Developing Improved Corrosion-Resistant AA5083—BN/WC Composites for Tribological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. AA5083-WC and AA5083-BN Composites
2.2. Electrochemical Techniques
3. Results
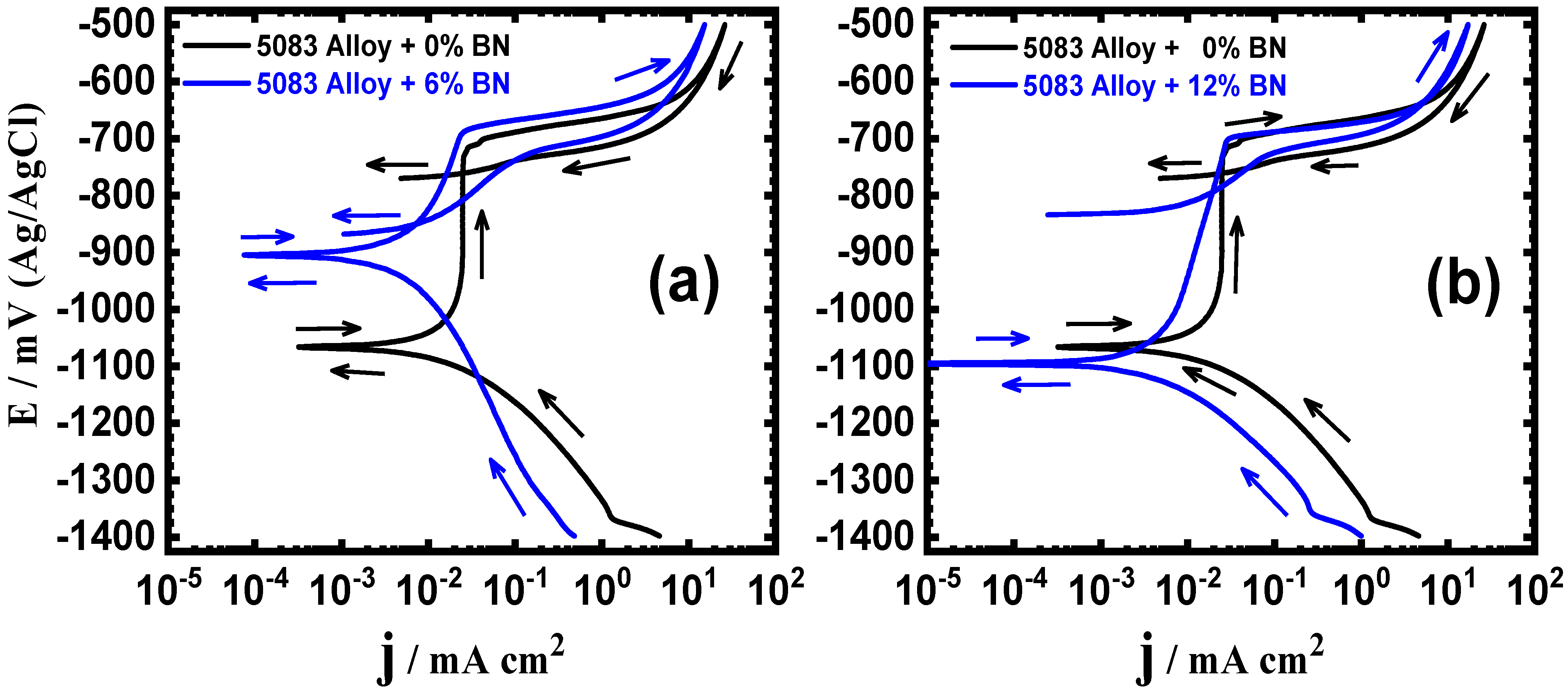
3.1. Cyclic Potentiodynamic Polarization (CPP) Results
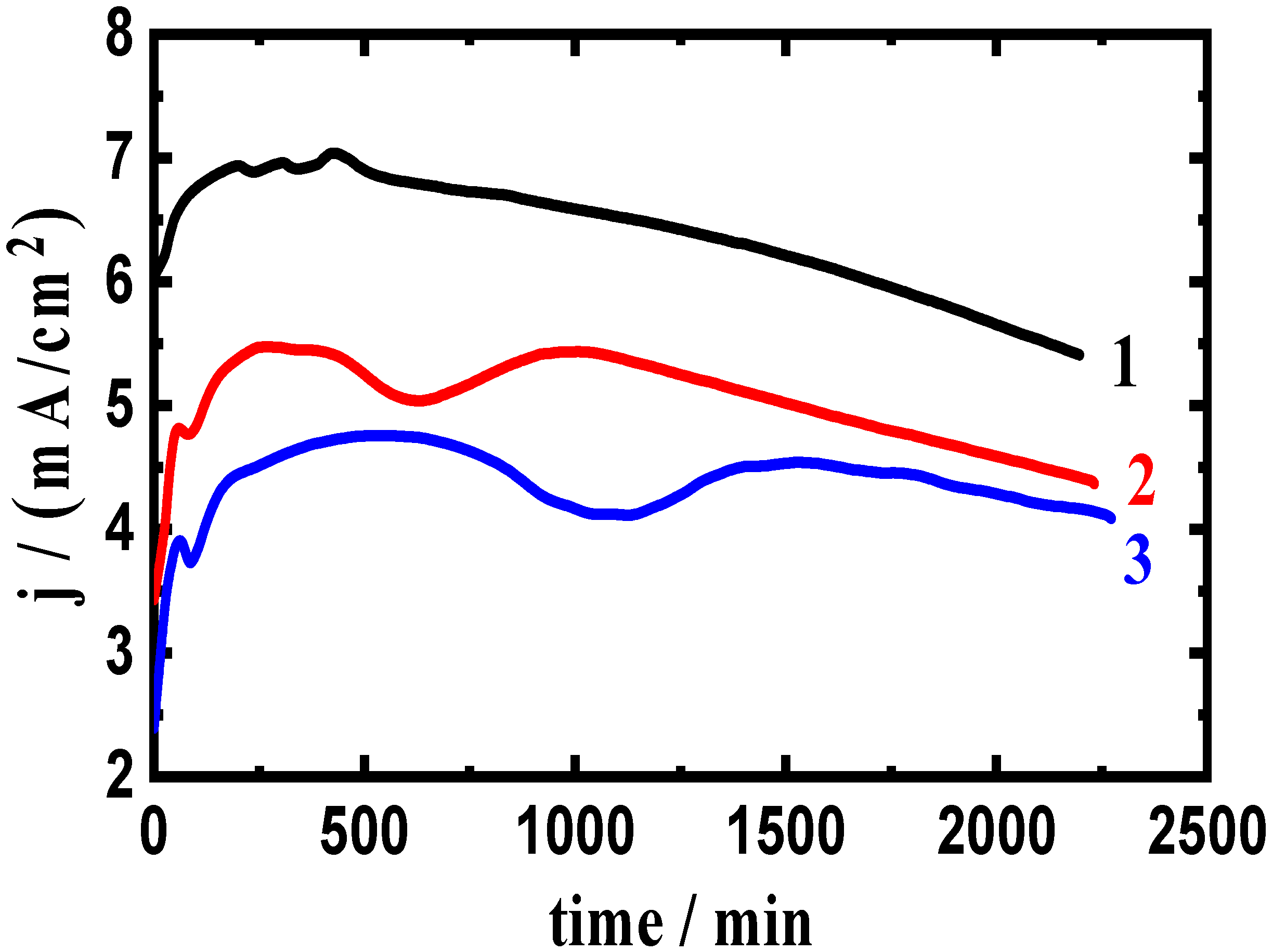
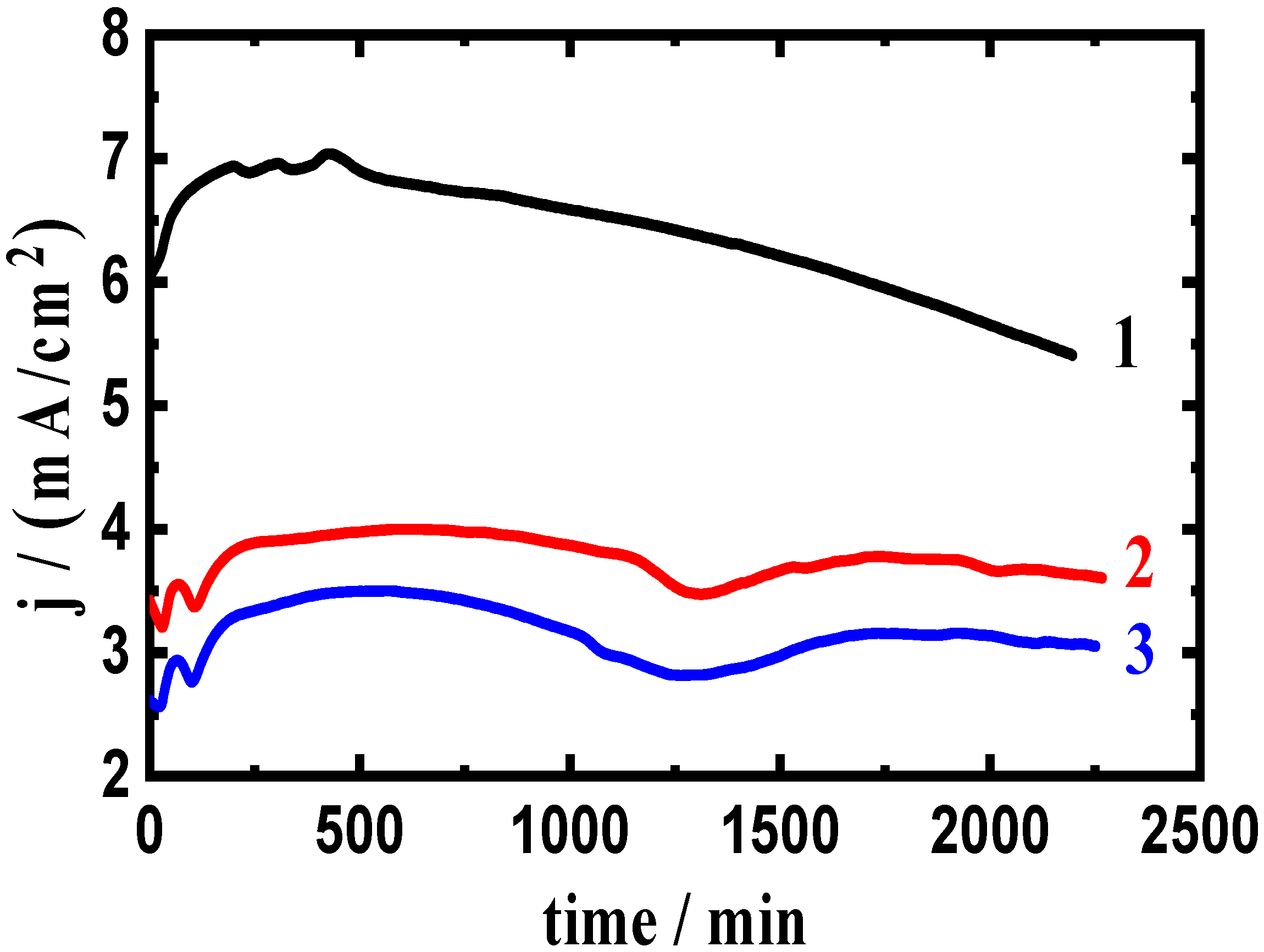
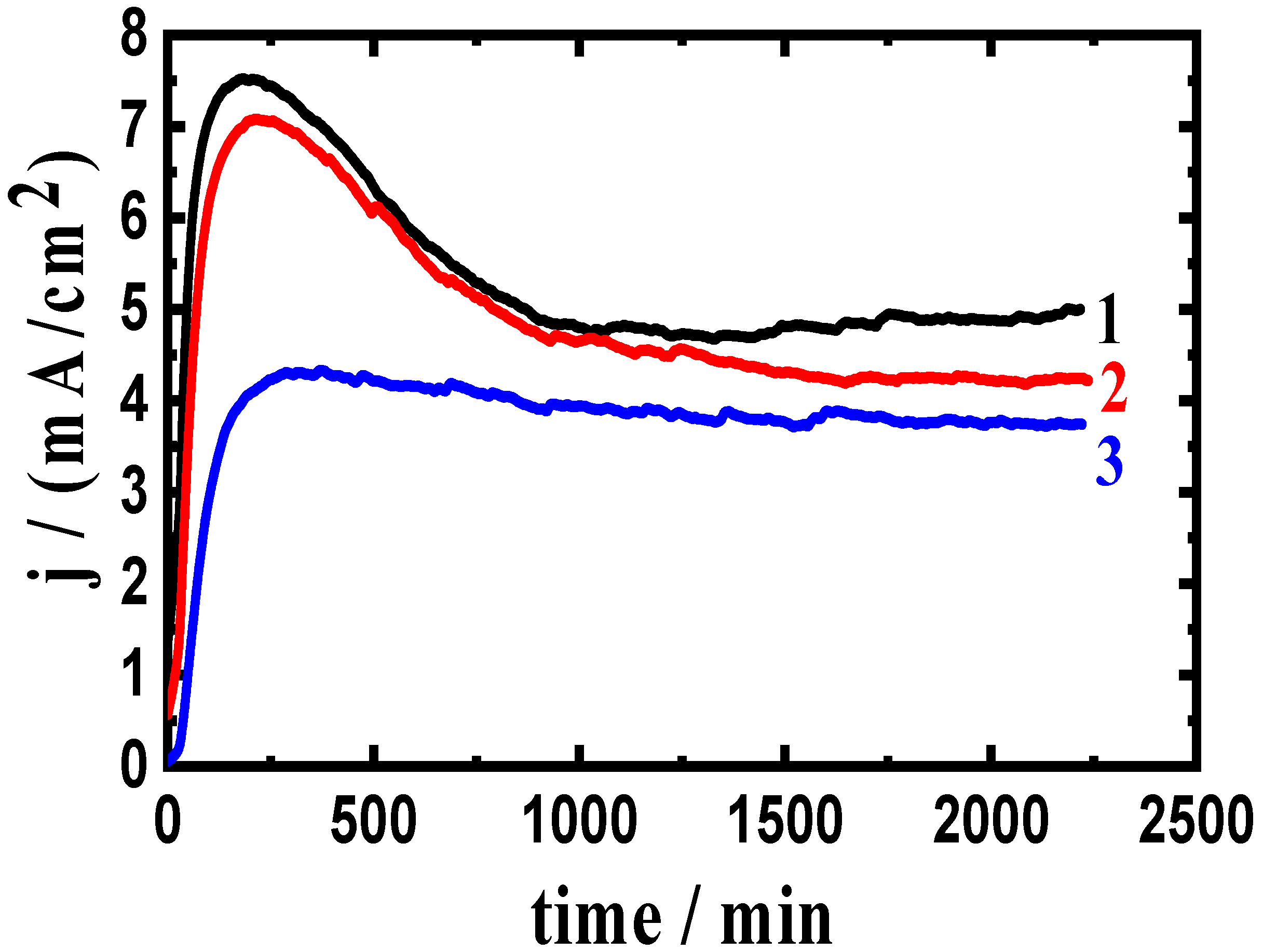
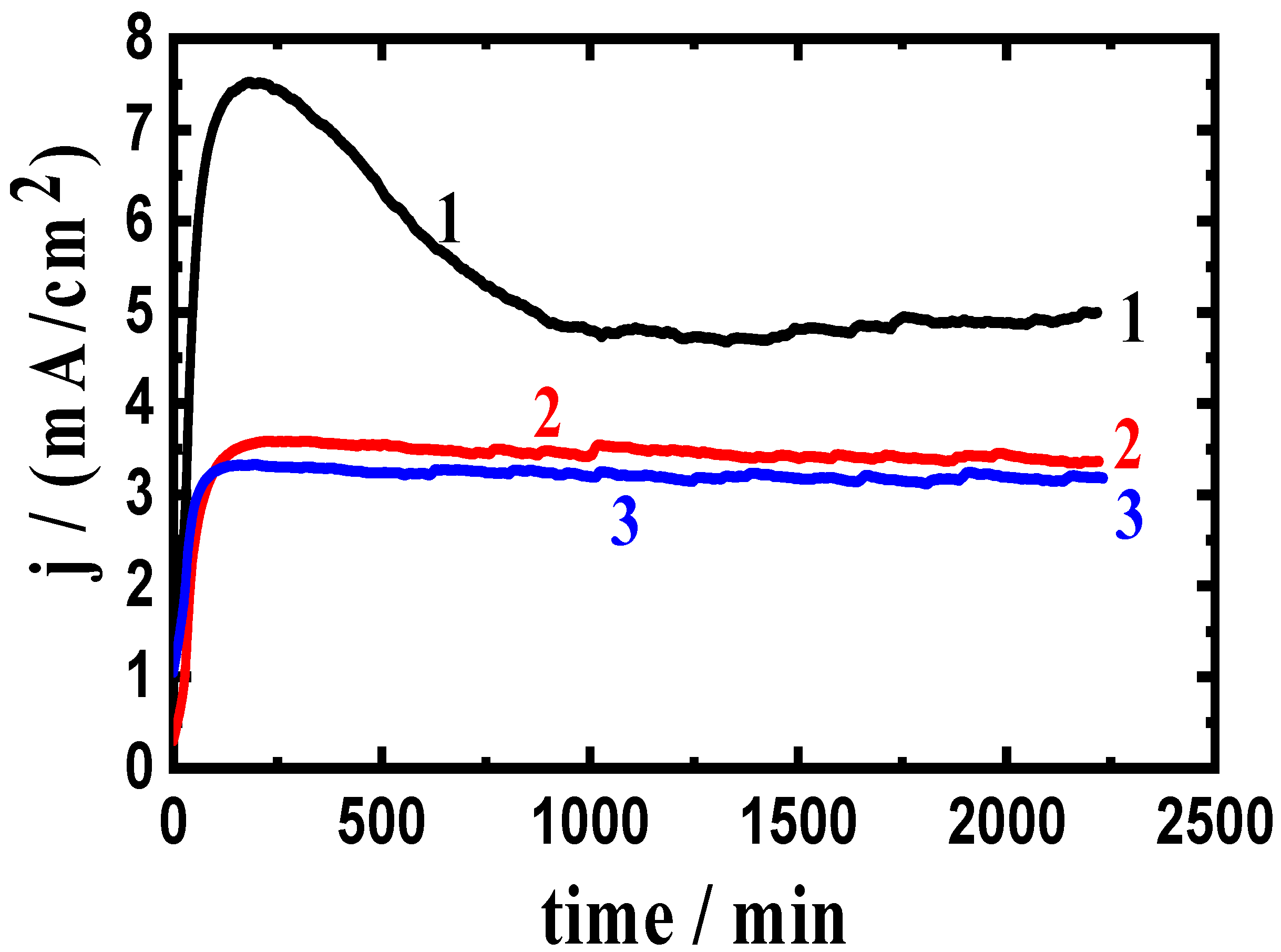
3.2. Change of Current with Time Results
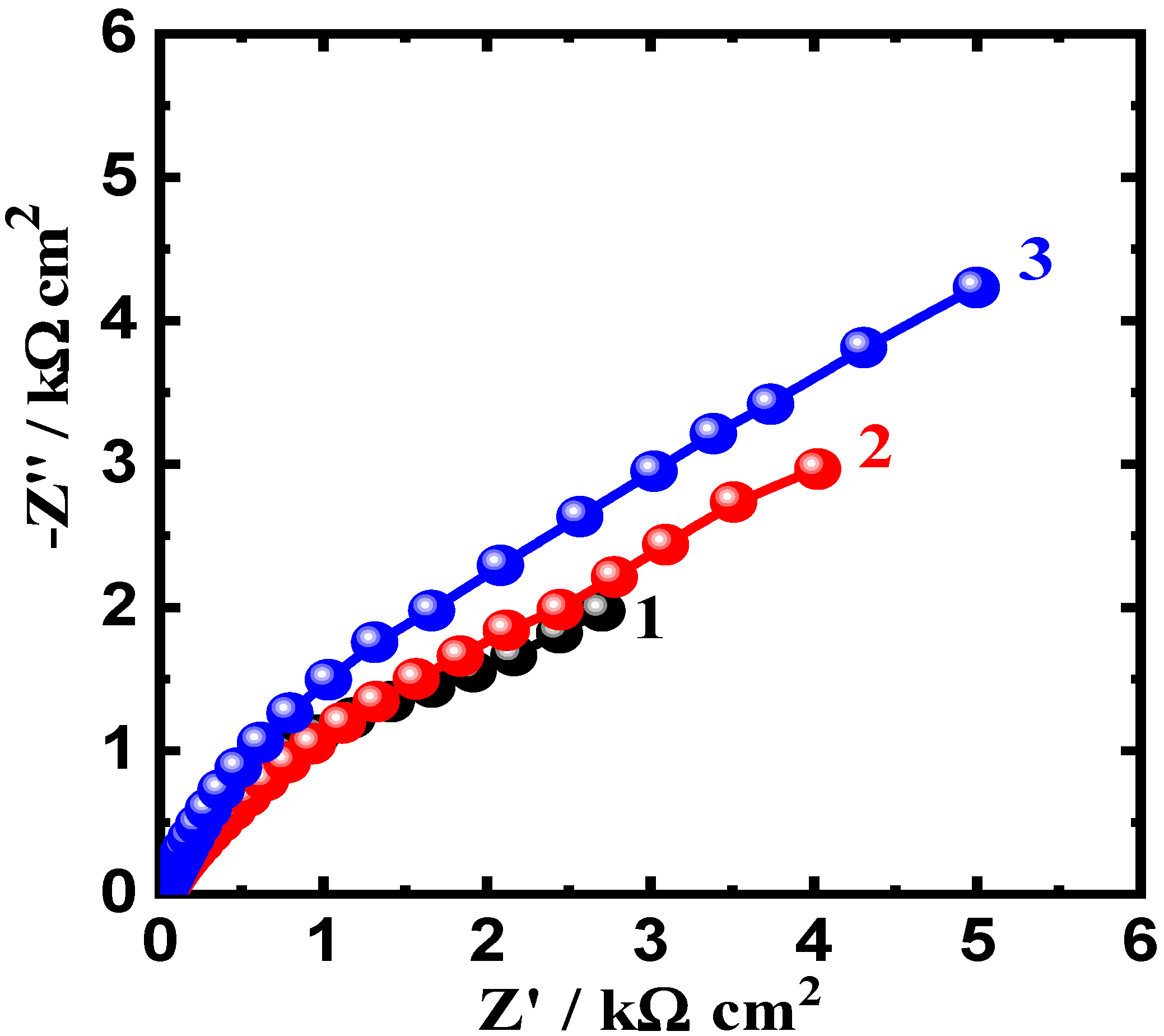
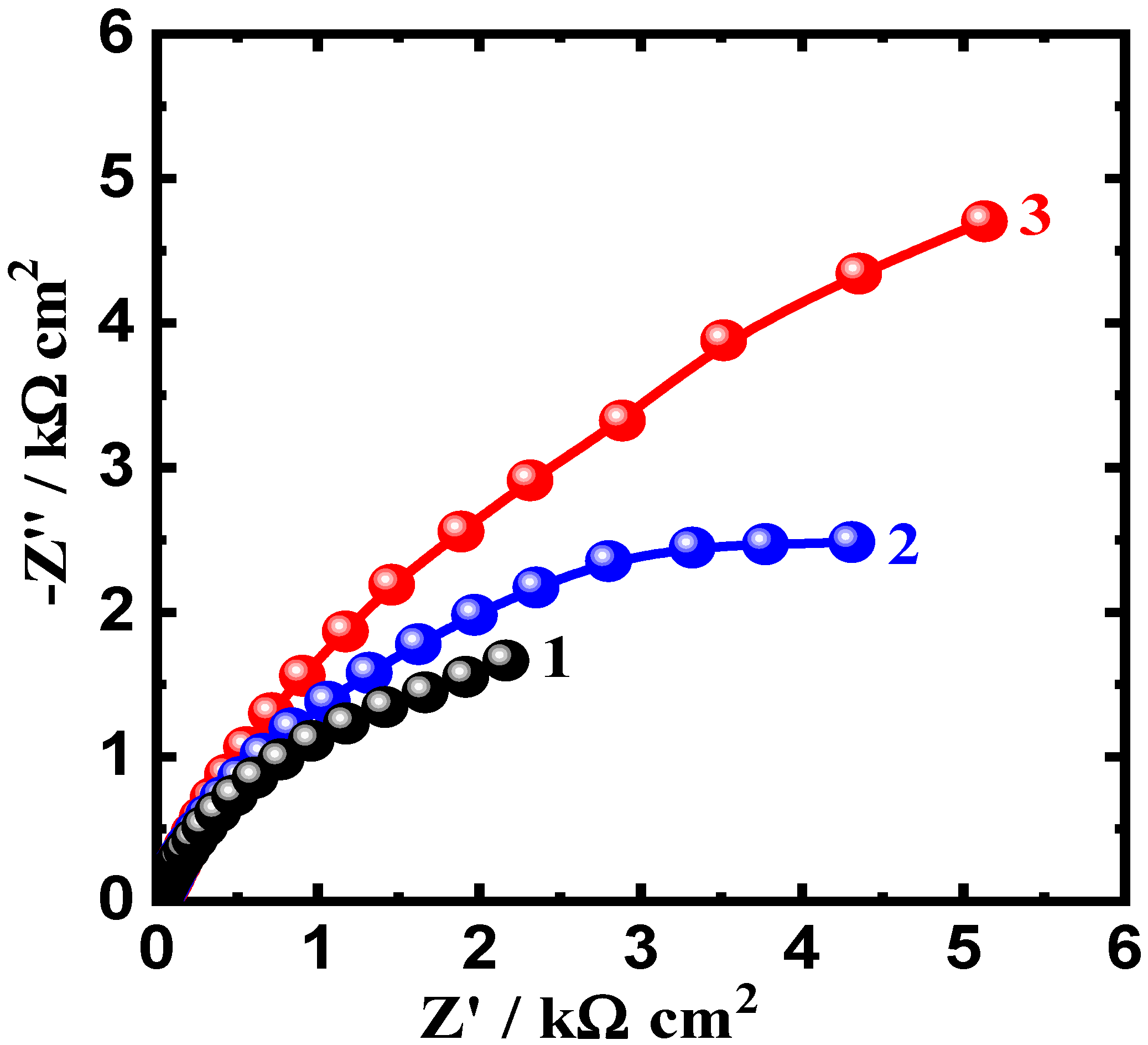
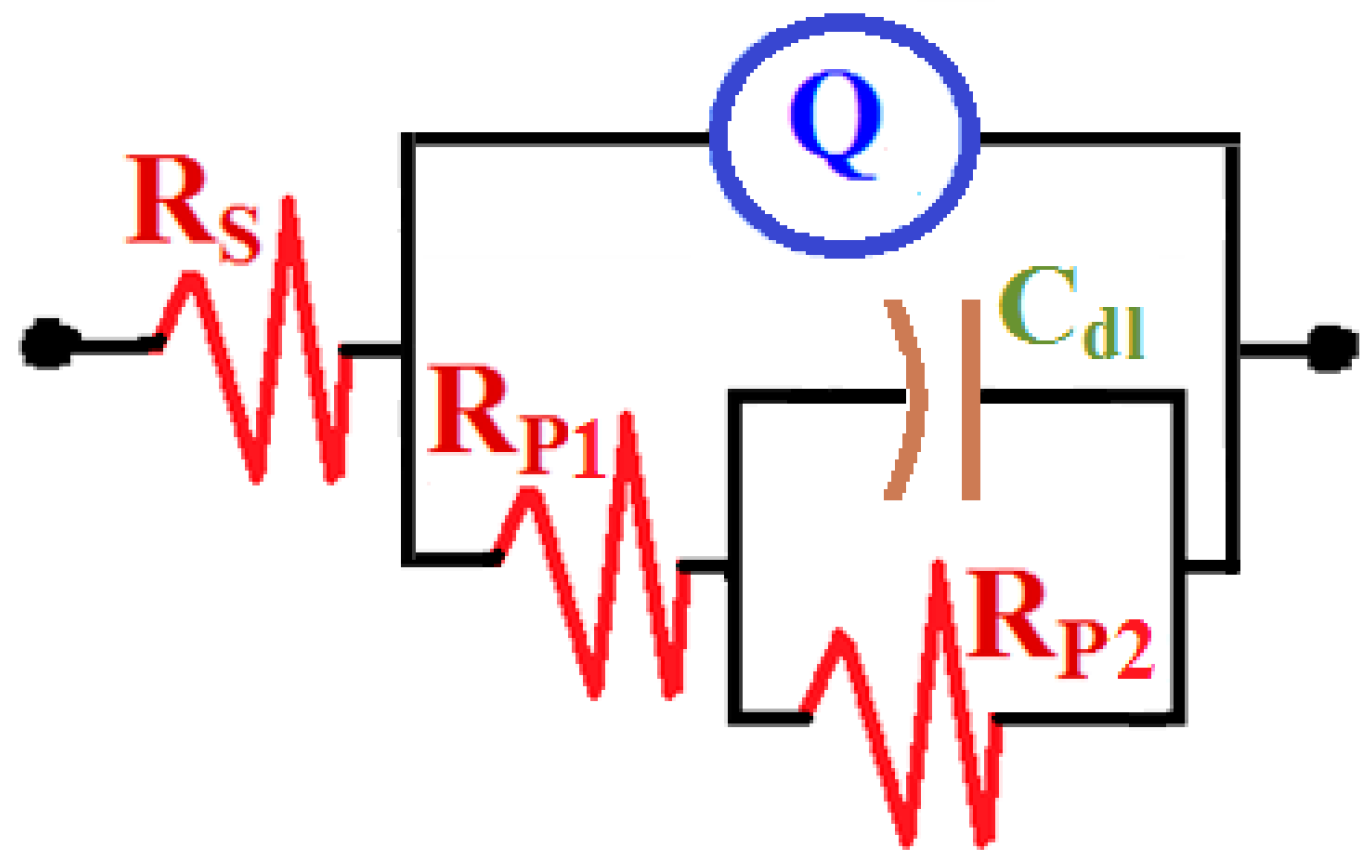
3.3. Electrochemical Impedance Spectroscopy (EIS) Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varshney, D.; Kumar, K. Application and use of different aluminium alloys with respect to workability, strength and welding parameter optimization. Ain Shams Eng. J. 2021, 12, 1143–1152. [Google Scholar] [CrossRef]
- Badawy, W.; Al-Kharafi, F.; El-Azab, A. Electrochemical behaviour and corrosion inhibition of Al, Al-6061 and Al–Cu in neutral aqueous solutions. Corros. Sci. 1999, 41, 709–727. [Google Scholar] [CrossRef]
- Mazhar, A.; Badawy, W.; Abou-Romia, M. Impedance studies of corrosion resistance of aluminium in chloride media. Surf. Coat. Technol. 1986, 29, 335–345. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Du, M.; Hou, J.; Peng, W.; Lin, C. Corrosion Mechanism of 5083 Aluminum Alloy in Seawater Containing Phosphate. J. Ocean Univ. China 2021, 20, 372–382. [Google Scholar] [CrossRef]
- Theivaprakasam, S.; Girard, G.; Howlett, P.; Forsyth, M.; Mitra, S.; MacFarlane, D. Passivation behaviour of aluminium current collector in ionic liquid alkyl carbonate (hybrid) electrolytes. NPJ Mater. Degrad. 2018, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Somers, A.; Howlett, P.C.; Forsyth, M. Film formation in trihexyl(tetradecyl)phosphonium diphenylphosphate ([P6,6,6,14][dpp]) ionic liquid on AA5083 aluminium alloy. Surf. Coat. Technol. 2016, 303, 385–395. [Google Scholar] [CrossRef]
- Nam, N.D.; Hung, T.V.; Ngan, D.T.; Hung, N.L.T.; Hoi, T.K.N. Film formation in Y(4NO2Cin)3 compound on 6061 aluminum alloy to protect against corrosion in chloride ion media. J. Taiwan Inst. Chem. Eng. 2016, 67, 495–504. [Google Scholar] [CrossRef]
- Zazi, N.; Chopart, J. Dissolution of Ag/AgCl reference electrode and deposition of silver onto the surface of 5083 H321 aluminum alloy, during corrosion in 3wt% NaCl solution at rest potential. Prot. Met. Phys. Chem. Surf. 2017, 53, 1114–1119. [Google Scholar] [CrossRef]
- Kaoru, M.; Anders, N.; Ingemar, O. Surface reactions during pickling of an aluminium-magnesium-silicon alloy in phosphoric acid. Corros. Sci. 2001, 43, 381–396. [Google Scholar]
- Nam, N.D.; Phung, V.D.; Thuy, P.T.P.; Dao, V.A.; Kim, S.H.; Yi, J.S. Corrosion behaviours of hot-extruded AlxMg alloys. J. Mater. Res. Technol. 2019, 8, 5246–5253. [Google Scholar] [CrossRef]
- Bach, L.X.; Son, D.L.; Phong, M.T.; Thang, L.V.; Bian, M.Z.; Nam, N.D. A study on Mg and ALN composite in microstructural and electrochemical characterizations of extruded aluminum alloy. Comp. B Eng. 2019, 156, 332–343. [Google Scholar] [CrossRef]
- Poovazhagan, L.; Kalaichelvan, K.; Sornakumar, T. Processing and performance characteristics of aluminum-nano boron carbide metal matrix nanocomposites. Mater. Manuf. Process. 2016, 31, 1275–1285. [Google Scholar] [CrossRef]
- Barenji, R.V.; Khojastehnezhad, V.M.; Pourasl, H.H.; Rabiezadeh, A. Wear properties of Al-Al2O3/TiB2 surface hybrid composite layer prepared by friction stir process. J. Comp. Mater. 2016, 50, 1457–1466. [Google Scholar] [CrossRef]
- Mirjavadi, S.S.; Alipour, M.; Emamian, S.; Kord, S.; Hamouda, A.M.S.; Koppad, P.G.; Keshavamurthy, R. Influence of TiO2 nanoparticles incorporation to friction stir welded 5083 aluminum alloy on the microstructure, mechanical properties and wear resistance. J. Alloys Compud. 2017, 712, 795–803. [Google Scholar] [CrossRef]
- Reddy, A.P.; Krishna, P.V.; Rao, R.N. Tribological behaviour of Al6061-2SiC-xGr hybrid metal matrix nanocomposites fabricated through ultrasonically assisted stir casting technique. Silicon 2019, 11, 2853–2871. [Google Scholar] [CrossRef]
- Jeyasimman, D.; Narayanasamy, R.; Ponalagusamy, R.; Anandakrishnan, V.; Kamaraj, M. The effects of various reinforcements on dry sliding wear behaviour of AA 6061 nanocomposites. Mater. Des. 2014, 64, 783–793. [Google Scholar] [CrossRef]
- Mirjavadi, S.S.; Alipour, M.; Hamouda, A.M.S.; Matin, A.; Kord, S.; Afshari, B.M.; Koppad, P.G. Effect of multi-pass friction stir processing on the microstructure, mechanical and wear properties of AA5083/ZrO2 nanocomposites. J. Alloys Compud. 2017, 726, 1262–1273. [Google Scholar] [CrossRef]
- Lekatoua, A.; Karantzalisa, A.E.; Evangeloua, A.; Gousiaa, V.; Kaptayb, G.; Gácsib, Z.; Baumlib, P.; Simon, A. Aluminium reinforced by WC and TiC nanoparticles (ex-situ) and aluminide particles (in-situ): Microstructure, wear and corrosion behaviour. Mater. Des. 2015, 65, 1121–1135. [Google Scholar] [CrossRef]
- Abdullah, H.A. Effect of the Reinforcing by WC on Microhardness, Roughness and Corrosion Behavior of Al-12Si Alloy. Eng. Technol. J. 2016, 34, 2891–2897. [Google Scholar] [CrossRef]
- Lei, B.; Peng, M.; Liu, L.; Hu, S.; Zhang, W.; Meng, G. Galvanic Corrosion Performance of an Al–BN Abradable Seal Coating System in Chloride Solution. Coatings 2021, 11, 9. [Google Scholar] [CrossRef]
- Zang, C.; Yang, M.; Liu, E.; Qian, Q.; Zhen, J.; Zhang, R.; Jia, Z.; Han, W. Synthesis, characterization and tribological behaviors of hexagonal boron nitride/copper nanocomposites as lubricant additives. Tribol. Int. 2022, 165, 107312. [Google Scholar] [CrossRef]
- Bondarev, A.; Fraile, A.; Polcar, T.; Shtansky, D. Mechanisms of friction and wear reduction by h-BN nanosheet and spherical W nanoparticle additives to base oil: Experimental study and molecular dynamics simulation. Tribol. Int. 2020, 151, 106493. [Google Scholar] [CrossRef]
- Mondal, S. Aluminum or Its Alloy Matrix Hybrid Nanocomposites. Met. Mater. Int. 2021, 27, 2188–2204. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, D.; Salameh, C.; Bechelany, M.; Miele, P. Nanostructured boron nitride based materials: Synthesis and applications. Mater. Today Adv. 2020, 8, 100107. [Google Scholar] [CrossRef]
- Harichandran, R.; Selvakumar, N. Microstructure and mechanical characterization of (B4C+h-BN)/Al hybrid nanocomposites processed by ultrasound assisted casting. Int. J. Mech. Sci. 2018, 144, 814–826. [Google Scholar] [CrossRef]
- Mahathanabodee, S.; Palathai, T.; Raadnui, S.; Tongsri, R.; Sombatsompop, N. Effects of hexagonal boron nitride and sintering temperature on mechanical and tribological properties of SS316L/h-BN composites. Mater. Des. 2013, 46, 588–597. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. Effects of exposure time on the anodic dissolution of Monel-400 in aerated stagnant sodium chloride solutions. J. Solid State Electrochem. 2011, 16, 891–899. [Google Scholar] [CrossRef]
- Darwish, N.A.; Hilbert, F.; Lorenz, W.J.; Rosswag, H. The influence of chloride ions on the kinetics of iron dissolution. Electrochim. Acta 1973, 18, 421–425. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim. Acta 2010, 55, 3657–3663. [Google Scholar]
- Sherif, E.-S.M.; Park, S.-M. Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim. Acta 2006, 51, 1313–1321. [Google Scholar] [CrossRef]
- Natishan, P.M.; McCafferty, E.; Hubler, G.K. Surface Charge Considerations in the Pitting of Ion-Implanted Aluminum. J. Electrochem. Soc. 1988, 135, 321–327. [Google Scholar] [CrossRef]
- Hunkeler, F.; Frankel, G.S.; Bohni, H. Technical Note: On the Mechanism of Localized Corrosion. Corrosion 1987, 43, 189–191. [Google Scholar] [CrossRef]
- Latief, F.; Sherif, E.-S.M.; Almajid, A.; Junaedi, H. Fabrication of exfoliated graphite nanoplatelets-reinforced aluminum composites and evaluating their mechanical properties and corrosion behavior. J. Anal. Appl. Pyrolysis 2011, 92, 485–492. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Ragab, S.A.; Abdo, H.S. Role of Vanadium Additions on the Corrosion Mitigation of Ti-6Al-xV Alloy in Simulated Body Fluid. Metals 2020, 10, 903. [Google Scholar] [CrossRef]
- Foley, R.T.; Nguyen, T.H. The Chemical Nature of Aluminum Corrosion: V. Energy Transfer in Aluminum Dissolution. J. Electrochem. Soc. 1982, 129, 464–467. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Bolzoni, F.M.; Ormellese, M.; Pérez-Rosales, E.A.; Pedeferri, M. Characterisation of titanium oxide films by potentiodynamic polarisation and electrochemical impedance spectroscopy. Corros. Eng. Sci. Technol. 2010, 45, 428–434. [Google Scholar] [CrossRef]
- Sato, N. The stability of localized corrosion. Corros. Sci. 1995, 37, 1947–1967. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Abdo, H.S.; Alharthi, N.H. Beneficial Effects of Vanadium Additions on the Corrosion of Ti6AlxV Alloys in Chloride Solutions. Metals 2020, 10, 264. [Google Scholar] [CrossRef] [Green Version]
- Şafak, S.; Duran, B.; Yurt, A.; Türkoğlu, G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012, 51, 251–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Meng, G.; Wei, L.; Zhang, T.; Shao, Y.; Wang, F.; Dong, C.; Li, X. Effect of microcrystallization on pitting corrosion of pure aluminium. Corros. Sci. 2009, 51, 2151–2157. [Google Scholar] [CrossRef]
- Mansfeld, F.; Lin, S.; Kim, S.; Shih, H. Pitting and surface modification of SIC/Al. Corros. Sci. 1987, 27, 997–1000. [Google Scholar] [CrossRef]



| Element | Al | Mg | Mn | Cr | Si | Fe | Cu | Zn | Ti | O |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard (wt.%) | Bal. | 4.0–4.9 | 0.4–1.0 | 0.05–0.25 | ≤0.4 | ≤0.4 | ≤0.4 | ≤0.4 | ≤0.4 | ≤0.4 |
| Actual (wt.%) | Bal. | 4.71 | 0.7 | 0.19 | 0.074 | 0.16 | 0.044 | 0.056 | ≤0.01 | 0.19 |
| Composite | Composition | ||
|---|---|---|---|
| AA5083 | WC | BN | |
| AA5083 alloy matrix | 100 | 0 | 0 |
| AA5083 + 6% WC | 94 | 6 | 0 |
| AA5083 + 12% WC | 88 | 12 | 0 |
| AA5083 + 6% BN | 94 | 0 | 6 |
| AA5083 + 12% BN | 88 | 0 | 12 |
| Composite | βc/ mV·dec−1 | ECorr/ mV | βa/ mV·dec−1 | jCorr/ µA·cm−2 | EProt./ mV | EPit./ mV | RP/ Ω·cm2 | RCorr/ mmpy |
|---|---|---|---|---|---|---|---|---|
| AA5083 alloy matrix | 95 | −1060 | 100 | 8.5 | −760 | −705 | 3492 | 0.2823 |
| AA5083 + 6% WC | 90 | −1035 | 80 | 0.75 | −970 | −708 | 24,552 | 0.0249 |
| AA5083 + 12% WC | 78 | −1055 | 130 | 2.5 | −755 | −698 | 8478 | 0.0830 |
| AA5083 + 6% BN | 120 | −910 | 135 | 1.9 | −840 | −685 | 14,538 | 0.0631 |
| AA5083 + 12% BN | 80 | −1095 | 120 | 2.3 | −800 | −695 | 9078 | 0.0764 |
| Composite | RS/Ωcm2 | Q | RP1/ Ω cm2 | Cdl/ F cm−2 | RP2/ Ω cm2 | |
|---|---|---|---|---|---|---|
| YQ/Fcm−2 | n | |||||
| AA5083 alloy matrix | 7.309 | 0.000233 | 0.68 | 12.83 | 0.0000240 | 7895 |
| AA5083 + 6% WC | 22.53 | 0.000061 | 0.73 | 30.33 | 0.0000020 | 11,580 |
| AA5083 + 12% WC | 16.82 | 0.000121 | 0.80 | 25.86 | 0.0000035 | 8980 |
| AA5083 + 6% BN | 13.28 | 0.000110 | 0.80 | 33.47 | 0.0000027 | 13,240 |
| AA5083+ 12% BN | 9.255 | 0.000149 | 0.80 | 26.15 | 0.0000034 | 9322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, H.R.; Sherif, E.M.; Sivasankaran, S.; Almufadi, F.A.; Mekky, A.-b.H. Developing Improved Corrosion-Resistant AA5083—BN/WC Composites for Tribological Applications. Materials 2023, 16, 1663. https://doi.org/10.3390/ma16041663
Ammar HR, Sherif EM, Sivasankaran S, Almufadi FA, Mekky A-bH. Developing Improved Corrosion-Resistant AA5083—BN/WC Composites for Tribological Applications. Materials. 2023; 16(4):1663. https://doi.org/10.3390/ma16041663
Chicago/Turabian StyleAmmar, Hany R., Elsayed M. Sherif, Subbarayan Sivasankaran, Fahad A. Almufadi, and Abdel-baset H. Mekky. 2023. "Developing Improved Corrosion-Resistant AA5083—BN/WC Composites for Tribological Applications" Materials 16, no. 4: 1663. https://doi.org/10.3390/ma16041663
APA StyleAmmar, H. R., Sherif, E. M., Sivasankaran, S., Almufadi, F. A., & Mekky, A.-b. H. (2023). Developing Improved Corrosion-Resistant AA5083—BN/WC Composites for Tribological Applications. Materials, 16(4), 1663. https://doi.org/10.3390/ma16041663











