Recent Advances in Synthesis of Graphite from Agricultural Bio-Waste Material: A Review
Abstract
:1. Introduction
2. Carbon Precursors from Agricultural Bio-Waste Materials
2.1. Importance of Utilizing Agricultural Bio-Waste Material
2.2. Types of Agricultural Bio-Waste Material as a Carbonaceous Precursor
2.3. Pre-Treatment Method for Preparation of Carbon Precursor
3. Synthesis Method of Graphite from Agricultural Bio-Waste Material
3.1. Carbonization of Agricultural Biomass Waste Material
3.2. Graphitization of Agricultural Carbon
3.2.1. Direct Heat Treatment Graphitization
Effect of Catalyst and Temperature on Graphitization
Effect of Activated Agent
3.2.2. Joule Heating Method
3.2.3. Ultrasonic-Assisted Graphitic Carbon Synthesis Method
4. Summary and Future Works
- It is more important to determine the carbon content of the biomass before starting, to avoid wasting resources.
- The workability of the study in terms of large-scale production is not known, as research is mostly performed on a laboratory scale.
- The safety of catalysts used in regard to large-scale production should be considered. Will this cause other environmental issues, e.g., creating more harmful waste or improper disposal, causing soil and water pollution?
- There is insufficient study of advanced graphitic carbon synthesis as research mostly focuses on the common use of one-step heating graphitization.
- Identification and utilization of novel biomass waste materials that are abundant, have high carbon content and can be easily processed.
- Development of cost-effective and environmentally friendly techniques for the purification of carbon precursor materials.
- Optimization of the carbonization and graphitization processes to increase efficiency and reduce energy consumption.
- Investigation of the properties and performance of the resulting graphite materials for different industrial applications.
- Development of new catalysts or additives that can improve the quality and yield of graphite.
- Recycling and reuse of waste products generated during the synthesis process to minimize waste and reduce the overall environmental impact.
- Study the feasibility of realizing the production of biomass-based graphitic carbon in the industry for large-scale production.
- Implementation of life cycle assessment (LCA) studies to evaluate the environmental sustainability of the synthesis process and identify areas for improvement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kupriyanova, E.; Pronina, N.; Los, D. Carbonic anhydrase—A universal enzyme of the carbon-based life. Photosynthetica 2017, 55, 3–19. [Google Scholar] [CrossRef]
- Mishra, R.; Pramanick, B.; Maiti, T.K.; Bhattacharyya, T.K. Glassy carbon microneedles—New transdermal drug delivery device derived from a scalable C-MEMS process. Microsyst. Nanoeng. 2018, 4, 38. [Google Scholar] [CrossRef]
- A Burchfield, L.; Al Fahim, M.; Wittman, R.S.; Delodovici, F.; Manini, N. Novamene: A new class of carbon allotropes. Heliyon 2017, 3, e00242. [Google Scholar] [CrossRef]
- Wang, Z.; Mizuseki, J.; Chen, H. Computational discovery of a new rhombohedral diamond phase. Phys. Rev. B 2018, 98, 094107. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Sun, H.; Gao, C. Superstructured Assembly of Nanocarbons: Fullerenes, Nanotubes, and Graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Seniutinas, G.; Brasselet, E.; Balčytis, A.; David, C.; Juodkazis, S. Diamond: A gem for micro-optics. Mater. Today 2018, 21, 798–799. [Google Scholar] [CrossRef]
- Erdemir, A.; Martin, J.M. Superior wear resistance of diamond and DLC coatings. Curr. Opin. Solid State Mater. Sci. 2018, 22, 243–254. [Google Scholar] [CrossRef]
- Junaidi; Suparno, J.; Ardiansyah, D.H.; Effendy, M. Jamari Improvement of Wear Resistant of Railway Wheel by Using Graphite in Various Speeds Based on Disc-on-Disc Contact System. AIP Conf. Proc. 2019, 2114, 40005. [Google Scholar] [CrossRef]
- Gangopadhyay, S. Sushil Scanning Tunneling Microscopy (STM) Imaging of Carbon Nanotropes: C60, CNT and Graphene; Springer: Singapore, 2021; pp. 47–75. [Google Scholar] [CrossRef]
- Lin, Z.; Shao, G.; Liu, W.; Wang, Y.; Wang, H.; Wang, H.; Fan, B.; Lu, H.; Xu, H.; Zhang, R. In-situ TEM observations of the structural stability in carbon nanotubes, nanodiamonds and carbon nano-onions under electron irradiation. Carbon 2022, 192, 356–365. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Aravindan, V. Graphene from Spent Lithium-Ion Batteries. Batter. Supercaps 2022, 5, e202200046. [Google Scholar] [CrossRef]
- Liang, C.; Chen, Y.; Wu, M.; Wang, K.; Zhang, W.; Gan, Y.; Huang, H.; Chen, J.; Xia, Y.; Zhang, J.; et al. Green synthesis of graphite from CO2 without graphitization process of amorphous carbon. Nat. Commun. 2021, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, N.; Chong, S.; Li, D.; Chen, Y.; Sun, C. Three-dimensional porous graphene-like sheets synthesized from biocarbon via low-temperature graphitization for a supercapacitor. Green Chem. 2018, 20, 694–700. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithiumion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Jayaraman, S.; Madhavi, S.; Aravindan, V. High energy Li-ion capacitor and battery using graphitic carbon spheres as an insertion host from cooking oil. J. Mater. Chem. A 2018, 6, 3242–3248. [Google Scholar] [CrossRef]
- Peng, J.; Chen, N.; He, R.; Wang, Z.; Dai, S.; Jin, X. Electrochemically Driven Transformation of Amorphous Carbons to Crystalline Graphite Nanoflakes: A Facile and Mild Graphitization Method. Angew. Chem. Int. Ed. 2017, 56, 1751–1755. [Google Scholar] [CrossRef]
- Amer, M.; Elwardany, A. Biomass Carbonization. In Renewable Energy-Resources, Challenges and Applications; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Roslam, W.N.; Isahak, W.; Wahab, N.R.M.; Hisham, M.W.M.; Yarmo, M.A. Highly Porous Carbon Materials from Biomass by Chemical and Carbonization Method: A Comparison Study. J. Chem. 2013, 2013, 620346. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, K.-H. Full graphitization of amorphous carbon by microwave heating. RSC Adv. 2016, 6, 24667–24674. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, C.; Cao, Y.; Huang, G.; Liu, Q.; Zhang, C.; Chen, Z.; Yi, G.; Chen, L.; Yu, J. Preparation of synthetic graphite from bituminous coal as anode materials for high performance lithium-ion batteries. Fuel Process. Technol. 2018, 172, 162–171. [Google Scholar] [CrossRef]
- Shi, M.; Song, C.; Tai, Z.; Zou, K.; Duan, Y.; Dai, X.; Sun, J.; Chen, Y.; Liu, Y. Coal-derived synthetic graphite with high specific capacity and excellent cyclic stability as anode material for lithium-ion batteries. Fuel 2021, 292, 120250. [Google Scholar] [CrossRef]
- Qiu, T.; Yang, J.-G.; Bai, X.-J.; Wang, Y.-L. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef] [PubMed]
- Neolaka, Y.A.; Lawa, Y.; Naat, J.N.; Riwu, A.A.; Iqbal, M.; Darmokoesoemo, H.; Kusuma, H.S. The adsorption of Cr(VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (Kusambi Wood or Schleichera oleosa): Study of kinetics, isotherms and thermodynamics. J. Mater. Res. Technol. 2020, 9, 6544–6556. [Google Scholar] [CrossRef]
- Jabarullah, N.H.; Kamal, A.S.; Othman, R. A Modification of Palm Waste Lignocellulosic Materials into Biographite Using Iron and Nickel Catalyst. Processes 2021, 9, 1079. [Google Scholar] [CrossRef]
- Kamal, A.S.; Jabarullah, N.H.; Othman, R. Catalytic graphitization of Oil Palm Frond using iron and silica. Mater. Today Proc. 2020, 31, 211–216. [Google Scholar] [CrossRef]
- Tetrick, M.A.; Odle, J. What Constitutes a Gluconeogenic Precursor? J. Nutr. 2020, 150, 2239–2241. [Google Scholar] [CrossRef]
- Seydibeyoğlu, M.Ö. A Novel Partially Biobased PAN-Lignin Blend as a Potential Carbon Fiber Precursor. J. Biomed. Biotechnol. 2012, 2012, 598324. [Google Scholar] [CrossRef]
- Karaboyaci, M.; Tama, B.; Şencan, A.; Kiliç, M. Recycling of Rose Wastes to Activated Carbon with Ecological Precursor. Bilge Int. J. Sci. Technol. Res. 2017, 1, 1–8. [Google Scholar]
- Panzella, L.; Moccia, F.; Toscanesi, M.; Trifuoggi, M.; Giovando, S.; Napolitano, A. Exhausted Woods from Tannin Extraction as an Unexplored Waste Biomass: Evaluation of the Antioxidant and Pollutant Adsorption Properties and Activating Effects of Hydrolytic Treatments. Antioxidants 2019, 8, 84. [Google Scholar] [CrossRef]
- Spiridon, I.; Anghel, N.C.; Darie-Nita, R.N.; Iwańczuk, A.; Ursu, R.G.; Spiridon, I.A. New composites based on starch/Ecoflex®/biomass wastes: Mechanical, thermal, morphological and antimicrobial properties. Int. J. Biol. Macromol. 2020, 156, 1435–1444. [Google Scholar] [CrossRef]
- Baysal, G.; Kasapbaşı, E.E.; Yavuz, N.; Hür, Z.; Genç, K.; Genç, M. Determination of Theoretical Calculations by DFT Method and Investigation of Antioxidant, Antimicrobial Properties of Olive Leaf Extracts from Different Regions. J. Food Sci. Technol. 2021, 58, 1909–1917. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Das, S.; Saha, B.; Paul, D.; Basu, B. Anti-microbial, anti-oxidant, and anti-breast cancer properties unraveled in yeast carotenoids produced via cost-effective fermentation technique utilizing waste hydrolysate. Front. Microbiol. 2023, 13, 1088477. [Google Scholar] [CrossRef] [PubMed]
- Decoupling Natural Resource Use and Environmental Impacts from Economic Growth—United Nations, International Resource Panel—Google Books. Available online: https://books.google.com.my/books?hl=en&lr=&id=dGt0Rogq6MIC&oi=fnd&pg=PA1&dq=Global+consumption+of+natural+resources+could+triple+to+140+billion+tons+a+year+by+2050&ots=jxJ1ryUQwZ&sig=oYFCARdnGDtca75yFZF2TfT8QWY&redir_esc=y#v=onepage&q=tripleresourcesuseby2050&f=false (accessed on 26 February 2022).
- Haluska, O.; Meščeriakovė, S.-M.; Murashko, K.; Meščeriakovas, A.; Kalidas, N.; Rantanen, J.; Liu, L.; Salami, A.; Lappalainen, R.; Lähde, A.; et al. Production of graphitic carbons from plant-based SiC/C nanocomposites for Li-ion batteries. Mater. Chem. Phys. 2023, 296, 127286. [Google Scholar] [CrossRef]
- Xia, S.; Yang, H.; Lu, W.; Cai, N.; Xiao, H.; Chen, X.; Chen, Y.; Wang, X.; Wang, S.; Wu, P.; et al. Fe–Co based synergistic catalytic graphitization of biomass: Influence of the catalyst type and the pyrolytic temperature. Energy 2022, 239, 122262. [Google Scholar] [CrossRef]
- Sun, Z.; Yao, D.; Cao, C.; Zhang, Z.; Zhang, L.; Zhu, H.; Yuan, Q.; Yi, B. Preparation and formation mechanism of biomass-based graphite carbon catalyzed by iron nitrate under a low-temperature condition. SSRN Electron. J. 2022, 318, 115555. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.; Yu, Y.; Wang, N.; Zhou, Z. Simultaneously tuning the hierarchical porous structure and graphitization degree of biomass derived carbon for supercapacitors. Electrochim. Acta 2022, 432, 141219. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, Z.; He, L.; Li, H. Three-dimensional high graphitic porous biomass carbon from dandelion flower activated by K2FeO4 for supercapacitor electrode. J. Energy Storage 2022, 52, 104889. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Nagendran, R. Agricultural Waste and Pollution. In Waste; Academic Press: Cambridge, MA, USA, 2011; pp. 341–355. [Google Scholar] [CrossRef]
- Hoekstra, J.; Beale, A.M.; Soulimani, F.; Versluijs-Helder, M.; Geus, J.W.; Jenneskens, L.W. Base Metal Catalyzed Graphitization of Cellulose: A Combined Raman Spectroscopy, Temperature-Dependent X-ray Diffraction and High-Resolution Transmission Electron Microscopy Study. J. Phys. Chem. C 2015, 119, 10653–10661. [Google Scholar] [CrossRef]
- McHenry, M.P. Agricultural bio-char production, renewable energy generation and farm carbon sequestration in Western Australia: Certainty, uncertainty and risk. Agric. Ecosyst. Environ. 2009, 129, 1–7. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Wang, X.; Holm, N.; Rajagopalan, K.; Chen, F.; Ma, S. Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Xie, X.; Goodell, B. Thermal Degradation and Conversion of Plant Biomass into High Value Carbon Products. In Deterioration and Protection of Sustainable Biomaterials; American Chemical Society: Washington, DC, USA, 2014; pp. 147–158. [Google Scholar] [CrossRef]
- Abdullah, R.F.; Rashid, U.; Ibrahim, M.L.; Hazmi, B.; Alharthi, F.A.; Nehdi, I.A. Bifunctional nano-catalyst produced from palm kernel shell via hydrothermal-assisted carbonization for biodiesel production from waste cooking oil. Renew. Sustain. Energy Rev. 2021, 137, 110638. [Google Scholar] [CrossRef]
- Kim, S.W.; Koo, B.S.; Lee, D.H. Catalytic pyrolysis of palm kernel shell waste in a fluidized bed. Bioresour. Technol. 2014, 167, 425–432. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Cocchi, S.; Manzo, A. Waste Wood Biomass Arising from Pruning of Urban Green in Viterbo Town: Energy Characterization and Potential Uses. In Proceedings, Part II 13, Proceedings of the Computational Science and Its Applications–ICCSA 2013: 13th International Conference, Ho Chi Minh City, Vietnam, 24–27 June 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 242–255. [Google Scholar] [CrossRef]
- Subramaniam, R.; Ponnusamy, S.K. Novel adsorbent from agricultural waste (cashew NUT shell) for methylene blue dye removal: Optimization by response surface methodology. Water Resour. Ind. 2015, 11, 64–70. [Google Scholar] [CrossRef]
- Martínez-Casillas, D.; Mascorro-Gutiérrez, I.; Arreola-Ramos, C.; Villafán-Vidales, H.; Arancibia-Bulnes, C.; Ramos-Sánchez, V.; Cuentas-Gallegos, A. A sustainable approach to produce activated carbons from pecan nutshell waste for environmentally friendly supercapacitors. Carbon 2019, 148, 403–412. [Google Scholar] [CrossRef]
- Wongcharee, S.; Aravinthan, V.; Erdei, L. Mesoporous activated carbon-zeolite composite prepared from waste macadamia nut shell and synthetic faujasite. Chin. J. Chem. Eng. 2019, 27, 226–236. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0 Introduction—“Snapshot of Solid Waste Management to 2050.” Overview Booklet. Urban Dev. Ser. 2018, 1–38. [Google Scholar] [CrossRef]
- A New Model of Circular Economy. European Commission 2020. p. 2. Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed on 26 February 2022).
- Periyasamy, S.; Karthik, V.; Kumar, P.S.; Isabel, J.B.; Temesgen, T.; Hunegnaw, B.M.; Melese, B.B.; Mohamed, B.A.; Vo, D.-V.N. Chemical, physical and biological methods to convert lignocellulosic waste into value-added products. A review. Environ. Chem. Lett. 2022, 20, 1129–1152. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Battisti, R.; Hafemann, E.; Claumann, C.A.; Machado, R.A.F.; Marangoni, C. Synthesis and characterization of cellulose acetate from royal palm tree agroindustrial waste. Polym. Eng. Sci. 2018, 59, 891–898. [Google Scholar] [CrossRef]
- Köseoğlu, E.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Angin, D. Utilization of activated carbon produced from fruit juice industry solid waste for the adsorption of Yellow 18 from aqueous solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- A Tahir, T.; Hamid, F.S. Vermicomposting of two types of coconut wastes employing Eudrilus eugeniae: A comparative study. Int. J. Recycl. Org. Waste Agric. 2012, 1, 7. [Google Scholar] [CrossRef]
- Sadorsky, P. Oil price shocks and stock market activity. Energy Econ. 1999, 21, 449–469. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Sahu, J.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef]
- Sun, D.; Hale, L.; Kar, G.; Soolanayakanahally, R.; Adl, S. Phosphorus recovery and reuse by pyrolysis: Applications for agriculture and environment. Chemosphere 2018, 194, 682–691. [Google Scholar] [CrossRef]
- Feng, Y.; Tao, L.; Zheng, Z.; Huang, H.; Lin, F. Upgrading agricultural biomass for sustainable energy storage: Bioprocessing, electrochemistry, mechanism. Energy Storage Mater. 2020, 31, 274–309. [Google Scholar] [CrossRef]
- Naik, P.B.; Yadav, P.; Nagaraj, R.; Puttaswamy, R.; Beere, H.K.; Maiti, U.N.; Mondal, C.; Kotrappanavar, N.S.; Ghosh, D. Developing High-Performance Flexible Zinc Ion Capacitors from Agricultural Waste-Derived Carbon Sheets. ACS Sustain. Chem. Eng. 2022, 10, 1471–1481. [Google Scholar] [CrossRef]
- Valdés-Rodríguez, E.; Mendoza-Castillo, D.; Reynel-Ávila, H.; Aguayo-Villarreal, I.; Bonilla-Petriciolet, A. Activated carbon manufacturing via alternative Mexican lignocellulosic biomass and their application in water treatment: Preparation conditions, surface chemistry analysis and heavy metal adsorption properties. Chem. Eng. Res. Des. 2022, 187, 9–26. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Q.; Xue, C.; Yang, J.; Hu, S. Chemical treatment of biomass wastes as carbon dot carriers for solar-driven water purification. J. Colloid Interface Sci. 2022, 621, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Qian, M.; Wang, C.; Mateo, W.; Wang, Y.; Dai, L.; Lin, X.; Zhao, Y.; Huo, E.; Wang, L.; et al. Biochar: From by-products of agro-industrial lignocellulosic waste to tailored carbon-based catalysts for biomass thermochemical conversions. Chem. Eng. J. 2022, 441, 135972. [Google Scholar] [CrossRef]
- Patnaik, S.; Martha, S.; Acharya, S.; Parida, K.M. An overview of the modification of g-C3N4 with high carbon containing materials for photocatalytic applications. Inorg. Chem. Front. 2016, 3, 336–347. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y. Biomass Digestion. Encycl. Sustain. Technol. 2017, 197–204. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Souto, F.; Calado, V.; Pereira, N., Jr. Lignin-based carbon fiber: A current overview. Mater Res Exp. 2018, 5, 072001. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Peng, H.-J.; Tang, C.; Lieder, M.; Zhang, Q.; Titirici, M.-M. Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro-/mesoporous hierarchy in hosting active species for lithium–sulphur batteries. Green Chem. 2016, 18, 5169–5179. [Google Scholar] [CrossRef]
- Destyorini, F.; Yudianti, R.; Irmawati, Y.; Hardiansyah, A.; Hsu, Y.-I.; Uyama, H. Temperature driven structural transition in the nickel-based catalytic graphitization of coconut coir. Diam. Relat. Mater. 2021, 117, 108443. [Google Scholar] [CrossRef]
- Abdeljaoued, A.; Querejeta, N.; Durán, I.; Álvarez-Gutiérrez, N.; Pevida, C.; Chahbani, M.H. Preparation and Evaluation of a Coconut Shell-Based Activated Carbon for CO2/CH4 Separation. Energies 2018, 11, 1748. [Google Scholar] [CrossRef]
- Bae, J.-S.; Su, S. Macadamia nut shell-derived carbon composites for post combustion CO2 capture. Int. J. Greenh. Gas Control 2013, 19, 174–182. [Google Scholar] [CrossRef]
- Ameen, A.; Saeed, H.; Harun, N.Y.; Nasef, M.M. Physicochemical Characterization of Different Agricultural Residues in Malaysia for Bio Char Production. Int. J. Civ. Eng. Technol. 2019, 2, 10–22. [Google Scholar]
- Mahmood, W.M.F.W.; Ariffin, M.A.; Harun, Z.; Ishak, N.A.I.; Ghani, J.A.; Rahman, M.N.A. Characterisation and potential use of biochar from gasified oil palm wastes. J. Eng. Sci. Technol. 2014, 10, 45–54. [Google Scholar]
- Xia, S.; Cai, N.; Wu, J.; Xiao, H.; Hu, J.; Chen, X.; Chen, Y.; Yang, H.; Wang, X.; Chen, H. Synthesis and formation mechanism of biomass-based mesoporous graphitic carbon. Fuel Process. Technol. 2020, 209, 106543. [Google Scholar] [CrossRef]
- Wu, C.; Qian, W.; Li, X.; Zhu, X.; Xu, K.; Zhao, Y.; Dacres, O.D.; Hu, Z.; Luo, G.; Liu, H.; et al. Preparation of carbon nanofiber with specific features by degradative solvent extraction product from biomass wastes. Fuel 2019, 258, 116149. [Google Scholar] [CrossRef]
- Janković, B.; Manić, N.; Dodevski, V.; Radović, I.; Pijović, M.; Katnić, Đ.; Tasić, G. Physico-chemical characterization of carbonized apricot kernel shell as precursor for activated carbon preparation in clean technology utilization. J. Clean. Prod. 2019, 236, 117614. [Google Scholar] [CrossRef]
- Kan, H.; Fan, F.; Yang, Z.; Li, H.; Shi, Z.; Kan, H. Preparation and properties of hydrochars from macadamia nut shell via hydrothermal carbonization. R. Soc. Open Sci. 2018, 5, 181126. [Google Scholar] [CrossRef]
- Rout, T.; Pradhan, D.; Singh, R.; Kumari, N. Exhaustive study of products obtained from coconut shell pyrolysis. J. Environ. Chem. Eng. 2016, 4, 3696–3705. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Jin, C.; Wang, Z.; Wang, T.; Cheng, X.; Ma, C. Effects of temperature, oxygen and steam on pore structure characteristics of coconut husk activated carbon powders prepared by one-step rapid pyrolysis activation process. Bioresour. Technol. 2020, 310, 123413. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Barin, G.B.; Gimenez, I.D.F.; da Costa, L.P.; Filho, A.G.S.; Barreto, L.S. Influence of hydrothermal carbonization on formation of curved graphite structures obtained from a lignocellulosic precursor. Carbon 2014, 78, 609–612. [Google Scholar] [CrossRef]
- Himmel, M.E. Biomass Recalcitrance: Deconstructing the Plant Cell Wall for Bioenergy; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 1–505. [Google Scholar] [CrossRef]
- Zeng, Y.; Himmel, M.E.; Ding, S.-Y. Visualizing chemical functionality in plant cell walls. Biotechnol. Biofuels 2017, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhao, C.; Zhou, T.; Chen, S.; Li, Q.; Wang, X.; Shen, D.; Wang, Y.; Xu, C. An analysis of the carbonization process of coal-based activated carbon at different heating rates. Energy 2023, 267, 126557. [Google Scholar] [CrossRef]
- Singh, S.; Cheng, G.; Sathitsuksanoh, N.; Wu, D.; Varanasi, P.; George, A.; Balan, V.; Gao, X.; Kumar, R.; Dale, B.E.; et al. Comparison of Different Biomass Pretreatment Techniques and Their Impact on Chemistry and Structure. Front. Energy Res. 2015, 3, 62. [Google Scholar] [CrossRef]
- Brito, J.O.; Barrichello, L.E.G.; Trevisan, J.F. Condicoes Climaticas e Suas Influencias Sobre a Producao de Resinas de Pinheiros Tropicais. 1978. Available online: https://www.ipef.br/publicacoes/scientia/nr16/cap03.pdf (accessed on 26 February 2022).
- Ceylan, Z.; Sungur, B. Estimation of coal elemental composition from proximate analysis using machine learning techniques. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 2576–2592. [Google Scholar] [CrossRef]
- Aboudi, K.; Fernández-Güelfo, L.A.; Álvarez-Gallego, C.J.; Romero-García, L.I. Biogas, biohydrogen, and polyhydroxyalkanoates production from organic waste in the circular economy context. In Sustainable Biofuels; Academic Press: Cambridge, MA, USA, 2021; pp. 305–343. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 1–19. [Google Scholar] [CrossRef]
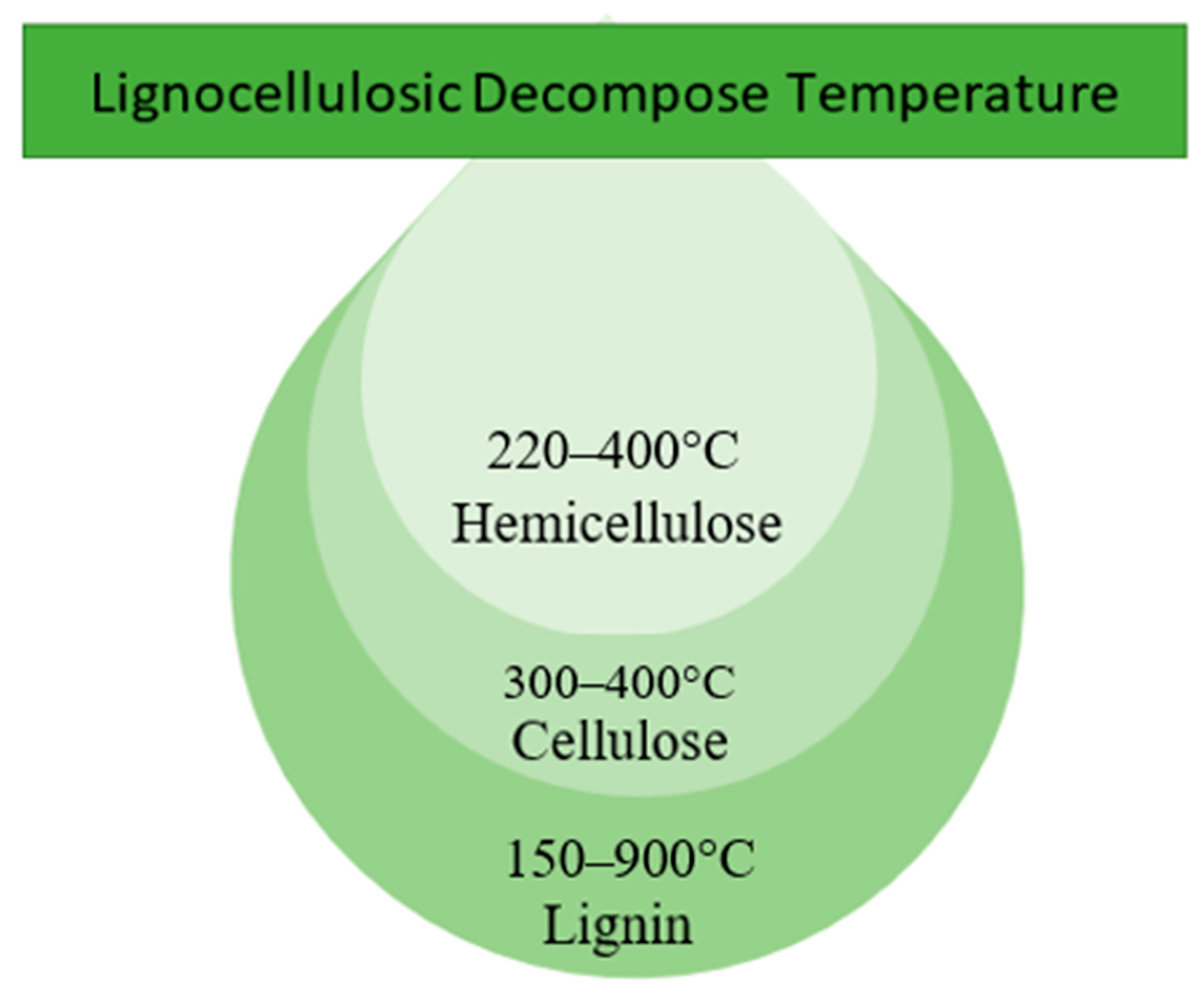
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Lv, D.; Xu, M.; Liu, X.; Zhan, Z.; Li, Z.; Yao, H. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Várhegyi, G.; Antal, M.J.; Jakab, E.; Szabó, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar] [CrossRef]
- Tarelho, L.; Hauschild, T.; Vilas-Boas, A.; Silva, D.; Matos, M. Biochar from pyrolysis of biological sludge from wastewater treatment. Energy Rep. 2020, 6, 757–763. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.-H.; Show, P.L. Biochar production via pyrolysis of citrus peel fruit waste as a potential usage as solid biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zou, R.; Qian, M.; Kong, X.; Huo, E.; Lin, X.; Wang, L.; Zhang, X.; Ruan, R.; Lei, H. Improvement of the carbon yield from biomass carbonization through sulfuric acid pre-dehydration at room temperature. Bioresour. Technol. 2022, 355, 127251. [Google Scholar] [CrossRef]
- Sieradzka, M.; Kirczuk, C.; Kalemba-Rec, I.; Mlonka-Mędrala, A.; Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Cheng, X.; Tang, Y.; Wang, B.; Jiang, J. Improvement of Charcoal Yield and Quality by Two-Step Pyrolysis on Rice Husks. Waste Biomass- Valorization 2018, 9, 123–130. [Google Scholar] [CrossRef]
- Chen, D.; Yu, X.; Song, C.; Pang, X.; Huang, J.; Li, Y. Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour. Technol. 2016, 218, 1303–1306. [Google Scholar] [CrossRef]
- Chang, G.; Huang, Y.; Xie, J.; Yang, H.; Liu, H.; Yin, X.; Wu, C. The lignin pyrolysis composition and pyrolysis products of palm kernel shell, wheat straw, and pine sawdust. Energy Convers. Manag. 2016, 124, 587–597. [Google Scholar] [CrossRef]
- Weldekidan, H.; Mohanty, A.K.; Misra, M. Upcycling of Plastic Wastes and Biomass for Sustainable Graphitic Carbon Production: A Critical Review. ACS Environ. Au 2022, 2, 510–522. [Google Scholar] [CrossRef]
- Gai, L.; Li, J.; Wang, Q.; Tian, R.; Li, K. Evolution of biomass to porous graphite carbon by catalytic graphitization. J. Environ. Chem. Eng. 2021, 9, 106678. [Google Scholar] [CrossRef]
- Destyorini, F.; Irmawati, Y.; Hardiansyah, A.; Widodo, H.; Yahya, I.N.D.; Indayaningsih, N.; Yudianti, R.; Hsu, Y.-I.; Uyama, H. Formation of nanostructured graphitic carbon from coconut waste via low-temperature catalytic graphitisation. Eng. Sci. Technol. Int. J. 2020, 24, 514–523. [Google Scholar] [CrossRef]
- Demir, M.; Kahveci, Z.; Aksoy, B.; Palapati, N.K.R.; Subramanian, A.; Cullinan, H.T.; El-Kaderi, H.M.; Harris, C.T.; Gupta, R.B. Graphitic Biocarbon from Metal-Catalyzed Hydrothermal Carbonization of Lignin. Ind. Eng. Chem. Res. 2015, 54, 10731–10739. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.; Liu, S.; Wang, Y.; Qiu, J. Sustainable Synthesis and Assembly of Biomass-Derived B/N Co-Doped Carbon Nanosheets with Ultrahigh Aspect Ratio for High-Performance Supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. [Google Scholar] [CrossRef]
- Ning, H.; Zhang, Z.; Shi, C.; Ma, X.; Li, J.; Zhu, H.; Hu, J. Fe/N codoped porous graphitic carbon derived from macadamia shells as an efficient cathode oxygen reduction catalyst in microbial fuel cells. RSC Adv. 2022, 12, 30145–30156. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, H.; Xu, Z.; Zhang, H.; Ding, L.; Wang, S.; Zhang, G.; Hou, H.; Xu, W.; Yang, F.; et al. Comparison of the heteroatoms-doped biomass-derived carbon prepared by one-step nitrogen-containing activator for high performance supercapacitor. Diam. Relat. Mater. 2021, 114, 108316. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, X.; Zhan, J.; Sun, W.; Si, Z.; Chen, H. Biomass-Based Nitrogen-Doped Hollow Carbon Nanospheres Derived Directly from Glucose and Glucosamine: Structural Evolution and Supercapacitor Properties. ACS Sustain. Chem. Eng. 2018, 6, 7380–7389. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.; Yu, C.; Zhong, W. Facile synthesis of high nitrogen-doped content, mesopore-dominated biomass-derived hierarchical porous graphitic carbon for high performance supercapacitors. Electrochim. Acta 2020, 334, 135615. [Google Scholar] [CrossRef]
- Xia, L.; Huang, H.; Fan, Z.; Hu, D.; Zhang, D.; Khan, A.S.; Usman, M.; Pan, L. Hierarchical macro-/meso-/microporous oxygen-doped carbon derived from sodium alginate: A cost-effective biomass material for binder-free supercapacitors. Mater. Des. 2019, 182, 108048. [Google Scholar] [CrossRef]
- Yan, H.; Li, Y.; Guo, X.; Zhou, M.; Wang, H.-Q.; Dai, Y.; Zheng, J.-C. Synergistic Supercritical Water ‘Wet’ Activated Biomass Carbon as High Performances Electrode Materials for Supercapacitor. J. Electrochem. Soc. 2018, 165, A2075–A2083. [Google Scholar] [CrossRef]
- Gelfond, J.; Meng, T.; Li, S.; Li, T.; Hu, L. Highly electrically conductive biomass-derived carbon fibers for permanent carbon sequestration. Sustain. Mater. Technol. 2023, 35, e00573. [Google Scholar] [CrossRef]
- Jiang, F.; Yao, Y.; Natarajan, B.; Yang, C.; Gao, T.; Xie, H.; Wang, Y.; Xu, L.; Chen, Y.; Gilman, J.; et al. Ultrahigh-temperature conversion of biomass to highly conductive graphitic carbon. Carbon 2019, 144, 241–248. [Google Scholar] [CrossRef]
- Mohamed, A.; Dong, S.; Elhefnawey, M.; Dong, G.; Gao, Y.; Zhu, K.; Cao, D. A comparison of the electrochemical performance of graphitized coal prepared by high-temperature heating and flash Joule heating as an anode material for lithium and potassium ion batteries. Chem. Phys. Lett. 2023, 815, 140362. [Google Scholar] [CrossRef]
- Hou, S.; Cheng, W.; Guo, F. Fast joule-heating synthesized heteroatom-doped carbon and its impressive electrochemical performance. Sustain. Mater. Technol. 2023, 35, e00570. [Google Scholar] [CrossRef]
- Poornima, B.; Vijayakumar, T. Hydrothermal synthesis of Boron -doped porous carbon from Azadirachta Indica wood for supercapacitor application. Inorg. Chem. Commun. 2022, 145, 109953. [Google Scholar] [CrossRef]
- Dong, S.; Song, Y.; Ye, K.; Yan, J.; Wang, G.; Zhu, K.; Cao, D. Ultra-fast, low-cost, and green regeneration of graphite anode using flash joule heating method. EcoMat 2022, 4, e12212. [Google Scholar] [CrossRef]
- Xu, M.; Xing, L.; Zhang, Q.; Pu, J. Ultrasonic-assisted Method of Graphite Preparation from Wheat Straw. Bioresources 2017, 12, 6405–6417. [Google Scholar] [CrossRef]
- Teng, Z.; Han, K.; Li, J.; Gao, Y.; Li, M.; Ji, T. Ultrasonic-assisted preparation and characterization of hierarchical porous carbon derived from garlic peel for high-performance supercapacitors. Ultrason. Sonochem. 2020, 60, 104756. [Google Scholar] [CrossRef]
- Bora, M.; Benoy, S.M.; Tamuly, J.; Saikia, B.K. Ultrasonic-assisted chemical synthesis of activated carbon from low-quality subbituminous coal and its preliminary evaluation towards supercapacitor applications. J. Environ. Chem. Eng. 2021, 9, 104986. [Google Scholar] [CrossRef]
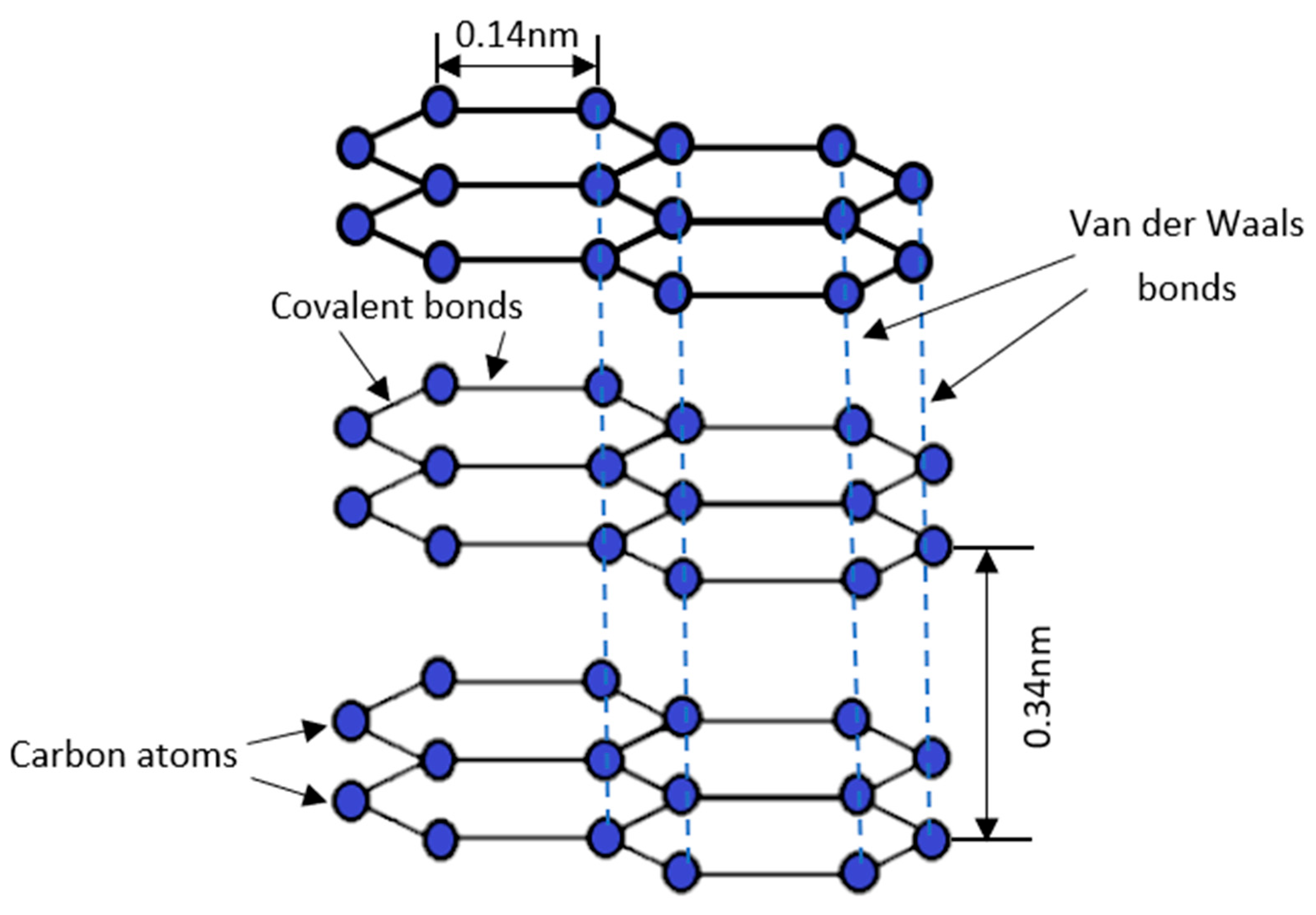
| Agricultural Waste | Proximate Analysis (% w/w) | Ultimate Analysis (% w/w) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture Content | Ash Content | Volatile Content | C | H | N | O | S | ||
| Coconut shell | 12.55 | 0.42 | NA | 51.60 | 5.60 | 0.10 | 42.70 | 0.00 | [78] |
| Macadamia nut shell | 10.00 | 0.40 | 71.0 | 57.50 | 5.95 | 0.33 | 36.20 | 0.06 | [79] |
| Coconut coir | 9.50 | 9.30 | 69.80 | 42.00 | 4.85 | 0.42 | 40.50 | 0.13 | [80] |
| Kenaf | 8.35 | 16.32 | 64.20 | 39.20 | 5.12 | 0.35 | 45.60 | 0.22 | [80] |
| Rice husk | 9.40 | 13.20 | 62.00 | 37.80 | 4.73 | 0.45 | 43.50 | 0.17 | [80] |
| Oil palm frond | 6.00 | 1.00 | 76.00 | 42.88 | 7.06 | 0.52 | 49.54 | NA | [81] |
| Palm kernel shell | 8.44 | 8.79 | 82.58 | 50.42 | 9.74 | 0.52 | 39.32 | NA | [81] |
| Chinese chestnut shell | NA | 0.00 | 73.23 | 46.68 | 5.94 | 0.62 | 46.76 | NA | [82] |
| Bamboo | NA | 1.02 | 82.08 | 54.30 | 5.57 | 0.21 | 38.91 | NA | [82] |
| Jatropha shell | NA | 6.69 | 70.94 | 47.35 | 5.88 | 1.00 | 39.08 | NA | [82] |
| Cotton stalk | NA | 3.14 | 80.54 | 47.12 | 6.25 | 0.57 | 42.79 | NA | [82] |
| Saw dust | NA | 0.42 | 85.14 | 50.64 | 6.00 | 0.07 | 43.29 | NA | [83] |
| Raw straw | NA | 9.98 | 74.39 | 50.01 | 5.54 | 0.81 | 43.64 | NA | [83] |
| Apricot kernel shell | 9.71 | 0.94 | 73.84 | 46.88 | 6.38 | 0.25 | 45.45 | 0.00 | [84] |
| Macadamia nut shell | NA | 2.51 | 77.68 | 49.15 | 5.51 | 0.59 | 42.12 | 0.12 | [85] |
| Biomass Material | Carbonization Temperature (°C) | Raw Carbon Content (wt%) | Highest Carbon Content after Pyrolysis (wt%) | Reference |
|---|---|---|---|---|
| Pulp and paper biological sludge | 525 | NA | 47.00 | [101] |
| Citrus peels | 500 | 50.85 | 81.80 | [102] |
| Douglas fir sawdust | 500 | 47.70 | 82.76 | [103] |
| Wheat straw | 500 | 43.23 | 67.66 | [104] |
| Spent coffee ground | 500 | 46.97 | 76.78 | [104] |
| Brewery grains | 500 | 48.80 | 70.86 | [104] |
| Rice husk | 600 | 42.50 | 58.60 | [105] |
| Bamboo | 700 | 48.13 | 86.34 | [106] |
| Palm kernel shell | 850 | 50.73 | 77.43 | [107] |
| Wheat straw | 850 | 43.00 | 55.83 | [107] |
| Pine sawdust | 800 | 46.73 | 91.73 | [107] |
| Biomass Material | Synthesis Method | Media | Temperature | Results | Ref. |
|---|---|---|---|---|---|
| Bamboo | Direct heating (catalyst and temperature) | Fe(NO3)3 Co(NO3)2 Ni(NO3)2 | 550–1300 °C 1 h |
| [37] |
| Pine sawdust | Direct heating (catalyst and temperature) | Fe(NO3)3 | 600–800 °C Nitrogen atmosphere |
| [38] |
| Coal | Direct heating (catalyst and temperature) | NiCl2 | 1400 °C 10 h Argon atmosphere |
| [121] |
| Palm kernel shell | Direct heating (catalyst and temperature) | Fe(NO3)3 ·9H2O H12N2NiO12 Fe(NO3) ·6H2O | 800 °C, 1000 °C Nitrogen atmosphere |
| [25] |
| Lignin | Direct heating (catalyst and temperature) | Fe(NO3)3 Co(NO3)2 Mn(NO3)2 | 900–1100 °C 3 h Argon gas at 15 psi |
| [111] |
| Pomelo peel | Direct heating (activated agent) | KOH C4H6NiO4 | 800 °C 4 h Argon atmosphere |
| [39] |
| Dandelion flower stem | Direct heating (activated agent) | K2FeO4 | 700–900 °C 2 h Nitrogen atmosphere |
| [40] |
| Bamboo | Direct heating (activated agent) | K2FeO4 | 800 °C 2 h Argon atmosphere |
| [41] |
| Camellia pollen | Direct heating (activated agent) | NH4Cl, (NH4)2CO3 urea | 800 °C 2 h |
| [114] |
| Pistachio nut shell | Direct heating (activated agent) | KOH | 900 °C 2 h |
| [118] |
| Bamboo | Joule heating | - | ~2000 °C 20–30 s Argon atmosphere |
| [119] |
| GO/lignin film | Joule heating | - | ~2500 K 1 h Argon atmosphere |
| [120] |
| Coal | Joule heating | NiCl2 | 300 V 5 s Vacuum atmosphere |
| [121] |
| Carbon Fiber Cloth (CFC) | Joule heating | - | 1.0–6.0 V 5.0–15.0 min |
| [122] |
| Used lithium battery graphite | Joule heating | - | 200 V |
| [123] |
| Wheat straw | Ultrasonic assisted | Ammonia (5%) Sodium dodecysuphate (0.5%) Silicate (3.5%) Magnesium sulfate (1.5%) | 16 kHz to 20 kHz 30 min, thrice |
| [125] |
| Garlic shell | Ultrasonic assisted | KOH | Impregnated at 40 kHz 500 W Heated at 800 °C, 2 h with inert gas flow |
| [126] |
| Low- quality sub-bituminous coals | Ultrasonic assisted | KOH and NaOH | 20–40 kHz 5–6 h Heated at 800 °C, 2 h |
| [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, Y.W.; Mahmed, N.; Norizan, M.N.; Abd Rahim, S.Z.; Ahmad Salimi, M.N.; Abdul Razak, K.; Mohamad, I.S.; Abdullah, M.M.A.-B.; Mohamad Yunus, M.Y. Recent Advances in Synthesis of Graphite from Agricultural Bio-Waste Material: A Review. Materials 2023, 16, 3601. https://doi.org/10.3390/ma16093601
Yap YW, Mahmed N, Norizan MN, Abd Rahim SZ, Ahmad Salimi MN, Abdul Razak K, Mohamad IS, Abdullah MMA-B, Mohamad Yunus MY. Recent Advances in Synthesis of Graphite from Agricultural Bio-Waste Material: A Review. Materials. 2023; 16(9):3601. https://doi.org/10.3390/ma16093601
Chicago/Turabian StyleYap, Yee Wen, Norsuria Mahmed, Mohd Natashah Norizan, Shayfull Zamree Abd Rahim, Midhat Nabil Ahmad Salimi, Kamrosni Abdul Razak, Ili Salwani Mohamad, Mohd Mustafa Al-Bakri Abdullah, and Mohd Yusry Mohamad Yunus. 2023. "Recent Advances in Synthesis of Graphite from Agricultural Bio-Waste Material: A Review" Materials 16, no. 9: 3601. https://doi.org/10.3390/ma16093601







