Environmental Protection Agency: Washington, DC, USA, 2007; Available online: https://settek.com/documents/EPA-Methods/PDF/EPA-Method-3051.pdf (accessed on 10 February 2023).
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber die chemische bodenanalyse als grundlage fur die beurteilung des nahrstoffzustandes der boden. II. Chemische extraktionsmethoden zur phosphorund kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- CEM Corporation. CEM Mars 6 Operation Manual; CEM Corporation: Matthews, NC, USA, 2017. [Google Scholar]
- Rolka, E.; Wyszkowski, M. Availability of trace elements in soil with simulated cadmium, lead and zinc pollution. Minerals 2021, 11, 879. [Google Scholar] [CrossRef]
- Burdzy, J. Statistical Tables; Lodz University of Technology Publishing House: Lublin, Poland, 1999. [Google Scholar]
- Microsoft. MS Excel® for Microsoft 365 MSO; Microsoft Corporation: Albuquerque, NM, USA, 2021. [Google Scholar]
- Tibco. Statistica Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA, 2021. [Google Scholar]
- Szostek, M.; Szpunar-Krok, E.; Ilek, A. Chemical speciation of trace elements in soil fertilized with biomass combustion ash and their accumulation in winter oilseed rape plants. Agronomy 2023, 13, 942. [Google Scholar] [CrossRef]
- Wang, W.; Song, W.; Zhou, T.; Wang, Z.; Christie, P.; Wu, L. Soil Metal Immobilization in Agricultural Land Contaminated with Cadmium and Lead: A Case Study of Effectiveness Evaluation in Lanping, Southwest China. Bull. Environ. Contam. Toxicol. 2021, 107, 1227–1235. Available online: https://link.springer.com/article/10.1007/s00128-021-03267-8 (accessed on 12 March 2024). [CrossRef]
- Xu, D.; Ji, P.; Wang, L.; Zhao, X.; Hu, X.; Huang, X.; Zhao, H.; Liu, F. Effect of modified fly ash on environmental safety of two soils contaminated with cadmium and lead. Ecotoxicol. Environ. Saf. 2021, 215, 112175. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ociepa, E.; Ociepa-Kubicka, A.; Okoniewska, E.; Lach, J. The immobilization of zinc and cadmium in the soil as a result of the use of waste substrates. Ann. Set Environ. Prot. 2013, 15, 1772–1786. [Google Scholar]
- Wang, R.; Mohammad Shafi, M.; Ma, J.; Zhong, B.; Guo, J.; Hu, X.; Xu, W.; Yang, Y.; Ruan, Z.; Wang, Y.; et al. Effect of amendments on contaminated soil of multiple heavy metals and accumulation of heavy metals in plants. Environ. Sci. Pollut. Res. 2018, 25, 28695–28704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; You, L.C.; Lyu, H.H.; Liu, Y.X.; He, L.L.; Hu, Y.D.; Luo, F.C.; Yang, S.M. Role of biochar–mineral composite amendment on the immobilization of heavy metals for Brassica chinensis from naturally contaminated soil. Environ. Technol. Innov. 2022, 28, 102622. [Google Scholar] [CrossRef]
- Ciećko, Z.; Żołnowski, A.C.; Chełstowski, A. Long-term effect of coal fly ash application on soil total nitrogen and organic carbon concentrations. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Kulakow, P.A., Pidlisnyuk, V.V., Eds.; Springer: Dodrecht, The Netherlands, 2010; pp. 147–158. [Google Scholar]
- Ciećko, Z.; Żołnowski, A.C.; Madej, M.; Wasiak, G.; Lisowski, J. Long-term effects of hard coal fly ash on selected soil 666 properties. Pol. J. Environ. Stud. 2015, 24, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Ciećko, Z.; Żołnowski, A.C.; Madej, M.; Wasiak, G.; Lisowski, J.; Rolka, E. The long-term impact of ameliorating doses of hard coal fly ash on shaping the content of selected microelements in agricultural soil. Pol. J. Soil Sci. 2015, 48, 671. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of Pb- and Cu-contaminated soil using coal fly ash and peat. Environ. Pollut. 2007, 145, 365–373. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Sun, Y.; Miao, W.; Liu, Z. Co-pyrolysis of corn stover with industrial coal ash for in situ efficient remediation of heavy metals in multi-polluted soil. Environ. Pollut. 2021, 289, 117840. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Chen, L.; Chen, Y.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, J.; Yang, W.; He, L.; Wei, W.; Tan, X.; Wang, J.; Lin, A. Evaluation of biochar pyrolyzed from kitchen waste, corn straw, and peanut hulls on immobilization of Pb and Cd in contaminated soil. Environ. Pollut. 2020, 261, 114133. [Google Scholar] [CrossRef] [PubMed]
- Barčauskaitė, K.; Anne, O.; Mockevičienė, I.; Repšienė, R.; Šiaudinis, G.; Karčauskienė, D. Determination of heavy metals immobilization by chemical fractions in contaminated soil amended with biochar. Sustainability 2023, 15, 8677. [Google Scholar] [CrossRef]
- Cui, L.; Li, L.; Zhang, A.; Pan, G.; Bao, D.; Chang, A.; Cui, L.; Li, L.; Zhang, A.; Pan, G.; et al. Biochar Amendment Greatly Reduces Rice Cd Uptake in a Contaminated Paddy Soil: A Two-Year Field Experiment. Bioresources 2011, 6, 2605–2618. Available online: https://bioresources.cnr.ncsu.edu/wp-content/uploads/2016/06/BioRes_06_3_2605_Cui_LZPB_Biochar_Amend_Rice_CD_Soil_Exper_1651.pdf (accessed on 26 February 2024). [CrossRef]
- Żolnowski, A.; Ciecko, Z.; Najmowicz, T. Arsenic content in and uptake by plants from arsenic-contaminated soil. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Kulakow, P.A., Pidlisnyuk, V.V., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 135–145. [Google Scholar]
- Beesley, L.; Marmiroli, M. The immobilization and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Hu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Ðurić, M.; Oprčkal, P.; Serjun, V.Z.; Pranjić, A.M.; Ščančar, J.; Milačič, R.; Mladenovič, A. Environmental impacts and immobilization mechanisms of cadmium, lead and zinc in geotechnical composites made from contaminated soil and paper-ash. Appl. Sci. 2021, 11, 11822. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Chen, Z.; Xue, Q.; Chen, X.; Mu, Y.; Poon, C.S. Immobilization of high-Pb contaminated soil by oxalic acid activated incinerated sewage sludge ash. Environ. Pollut. 2021, 284, 117720. [Google Scholar] [CrossRef]

| BA Doses g pot−1 | BA | BA + Cd | BA + Pb | BA + Zn | Mean for Doses |
|---|---|---|---|---|---|
| 0 | 0.520 a | 1.873 e | 0.593 abc | 0.627 abcd | 0.903 a |
| ±0.028 | ±0.084 | ±0.041 | ±0.038 | ±0.019 | |
| 30 | 0.533 a | 1.840 e | 0.600 abc | 0.767 d | 0.935 ab |
| ±0.038 | ±0.114 | ±0.033 | ±0.062 | ±0.058 | |
| 60 | 0.567 ab | 2.007 f | 0.760 d | 0.727 cd | 1.015 c |
| ±0.025 | ±0.066 | ±0.033 | ±0.009 | ±0.023 | |
| 90 | 0.733 cd | 1.920 ef | 0.627 abcd | 0.693 bcd | 0.993 bc |
| ±0.019 | ±0.150 | ±0.034 | ±0.034 | ±0.046 | |
| Mean for series | 0.588 a | 1.910 c | 0.645 ab | 0.703 b | 0.962 |
| ±0.090 | ±0.125 | ±0.076 | ±0.065 | ±0.060 | |
| r | 0.887 ** | 0.274 n.s. | 0.381 n.s. | 0.274 n.s. | 0.070 n.s. |
| BA Doses g pot−1 | BA | BA + Cd | BA + Pb | BA + Zn | Mean for Doses |
|---|---|---|---|---|---|
| 0 | 12.49 a | 13.50 ab | 76.41 d | 13.69 ab | 29.02 b |
| ±0.58 | ±0.53 | ±1.83 | ±0.37 | ±0.55 | |
| 30 | 12.58 a | 13.05 ab | 94.33 f | 14.87 b | 33.71 d |
| ±0.38 | ±0.21 | ±1.66 | ±0.31 | ±0.53 | |
| 60 | 13.31 ab | 13.51 ab | 85.58 e | 14.07 ab | 31.62 c |
| ±0.14 | ±0.35 | ±1.15 | ±0.36 | ±0.25 | |
| 90 | 13.12 ab | 13.11 ab | 70.99 c | 14.44 ab | 27.92 a |
| ±0.12 | ±0.04 | ±1.42 | ±0.00 | ±0.37 | |
| Mean for series | 12.88 a | 13.29 a | 81.83 c | 14.27 b | 30.57 |
| ±0.50 | ±0.40 | ±9.04 | ±0.53 | ±2.30 | |
| r | 0.585 n.s. | −0.200 n.s. | −0.310 n.s. | 0.308 n.s. | −0.020 n.s. |
| BA Doses g pot−1 | BA | BA + Cd | BA + Pb | BA + Zn | Mean for Doses |
|---|---|---|---|---|---|
| 0 | 21.69 b | 15.31 a | 17.31 ab | 188.0 c | 60.59 a |
| ±0.90 | ±0.09 | ±0.50 | ±4.66 | ±1.31 | |
| 30 | 18.96 ab | 18.52 ab | 17.64 ab | 189.6 c | 61.17 a |
| ±0.65 | ±0.16 | ±0.26 | ±5.46 | ±1.50 | |
| 60 | 18.64 ab | 19.06 ab | 18.74 ab | 189.8 c | 61.57 a |
| ±0.47 | ±0.75 | ±0.41 | ±5.98 | ±1.29 | |
| 90 | 18.69 ab | 20.79 ab | 20.90 ab | 212.66 d | 68.26 b |
| ±0.53 | ±0.44 | ±0.20 | ±1.68 | ±0.42 | |
| Mean for series | 19.50 a | 18.42 a | 18.65 a | 195.0 b | 62.90 |
| ±1.44 | ±2.03 | ±1.45 | ±11.26 | ±3.34 | |
| r | −0.728 * | 0.935 ** | 0.915 ** | 0.737 * | 0.034 n.s. |
| BA Doses g pot−1 | BA | BA + Cd | BA + Pb | BA + Zn | Mean for Doses |
|---|---|---|---|---|---|
| 0 | 0.113 a | 1.519 b | 0.128 a | 0.142 a | 0.476 a |
| ±0.006 | ±0.044 | ±0.016 | ±0.016 | ±0.015 | |
| 30 | 0.125 a | 1.497 b | 0.128 a | 0.138 a | 0.472 a |
| ±0.008 | ±0.069 | ±0.004 | ±0.001 | ±0.019 | |
| 60 | 0.131 a | 1.581 b | 0.141 a | 0.146 a | 0.500 a |
| ±0.009 | ±0.140 | ±0.002 | ±0.003 | ±0.037 | |
| 90 | 0.137 a | 1.556 b | 0.148 a | 0.159 a | 0.500 a |
| ±0.001 | ±0.094 | ±0.004 | ±0.001 | ±0.024 | |
| Mean for series | 0.126 a | 1.538 b | 0.136 a | 0.146 a | 0.487 |
| ±0.011 | ±0.099 | ±0.012 | ±0.012 | ±0.028 | |
| r | 0.784 ** | 0.220 n.s. | 0.663 * | 0.575 n.s. | 0.019 n.s. |
| BA Doses g pot−1 | BA | BA + Cd | BA + Pb | BA + Zn | Mean for Doses |
|---|---|---|---|---|---|
| 0 | 6.031 a | 5.905 a | 46.69 b | 7.174 a | 16.45 b |
| ±0.143 | ±0.063 | ±3.03 | ±0.415 | ±0.71 | |
| 30 | 6.430 a | 6.218 a | 63.53 d | 6.619 a | 20.70 a |
| ±0.063 | ±0.149 | ±1.56 | ±0.073 | ±0.36 | |
| 60 | 6.536 a | 6.224 a | 61.85 c | 6.806 a | 20.35 a |
| ±0.374 | ±0.072 | ±2.76 | ±0.080 | ±0.76 | |
| 90 | 6.432 a | 6.412 a | 60.15 c | 6.994 a | 20.00 a |
| ±0.146 | ±0.209 | ±3.66 | ±0.075 | ±0.89 | |
| Mean for series | 6.357 a | 6.190 a | 58.06 b | 6.899 a | 19.38 |
| ±0.289 | ±0.228 | ±7.26 | ±0.300 | ±1.85 | |
| r | 0.507 n.s. | 0.730 ** | 0.596 n.s. | −0.131 n.s. | 0.051 n.s. |
| BA Doses g pot−1 | BA | BA + Cd | BA + Pb | BA + Zn | Mean for Doses |
|---|---|---|---|---|---|
| 0 | 9.917 bcd | 9.124 ab | 8.167 a | 28.68 f | 13.97 a |
| ±0.49 | ±0.43 | ±0.73 | ±0.69 | ±0.47 | |
| 30 | 11.00 d | 9.549 abc | 9.493 abc | 26.35 e | 14.10 a |
| ±0.46 | ±0.38 | ±0.55 | ±1.00 | ±0.16 | |
| 60 | 11.08 d | 8.808 ab | 8.569 ab | 26.68 e | 13.79 a |
| ±0.25 | ±0.96 | ±0.24 | ±1.29 | ±0.15 | |
| 90 | 10.79 cd | 9.412 abc | 8.806 ab | 26.09 e | 13.78 a |
| ±0.60 | ±0.60 | ±0.15 | ±0.38 | ±0.17 | |
| Mean for series | 10.70 b | 9.223 a | 8.759 a | 26.95 c | 13.91 |
| ±0.66 | ±0.69 | ±0.68 | ±1.36 | ±0.30 | |
| r | 0.460 n.s. | 0.020 n.s. | 0.164 n.s. | −0.609 n.s. | −0.013 n.s. |
| Elements | Cdtot | Cdav | Pbtot | Pbav | Zntot |
|---|---|---|---|---|---|
| Cdav | 0.988 ** | ||||
| Pbtot | −0.325 ** | −0.332 ** | |||
| Pbav | −0.328 ** | −0.335 ** | 0.986 ** | ||
| Zntot | −0.271 n.s. | −0.325 ** | −0.316 ** | −0.318 ** | |
| Znav | −0.295 ** | −0.342 ** | −0.368 ** | −0.371 ** | 0.982 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolka, E.; Wyszkowski, M.; Żołnowski, A.C.; Skorwider-Namiotko, A. Role of Woody Biomass Ash Material in Immobilization of Cadmium, Lead and Zinc in Soil. Materials 2024, 17, 2206. https://doi.org/10.3390/ma17102206
Rolka E, Wyszkowski M, Żołnowski AC, Skorwider-Namiotko A. Role of Woody Biomass Ash Material in Immobilization of Cadmium, Lead and Zinc in Soil. Materials. 2024; 17(10):2206. https://doi.org/10.3390/ma17102206
Chicago/Turabian StyleRolka, Elżbieta, Mirosław Wyszkowski, Andrzej Cezary Żołnowski, and Anna Skorwider-Namiotko. 2024. "Role of Woody Biomass Ash Material in Immobilization of Cadmium, Lead and Zinc in Soil" Materials 17, no. 10: 2206. https://doi.org/10.3390/ma17102206








