Composition Effect on the Formation of Oxide Phases by Thermal Decomposition of CuNiM(III) Layered Double Hydroxides with M(III) = Al, Fe
Abstract
:1. Introduction
2. Materials and Methods
3. Results
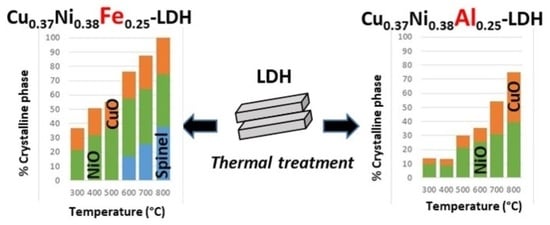
3.1. (Cu,Ni)Al Series
3.2. (Cu,Ni)Fe Series
4. Discussion
4.1. Composition Effects in the Decomposition of LDHs
4.2. Formation of Oxide Phases by Crystallization of the Products of LDH Decomposition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Dasha, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Ameena Shirin, V.K.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Rives, V.; Del Arco, M.; Martín, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88–89, 239–269. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Shakir, I. Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices. J. Energy Storage 2017, 13, 103–122. [Google Scholar] [CrossRef]
- Xie, W.; Song, Y.; Li, S.; Shao, M.; Wei, M. Integrated Nanostructural Electrodes Based on Layered Double Hydroxides. Energy Environ. Mater. 2019, 2, 158–171. [Google Scholar] [CrossRef]
- Mousty, C. Biosensing applications of clay-modified electrodes: A review. Anal. Bioanal. Chem. 2010, 396, 315–325. [Google Scholar] [CrossRef]
- Posati, T.; Costantino, F.; Latterini, L.; Nocchetti, M.; Paolantoni, M.; Tarpani, L. New Insights on the Incorporation of Lanthanide Ions into Nanosized Layered Double Hydroxides. Inorg. Chem. 2012, 51, 13229–13236. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Şen, S.; Salak, A.N.; Ferreira, M.G.S.; Beganskiene, A.; Kareiva, A. Sol–Gel Derived Lanthanide-Substituted Layered Double Hydroxides Mg3/Al1−xLnx. Acta Phys. Pol. A 2018, 133, 884–886. [Google Scholar] [CrossRef]
- Golovin, S.N.; Yapryntsev, M.N.; Lebedeva, O.E. Hydrothermal Synthesis of Layered Double Hydroxides Doped with Holmium, Thulium and Lutetium. Inorganics 2022, 10, 217. [Google Scholar] [CrossRef]
- Velu, S.; Ramaswamy, V.; Ramani, A.; Chanda, B.M.; Sivasanker, S. New hydrotalcite-like anionic clays containing Zr4+ in the layers. Chem. Commun. 1997, 21, 2107. [Google Scholar] [CrossRef]
- Saber, O.; Tagaya, H. Preparation and Intercalation Reactions of Zn-Sn LDH and Zn-Al-Sn LDH. J. Porous Mater. 2003, 10, 83–91. [Google Scholar] [CrossRef]
- Saber, O. Preparation and characterization of a new nano-structural material, Co-Sn LDH. J. Phys. Confer. Ser. 2007, 61, 825. [Google Scholar] [CrossRef]
- Saber, O.; Hatano, B.; Tagaya, H. Preparation of New Layered Double Hydroxide, Co-Ti LDH. J. Incl. Phen. Macroc. Chem. 2005, 51, 17–25. [Google Scholar] [CrossRef]
- Saber, O.; Tagaya, H. Preparation of a new nano-layered materials and organic–inorganic nano-hybrid materials, Zn–Si LDH. J. Porous Mater. 2009, 16, 81–89. [Google Scholar] [CrossRef]
- Intissar, M.; Holler, S.; Malherbe, F.; Besse, J.P.; Leroux, F. Incorporation of Ti4+ into layered double hydroxide sheets? The response by X-ray diffraction and absorption study. J. Phys. Chem. Solids 2004, 65, 453–457. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; Van Tendeloo, G.; Cool, P.; Vansant, E.F. The influence of the cationic ratio on the incorporation of Ti4+ in the brucite-like sheets of layered double hydroxides. Microp. Mesop. Mater. 2008, 111, 12–17. [Google Scholar] [CrossRef]
- Ho, P.H.; Sanghez de Luna, G.; Schiaroli, N.; Natoli, A.; Ospitali, F.; Battisti, M.; Di Renzo, F.; Lucarelli, C.; Vaccari, A.; Fornasari, G.; et al. Effect of Fe and La on the Performance of NiMgAl HT-Derived Catalysts in the Methanation of CO2 and Biogas. Ind. Eng. Chem. Res. 2022, 61, 10511–10521. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chaib, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; He, Y.; Liu, Y.; Du, Y.; Li, D. Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: General functionality and promising application prospects. Chem. Soc. Rev. 2015, 44, 5291–5319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Zhang, J.; Adebajo, M.O.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Kaneda, K.; Mizugaki, T. Design of high-performance heterogeneous catalysts using hydrotalcite for selective organic transformations. Green Chem. 2019, 21, 1361–1389. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Lauwaert, J.; Van Der Voort, P.; Verberckmoes, A. Recent advances on the utilization of layered double hydroxides (LDHs) and related heterogeneous catalysts in a lignocellulosic-feedstock biorefinery scheme. Green Chem. 2017, 19, 5269–5302. [Google Scholar] [CrossRef]
- Tichit, D.; Gérardin, C.; Durand, R.; Coq, B. Layered double hydroxides: Precursors for multifunctional catalyst. Top. Catal. 2006, 39, 89–96. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Chen, J.; Zhang, R.; Guo, L.; Gan, H.; Song, B.; Zhu, W.; Hua, L.; Hou, Z. One-pot tandem catalytic synthesis of α, β-unsaturated nitriles from alcohol with nitriles in aqueous phase. Catal. Commun. 2014, 47, 49–53. [Google Scholar] [CrossRef]
- Badhe, K.; Dabholkar, V.; Kurade, S. One-pot Synthesis of 5-Amino-1H-pyrazole-4-carbonitrile Using Calcined Mg-Fe Hydrotalcite Catalyst. Curr. Organocatal. 2018, 5, 3–12. [Google Scholar] [CrossRef]
- Motokura, K.; Fujita, N.; Mori, K.; Mizugaki, T.; Ebitani, K.; Jitsukawa, K.; Kaneda, K. Environmentally Friendly One-Pot Synthesis of α-Alkylated Nitriles Using Hydrotalcite-Supported Metal Species as Multifunctional Solid Catalysts. Chem. Eur. J. 2006, 12, 8228–8239. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, D. Multifunctional nanocatalysts for tandem reactions: A leap toward sustainability. Appl. Catal. A Gen. 2016, 511, 59–77. [Google Scholar] [CrossRef]
- Gherardi, P.; Ruggeri, O.; Trifiro, F.; Vaccari, A.; Del Piero, G.; Manara, G.; Notari, B. Preparation of Cu-Zn-Al Mixed Hydroxycarbonates Precursors of Catalysts for the Synthesis of Methanol at Low Pressure. Stud. Surf. Sci. Catal. 1983, 16, 723–733. [Google Scholar]
- Ginés, M.J.L.; Amadeo, N.; Laborde, M.; Apesteguia, C.R. Activity and structure-sensitivity of the water-gas shift reaction over Cu-Zn-Al mixed oxide catalysts. Appl. Catal. A Gen. 1995, 131, 283–296. [Google Scholar] [CrossRef]
- Antoniak-Jurak, K.; Kowalik, P.; Bicki, R.; Michalska, K.; Prochniak, W.; Wiercioch, P. Cu substituted ZnAl2O4 ex-LDH catalysts for medium-temperature WGS—Effect of Cu/Zn ratio and thermal treatment on catalyst efficiency. Int. J. Hydrogen Energy 2019, 44, 27390–27400. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Osaki, T. Selective production of hydrogen by partial oxidation of methanol over catalysts derived from CuZnAl-layered double hydroxides. Catal. Lett. 1999, 62, 159–167. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Okazaki, M.; Kapoor, M.P.; Osaki, T.; Ohashi, F. Oxidative Steam Reforming of Methanol over CuZnAl(Zr)-Oxide Catalysts for the Selective Production of Hydrogen for Fuel Cells: Catalyst Characterization and Performance Evaluation. J. Catal. 2000, 194, 373–384. [Google Scholar] [CrossRef]
- Cunha, F.; Wu, Y.J.; Santos, J.C.; Rodrigues, A.E. Steam Reforming of Ethanol on Copper Catalysts Derived from Hydrotalcite-like Materials. Ind. Eng. Chem. Res. 2012, 51, 13132–13143. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; An, K.; Zhang, S.; Liu, G.; Liu, Y. Highly Dispersed Ni–Fe Alloy Catalysts on MgAl2O4 Derived from Hydrotalcite for Direct Ethanol Synthesis from Syngas. Energy Technol. 2020, 8, 2000205. [Google Scholar] [CrossRef]
- Wu, J.; Gao, G.; Li, J.; Sun, P.; Long, X.; Li, F. Efficient and versatile CuNi alloy nanocatalysts for the highly selective hydrogenation of furfural. Appl. Catal. B Environ. 2017, 203, 227–236. [Google Scholar] [CrossRef]
- Wen, Y.; Zhao, S.; Yi, H.; Gao, F.; Yu, Q.; Liu, J.; Tang, T.; Tang, X. Efficient catalytic oxidation of methyl mercaptan to sulfur dioxide with NiCuFe mixed metal oxides. Environ. Tech. Innov. 2022, 26, 102252. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Giorgianni, G.; Abate, S.; Perathoner, S.; Centi, G.; Large, A.I.; Held, G.; Arrigo, R.; Palkovits, R. Insights by in-situ studies on the nature of highly-active hydrotalcite-based Ni-Fe catalysts for CO2 methanation. Chem. Eng. Res. Des. 2023, 193, 320–339. [Google Scholar] [CrossRef]
- Awan, I.Z.; Beltrami, G.; Bonincontro, D.; Gimello, O.; Cacciaguerra, T.; Tanchoux, N.; Martucci, A.; Albonetti, S.; Cavani, F.; Di Renzo, F. Copper-nickel mixed oxide catalysts from layered double hydroxides for the hydrogen-transfer valorisation of lignin in organosolv pulping. Appl. Catal. A Gen. 2021, 609, 117929. [Google Scholar] [CrossRef]
- Awan, I.Z.; Hoang Ho, P.; Beltrami, G.; Gimello, O.; Cacciaguerra, T.; Gaudin, P.; Tanchoux, N.; Albonetti, S.; Martucci, A.; Cavani, F.; et al. Design of Multicationic Copper-Bearing Layered Double Hydroxides for Catalytic Application in Biorefinery. ChemCatChem 2023, 15, e202201622. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaïm, E. Hydrotalcite Decomposition Mechanism: A Clue to the Structure and Reactivity of Spinel-like Mixed Oxides. J. Phys. Chem. 1996, 100, 8535–8542. [Google Scholar] [CrossRef]
- Stanimirova, T.S.; Kirov, G.; Dinolova, E. Mechanism of hydrotalcite regeneration. J. Mater. Sci. Lett. 2001, 20, 453–455. [Google Scholar] [CrossRef]
- Mascolo, G.; Mascolo, M.C. On the synthesis of layered double hydroxides (LDHs) by reconstruction method based on the “memory effect”. Microp. Mesop. Mater. 2015, 214, 246–248. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, X.; Wang, F.; Ning, X.; Wen, Y.; Song, B.; Yang, C.; Wu, D.; Ke, X.; Peng, L. Insights into memory effect mechanisms of layered double hydroxides with solid-state NMR spectroscopy. Nat. Commun. 2022, 13, 6093. [Google Scholar] [CrossRef]
- Ye, H.; Liu, S.; Yu, D.; Zhou, X.; Qin, L.; Lai, C.; Qin, F.; Zhang, M.; Chen, W.; Chen, W.; et al. Regeneration mechanism, modification strategy, and environment application of layered double hydroxides: Insights based on memory effect. Coord. Chem. Rev. 2022, 450, 214253. [Google Scholar] [CrossRef]
- Yamaoka, T.; Abe, M.; Tsuji, M. Synthesis of Cu-Al hydrotalcite like compound and its ion exchange property. Mat. Res. Bull. 1989, 24, 1183–1199. [Google Scholar] [CrossRef]
- Rives, V. (Ed.) Study of layered double hydroxides by thermal methods. In Layered Double Hydroxides: Present and Future; Publisher Nova Science Inc.: New York, NY, USA, 2001; pp. 115–137. [Google Scholar]
- Rey, F.; Fornés, V.; Rojo, J.M. Thermal Decomposition of Hydrotalcites–An Infrared and Nuclear Magnetic Resonance Spectroscopic Study. J. Chem. Soc. Faraday Trans. 1992, 88, 2233–2238. [Google Scholar] [CrossRef]
- Labajos, F.M.; Rives, V.; Ulibarri, M.A. Effect of hydrothermal and thermal treatments on the physicochemical properties of Mg-Al hydrotalcite-like materials. J. Mater. Sci. 1992, 27, 1546–1552. [Google Scholar] [CrossRef]
- Velu, S.; Swamy, C.S. Synthesis and physicochemical properties of a new copper-manganese-aluminium ternary hydrotalcite-like compound. J. Mater. Sci. Lett. 1996, 15, 1674–1677. [Google Scholar] [CrossRef]
- Alejandre, A.; Medina, F.; Salagre, P.; Correig, X.; Sueiras, J.E. Preparation and Study of Cu-Al Mixed Oxides via Hydrotalcite-like Precursors. Chem. Mater. 1999, 11, 939–948. [Google Scholar] [CrossRef]
- Britto, S.; Kamath, P.V. Thermal, solution and reductive decomposition of Cu–Al layered double hydroxides into oxide products. J. Solid State Chem. 2009, 182, 1193–1199. [Google Scholar] [CrossRef]
- Kameda, T.; Hoshi, K.; Yoshioka, T. Thermal decomposition behavior of Cu–Al layered double hydroxide, and ethylenediaminetetraacetate-intercalated Cu–Al layered double hydroxide reconstructed from Cu–Al oxide for uptake of Y3+ from aqueous solution. Mat. Res. Bull. 2012, 47, 4216–4219. [Google Scholar] [CrossRef]
- Voyer, N.; Soisnard, A.; Palmer, S.J.; Martens, W.N.; Frost, R.L. Thermal decomposition of the layered double hydroxides of formula Cu6Al2(OH)16CO3 and Zn6Al2(OH)16CO3. J. Therm. Anal. Calorim. 2009, 96, 481–485. [Google Scholar] [CrossRef]
- Günter, J.R.; Oswald, H.R. Topotactic electron induced and thermal decomposition of copper(II) hydroxide. J. Appl. Cryst. 1970, 3, 21–26. [Google Scholar] [CrossRef]
- Carpenter, G.J.C.; Wronski, Z.S. Nanocrystalline NiO and NiO-Ni(OH)2 composite powders prepared by thermal and mechanical dehydroxylation of nickel hydroxide. Nanostruct. Mater. 1999, 11, 67–80. [Google Scholar] [CrossRef]
- Stanimirova, T.S.; Vergilov, I.; Kirov, G.; Petrova, N. Thermal decomposition products of hydrotalcite-like compounds: Low-temperature metaphases. J. Mater. Sci. 1999, 34, 4153–4161. [Google Scholar] [CrossRef]
- Kanezaki, E. Direct Observation of a Metastable Solid Phase of Mg/Al/CO3-Layered Double Hydroxide by Means of High Temperature in Situ Powder XRD and DTA/TG. Inorg. Chem. 1998, 37, 2588–2590. [Google Scholar] [CrossRef]
- Rives, V. Comment on “Direct Observation of a Metastable Solid Phase of Mg/Al/CO3-Layered Double Hydroxide by Means of High-Temperature in Situ Powder XRD and DTA/TG”. Inorg. Chem. 1999, 38, 406–407. [Google Scholar] [CrossRef]
- Vaysse, C.; Guerlou-Demourgues, L.; Delmas, C. Thermal Evolution of Carbonate Pillared Layered Hydroxides with (Ni, L) (L = Fe, Co) Based Slabs: Grafting or Nongrafting of Carbonate Anions? Inorg. Chem. 2002, 41, 6905–6913. [Google Scholar] [CrossRef] [PubMed]
- Gazzano, M.; Kagunya, W.; Matteuzzi, D.; Vaccari, A. Neutron Diffraction Studies of Polycrystalline Ni/Mg/Al Mixed Oxides Obtained from Hydrotalcite-like Precursors. J. Phys. Chem. B 1997, 101, 4514–4519. [Google Scholar] [CrossRef]
- Hill, R.J.; Craig, J.R.; Gibbs, G.V. Systematics of the spinel structure type. Phys. Chem. Miner. 1979, 4, 317–339. [Google Scholar] [CrossRef]
- Reichle, W.T. Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ion. 1986, 22, 135–142. [Google Scholar] [CrossRef]
- Carpentier, J.; Siffert, S.; Lamonier, J.F.; Laversin, H.; Aboukaïs, A. Synthesis and characterization of Cu–Co–Fe hydrotalcites and their calcined products. J. Porous Mater. 2007, 14, 103–110. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Shiva Prasada, K.; Krishna Chaitanya, V.; Babu, E.V.S.S.K.; Sreedhar, B.; Ramana Murthy, S. In situ FTIR study on the dehydration of natural goethite. J. Asian Earth Sci. 2006, 27, 503–511. [Google Scholar] [CrossRef]
- Jagtap, S.B.; Pande, A.R.; Gokarn, A.N. Kinetics of thermal decomposition of siderite: Effect of particle size. Int. J. Min. Process. 1992, 36, 113–124. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R.L. Thermal treatment of natural goethite: Thermal transformation and physical properties. Thermochim. Acta 2013, 568, 115–121. [Google Scholar] [CrossRef]
- Kester, E.; Gillot, B.; Perriat, P.; Dufour, P.; Villette, C.; Tailhades, P.; Rousset, A. Thermal Behavior and Cation Distribution of Submicron Copper Ferrite Spinels CuxFe3−xO4(0 ≤x≤ 0.5) Studied by DTG, FTIR, and XPS. J. Solid State Chem. 1996, 126, 7–14. [Google Scholar] [CrossRef]
- Rives, V.; Kannan, S. Layered double hydroxides with the hydrotalcite-type structure containing Cu2+, Ni2+ and Al3+. J. Mater. Chem. 2000, 10, 489–495. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and calchogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lebedeva, O.; Tichit, D.; Coq, B. Influence of the compensating anions of Ni/Al and Ni/Mg/Al layered double hydroxides on their activation under oxidising and reducing atmospheres. Appl. Catal. A Gen. 1999, 183, 61–71. [Google Scholar] [CrossRef]
- Lin, Y.H.; Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T. Thermogravimetric analysis of hydrotalcites based on the takovite formula NixZn6-xAl2(OH)16(CO3).4H2O. J. Therm. Anal. Calorim. 2005, 81, 83–89. [Google Scholar] [CrossRef]
- Clause, O.; Gazzano, M.; Trifiro, F.; Vaccari, A.; Zatorski, L. Preparation and thermal reactivity of nickel/chromium and nickel/aluminium hydrotalcite-type precursors. Appl. Catal. 1991, 73, 217–236. [Google Scholar] [CrossRef]
- Trifirò, F.; Vaccari, A.; Clause, O. Nature and properties of nickel-containing mixed oxides obtained from hydrotalcite-type anionic clays. Catal. Today 1994, 21, 185–195. [Google Scholar] [CrossRef]
- Clause, O.; Rebours, B.; Merlen, E.; Trifirò, F.; Vaccari, A. Preparation and characterization of nickel-aluminum mixed oxides obtained by thermal decomposition of hydrotalcite-type precursors. J. Catal. 1992, 133, 231–246. [Google Scholar] [CrossRef]
- Beccat, P.; Roussel, J.C.; Clause, O.; Vaccari, A.; Trifirò, F. High thermal stability of nickel-aluminium mixed oxides obtained from hydrotalcite-type precursors. In Catalysis and Surface Characterization; Dines, T.J., Rochester, C.H., Thomson, J., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1992; pp. 32–41. [Google Scholar]
- Zhang, Z.; Zhou, D.; Bao, X.; Yu, H.; Huang, B. Thermal decomposition behavior of nickel-iron hydrotalcite and its electrocatalytic properties of oxygen reduction and oxygen evolution reactions. Int. J. Hydrogen Energy 2018, 43, 20734–20738. [Google Scholar] [CrossRef]










| Sample | d003 (Å) | a (Å) | ||
|---|---|---|---|---|
| 25 °C | 100 °C | 200 °C | 25 °C | |
| Ni75Al25 | 7.61 | 7.39 | 6.58 | 3.040 |
| Cu07Ni68Al25 | 7.63 | 7.39 | 6.53 | 3.040 |
| Cu38Ni37Al25 | 7.52 | 6.62 | 6.42 | 3.059 |
| Cu68Ni07Al25 * | 7.52 | 6.52 | 5.64 | - |
| Cu75Al25 * | 7.52 - | 7.49 6.31 | - 5.61 | - - |
| Ni75Fe25 | 7.73 | 7.14 | 6.41 | 3.082 |
| Cu07Ni68Fe25 | 7.73 | 6.73 | 6.37 | 3.086 |
| Cu38Ni37Fe25 | 7.59 | 6.46 | 5.5 | 3.093 |
| Cu68Ni07Fe25 | 7.56 | 6.39 | - | - |
| Cu75Fe25 | - | - | - | - |
| Sample | Phases | 1st Step | 2nd Step | 3rd Step | 4th Step | Total Loss (%) | ||||||||
| (°C) | ∆m1 (wt%) | DTG1 (°C) | T (°C) | ∆m2 (wt%) | DTG2 (°C) | T (°C) | ∆m3 (wt%) | DTG3 (°C) | T (°C) | ∆m4 (wt%) | DTG4 (°C) | |||
| Ni75Al25 | LDH | 25–213 | 14.3 | 85w 140w 177s | 213–400 | 17.7 | 283m 321s | 400–700 | 3.6 | 545 | 35.6 | |||
| Cu07Ni68Al25 | LDH | 25–235 | 14.8 | 196 | 235–444 | 18.4 | 310m 337s | 444–800 | 2.8 | 510 | 36 | |||
| Cu38Ni37Al25 | LDH | 25–175 | 13 | 80w 133s | 175–450 | 19 | 251s 305m | 450–700 | 3.4 | 527 | 35.4 | |||
| Cu68Ni07Al25 | LDH, CuOw | 25–165 | 10 | 152 | 165–450 | 14 | 213 | 450–750 | 5 | 601 | 29 | |||
| Cu75Al25 | LDH | 25–167 | 13.6 | 142 | 167–400 | 11.1 | 240 | 400–680 | 5.2 | 604 | 680–800 | 1.6 | 737 | 31.5 |
| Ni75Fe25 | LDH | 25–210 | 14.7 | 96w 168s | 210–405 | 15.6 | 250w 294s | 405–650 | 2.2 | - | 32.5 | |||
| Cu07Ni68Fe25 | LDH | 25–195 | 14.4 | 92w 154s | 195–390 | 15.6 | 247w 285s | 390–600 | 3 | - | 33 | |||
| Cu38Ni37Fe25 | LDH | 25–190 | 13 | 91w 153s | 190–470 | 15 | 243m 283m | 470–800 | 2 | - | 30 | |||
| Cu68Ni07Fe25 | CuO, LDHw | 25–115 | 5.4 | 82 | 115–365 | 9.1 | 143s 230s | 365–600 | 1.5 | 405 | 16 | |||
| Cu75Fe25 | CuO | 25–134 | 6 | 77 | 134–350 | 4.7 | 190 | 350–800 | 1.3 | 427 | 12 | |||
| Sample | LDH Formula | ∆m Experimental (%) | ∆m Theoretical (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | Step 4 | Total | Dehydration | Dehydroxylation | Decarbonation | ||
| Ni75Al25 | Ni0.75Al0.25(OH)2(CO3)0.125·0.81 H2O | 14.3 | 17.7 | 3.6 | - | 35.6 | 13.6 | 16.9 | 5.2 |
| Cu08Ni67Al25 | Cu0.08Ni0.67Al0.25(OH)2(CO3)0.125·0.85 H2O | 14.8 | 19.4 | 2.8 | - | 36 | 14.2 | 16.7 | 5.1 |
| Cu38Ni37Al25 | Cu0.38Ni0.37Al0.25(OH)2 (CO3)0.125·0.84 H2O | 13 | 19 | 3.4 | - | 35.4 | 13.9 | 16.5 | 5 |
| Cu75Al25 | Cu0.75 Al0.25(OH)2 (CO3)0.125·0.54 H2O | 13.6 | 11.1 | 5.2 | 1.6 | 31.5 | 9.3 | 17 | 5.2 |
| Ni75Fe25 | Ni0.75Fe0.25(OH)2 (CO3)0.125·0.73 H2O | 14.7 | 15.6 | 2.2 | - | 32.5 | 11.6 | 16 | 4.9 |
| Cu07Ni68Fe25 | Cu0.07Ni0.68 Fe0.25(OH)2 (CO3)0.125·0.78 H2O | 14.4 | 15.6 | 3 | - | 33 | 12.4 | 15.8 | 4.8 |
| Cu38Ni37Fe25 | Cu0.38Ni0.37 Fe0.25(OH)2 (CO3)0.125·0.55 H2O | 13 | 15 | 2 | - | 30 | 8.8 | 16.2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awan, I.Z.; Ho, P.H.; Beltrami, G.; Fraisse, B.; Cacciaguerra, T.; Gaudin, P.; Tanchoux, N.; Albonetti, S.; Martucci, A.; Cavani, F.; et al. Composition Effect on the Formation of Oxide Phases by Thermal Decomposition of CuNiM(III) Layered Double Hydroxides with M(III) = Al, Fe. Materials 2024, 17, 83. https://doi.org/10.3390/ma17010083
Awan IZ, Ho PH, Beltrami G, Fraisse B, Cacciaguerra T, Gaudin P, Tanchoux N, Albonetti S, Martucci A, Cavani F, et al. Composition Effect on the Formation of Oxide Phases by Thermal Decomposition of CuNiM(III) Layered Double Hydroxides with M(III) = Al, Fe. Materials. 2024; 17(1):83. https://doi.org/10.3390/ma17010083
Chicago/Turabian StyleAwan, Iqra Zubair, Phuoc Hoang Ho, Giada Beltrami, Bernard Fraisse, Thomas Cacciaguerra, Pierrick Gaudin, Nathalie Tanchoux, Stefania Albonetti, Annalisa Martucci, Fabrizio Cavani, and et al. 2024. "Composition Effect on the Formation of Oxide Phases by Thermal Decomposition of CuNiM(III) Layered Double Hydroxides with M(III) = Al, Fe" Materials 17, no. 1: 83. https://doi.org/10.3390/ma17010083