An Efficient Laser Decontamination Process Based on Non-Radioactive Specimens of Nuclear Power Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
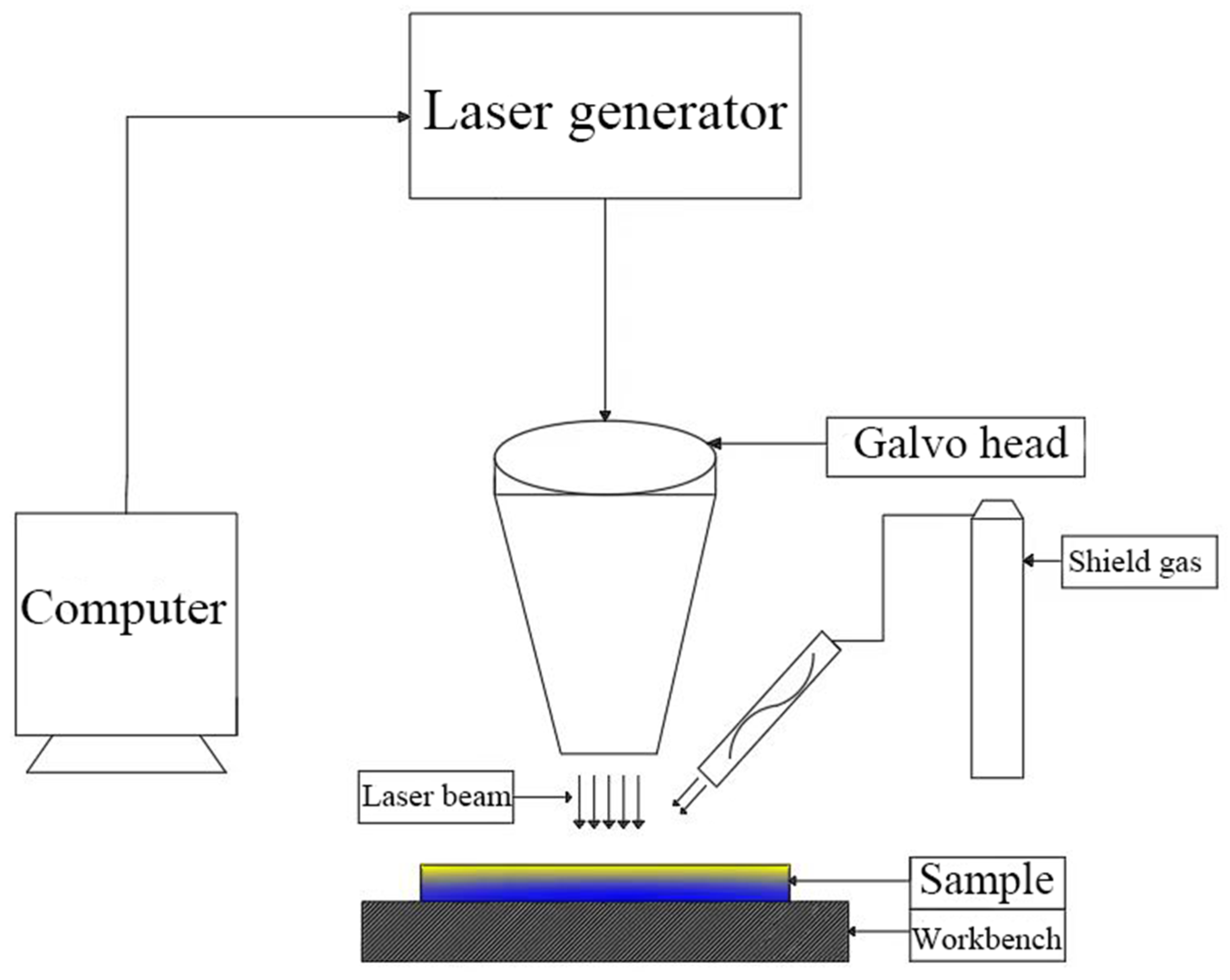
2.2. Equipment
2.3. Methods
2.3.1. Single-Pulse Experimental Method
2.3.2. Experimental Method of Laser Decontamination
2.4. Characterization Methods
3. Results
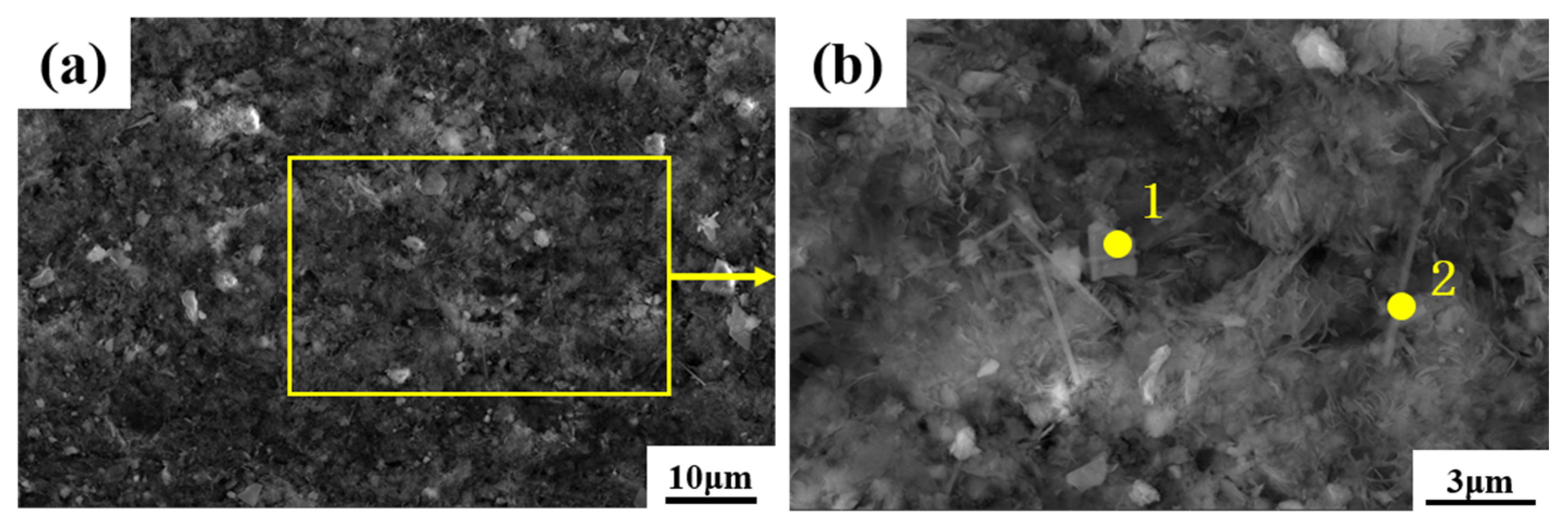
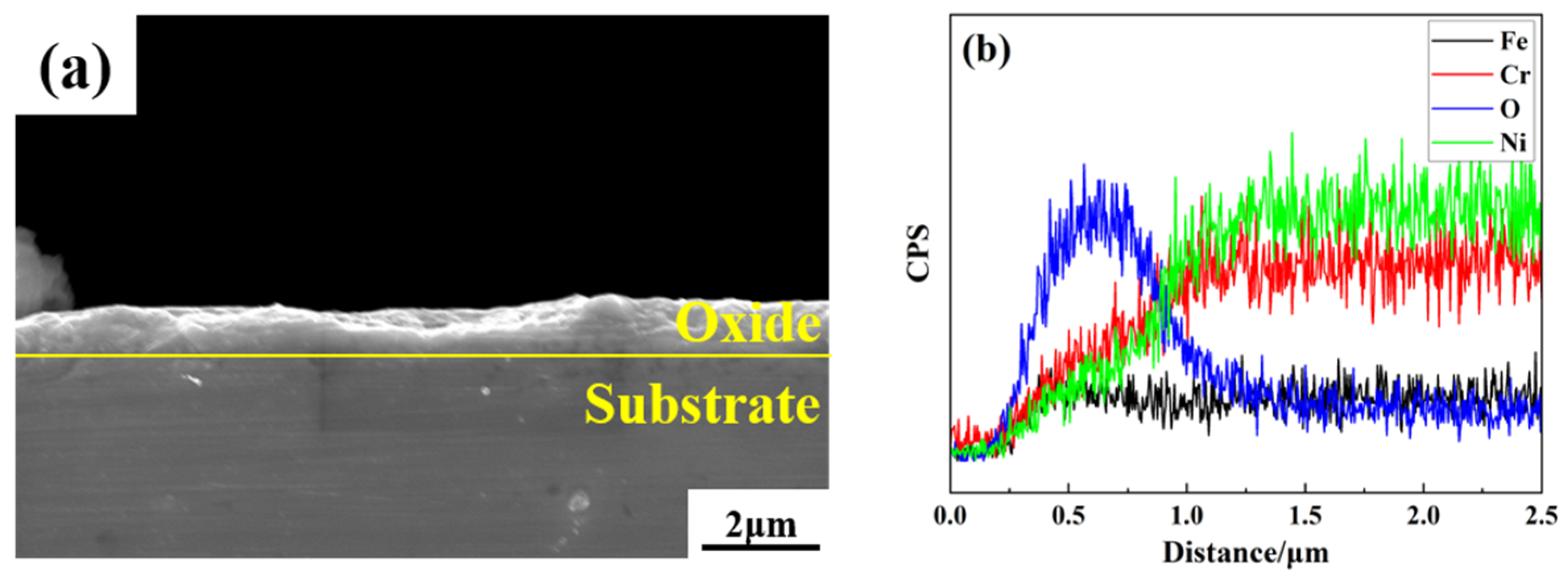
3.1. Characterization of Non-Radioactive Specimen Oxides
3.2. Results of Single-Pulse Experiments
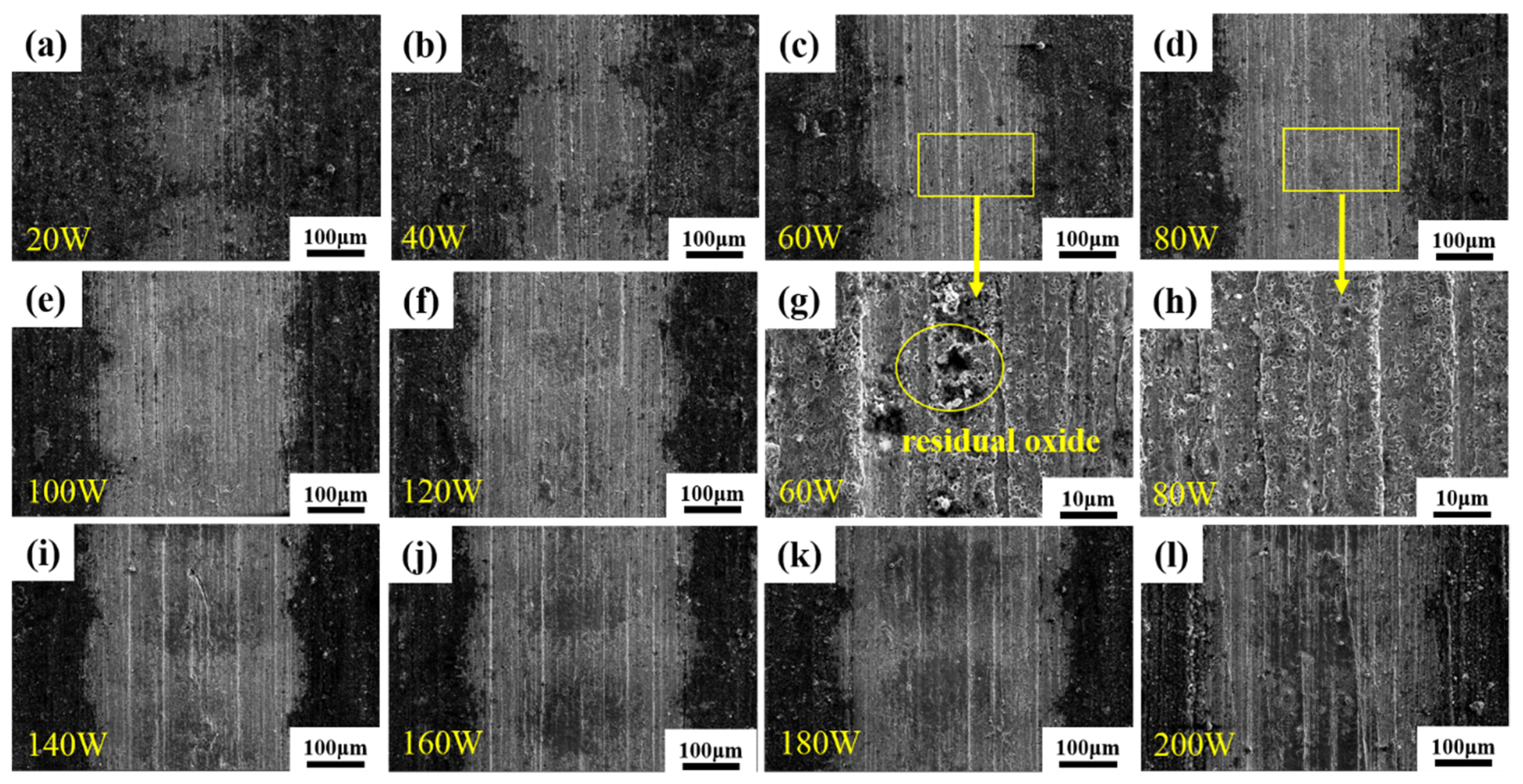
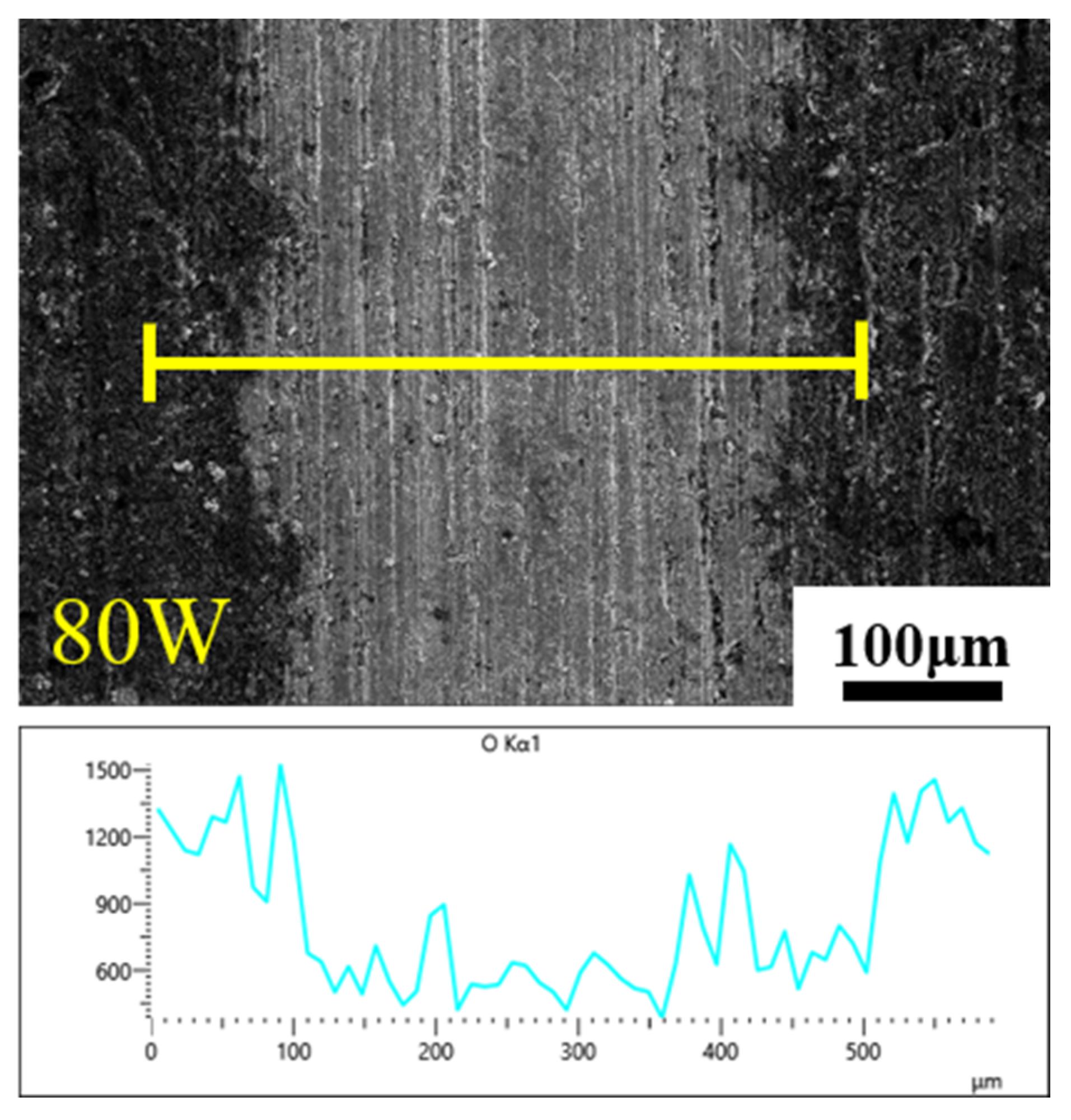
3.3. Surface Morphology after Laser Decontamination
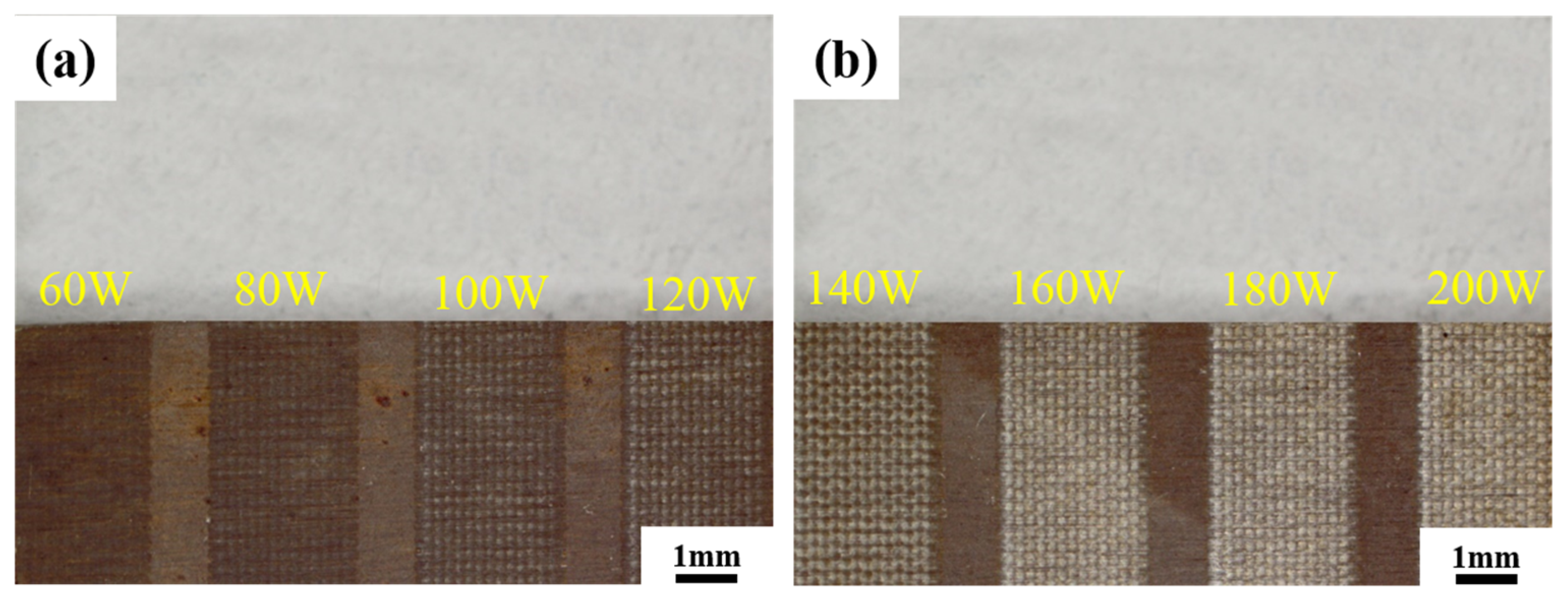
3.3.1. Macroscopic Morphology
3.3.2. Microscopic Morphology
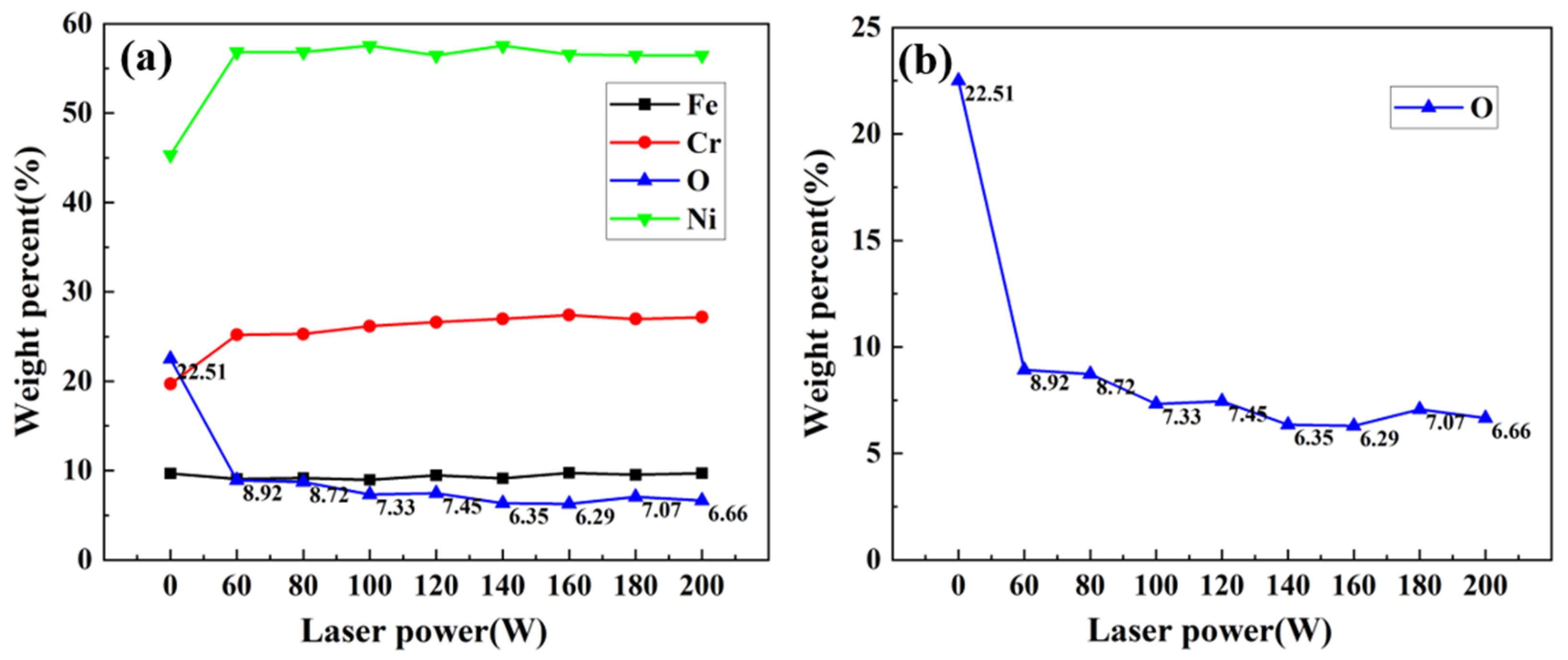
3.4. Elemental Analysis
4. Discussion
5. Conclusions
- Alloy 690 specimens with a boron mass concentration of 1200 mg/L and a lithium mass concentration of 2.2 mg/L, under conditions of a temperature of 300 °C, a pressure of 15.6 MPa, and continuous oxidation for 960 h in a autoclave, produced an oxide layer consisting of polyhedral and acicular particles of nickel–chromium–iron oxides with a thickness of about 1.2 μm, which consisted of two composition phases: spinel and Ni(OH)2.
- The laser pulse width was set as 500 ns, the frequency was 40 kHz, the scanning speed was 15,000 mm/s, and the line spacing was 1 mm for the laser single-pulse experiments. The laser power levels ranged from 20 W to 200 W, corresponding to the laser fluence of 50.93 J/cm2 to 509.3 J/cm2, and changes in the average diameter of the single-laser-pulse area ranged from 205.2 μm to 397.9 μm, which is larger than the diameter of the laser spot. The laser fluence of 203.72 J/cm2, corresponding to a laser power of 80 W, was determined as the oxide removal threshold.
- Keeping the above parameters unchanged, the line spacing was changed to 0.2 mm for the laser decontamination experiments, at which time the decontamination efficiency was 10.8 m2/h. When the laser power was changed from 60 W to 200 W, the surface of the samples became brighter and its color was close to that of the substrate, while the microscopic oxide particles were gradually reduced and the content of the elemental oxygen was gradually reduced from the original 22.51% to 6.29%.
- By comparing the effects of different power levels after decontamination, it was determined that the laser power level of 160 W was the best power level. At this time, the oxides on the surface of the sample were removed, and the oxygen content was 6.29%. The XRD spectrum after decontamination was consistent with that of the original substrate, with a good decontamination effect. It is proved that the high decontamination efficiency of 10.8 m2/h can remove the surface oxides from a non-radioactive Alloy 690 specimen oxidized for 960 h.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.C.; Xie, J.X.; Song, J.; Zhang, X.W. Test of dissolution decontamination of simulated corrosion-oxides in primary loop of PWR. Radiat. Prot. 2003, 1, 42–48, 54. [Google Scholar]
- Kinnunen, P. ANTIOXI–Decontamination Techniques for Activity Removal in Nuclear Environments; VTT Technical Research Centre of Finland: Espoo, Finland, 2008. [Google Scholar]
- Cetnar, J.; Stanisz, P.; Oettingen, M. Linear Chain Method for Numerical Modelling of Burnup Systems. Energies 2021, 14, 1520. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, J.J.; Wang, S.; Wen, X.J.; Zhang, Y.L.; Ding, R.; Peng, J. Study on Laser Decontamination Technology for Metal Scraps with Radioactively Contaminated Surfaces. Nucl. Pow. Eng. 2021, 5, 250–255. [Google Scholar]
- Wang, Q.; Wang, F.; Cai, C.; Chen, H.; Ji, F.; Wen, T.; Liao, D. Laser decontamination for radioactive contaminated metal surface: A review. Nucl. Eng. Technol. 2022, 55, 12–24. [Google Scholar] [CrossRef]
- Kumar, A.; Bhatt, R.B.; Behere, P.G.; Afzal, M. Ultrasonic decontamination of prototype fast breeder reactor fuel pins. Ultrasonics 2014, 54, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.; Gallagher, M.; Choularton, T. The characterisation and removal of water droplets in high pressure water jetting nuclear decontamination (16036). In Proceedings of the Waste Management Conference 2016, Phoenix, AZ, USA, 6–10 March 2016; pp. 1–19. [Google Scholar]
- Li, Y.; Sun, Y.; Zhang, D.; Wang, P.; Li, T.W.; Zhao, D.; Tan, Z.Y. Decontamination research of ventilation pipes by dry ice jet. At. Energy Sci. Technol. 2012, 46, 164–167. [Google Scholar]
- Zhao, P.; Chung, W.; Lee, M.; Ahn, S. Experimental Study on Melt Decontamination of Stainless Steel and Carbon Steel Using Induction Melting. Metals 2021, 11, 1218. [Google Scholar] [CrossRef]
- Garcia, D.; Bruyere, V.I.E.; Bordoni, R.; Olmedo, A.M.; Morando, P.J. Malonic acid: A potential reagent in decontamination processes for Ni-rich alloy surfaces. J. Nucl. Mater. 2011, 412, 72–81. [Google Scholar] [CrossRef]
- Moore, J.J.; Raine, T.P.; Jenkins, A.; Livens, F.R.; Law, K.A.; Morris, K.; Law, G.T.W.; Yeates, S.G. Decontamination of caesium and strontium from stainless steel surfaces using hydrogels. React. Funct. Polym. 2019, 142, 7–14. [Google Scholar] [CrossRef]
- Park, H.; Yoo, H.J.; Park, C. Wear and corrosion behaviors of high-power laser surface-cleaned 304L stainless steel. Opt. Laser Technol. 2024, 168, 109640. [Google Scholar] [CrossRef]
- Chen, G.X.; Kwee, T.J.; Tan, K.P.; Choo, Y.S.; Hong, M.H. Laser cleaning of steel for paint removal. Appl. Phys. A 2010, 101, 249–253. [Google Scholar] [CrossRef]
- Tawfeeq, I.S.; Taha, Z.A. The effect of laser parameters on the angular cleaning of aluminum 4004 alloy. Optik 2022, 271, 170078. [Google Scholar] [CrossRef]
- Yoo, H.J.; Baek, S.; Kim, J.H.; Choi, J.; Kim, Y.J.; Park, C. Effect of laser surface cleaning of corroded 304L stainless steel on microstructure and mechanical properties. J. Mater. Res. Technol. 2022, 16, 373–385. [Google Scholar] [CrossRef]
- Maingi, E.M.; Alonso, M.P.; de la Fuente, G.F.; Dubernet, S.; Lefrais, Y.; Chapoulie, R.; Vally, E.; Angurel, L.A. UV femtosecond laser cleaning of encrusted historical stained-glasses. J. Cult. Heritage 2023, 61, 100–108. [Google Scholar] [CrossRef]
- Delaporte, P.; Gastaud, M.; Marine, W.; Sentis, M.; Uteza, O.; Thouvenot, P.; Alcaraz, J.L.; Le Samedy, J.M.; Blin, D. Dry excimer laser cleaning applied to nuclear decontamination. Appl. Surf. Sci. 2003, 208, 298–305. [Google Scholar] [CrossRef]
- Zhou, X.; Imasaki, K.; Umino, H.; Sakagishi, K.; Nakai, S.; Yamanaka, C. Laser surface ablation cleaning of nuclear facilities. In High-Power Lasers in Civil Engineering and Architecture; SPIE: Bellingham, DC, USA, 2000; Volume 3887, pp. 326–334. [Google Scholar]
- Kumar, A.; Prakash, T.; Prasad, M.; Shail, S.; Bhatt, R.B.; Behere, P.G.; Biswas, D.J. Laser assisted removal of fixed radioactive contamination from metallic substrate. Nucl. Eng. Des. 2017, 320, 183–186. [Google Scholar] [CrossRef]
- Carvalho, L.; Pacquentin, W.; Tabarant, M.; Semerok, A.; Maskrot, H. Metal decontamination by high repetition rate nanosecond fiber laser: Application to oxidized and Eu-contaminated stainless steel. Appl. Surf. Sci. 2020, 526, 146654. [Google Scholar] [CrossRef]
- Xie, Y.P.; Wang, J.; Hu, Y.; Sun, Q.; Li, X.; Wang, S.; Liu, G. Numerical simulation and experiments study on laser ablation decontamination for strontium and cesium from contaminated 316L stainless steels in spent nuclear fuel reprocessing. Prog. Nucl. Energy 2023, 161, 104760. [Google Scholar] [CrossRef]
- Stipp, W.; Morais, N.W.S.; Martins, J.V.; Matos, P.; Rossi, J.L.; de Rossi, W.; Raele, M.P. Surface contaminants’ incorporation after nanosecond laser ablation. J. Radioanal. Nucl. Chem. 2023, 332, 4535–4540. [Google Scholar] [CrossRef]
- Costa, P.; Vicente, R.; Genezini, F.A.; de Rossi, W.; Raele, M.P. Laser decontamination of surface impregnated with radioactive material. J. Radioanal. Nucl. Chem. 2022, 331, 4553–4561. [Google Scholar] [CrossRef]
- Oh, S.Y.; Lim, G.; Nam, S.; Kim, T.; Park, H.; Kim, S. Laser decontamination of high-strength concrete using a high-power fiber laser. In Proceedings of the Transactions of the Korean Nuclear Society Virtual Autumn Meeting, Virtual, 21–22 October 2021. [Google Scholar]
- Greifzu, G.; Kahl, T.; Herrmann, M.; Lippmann, W.; Hurtado, A. Laser-based decontamination of metal surfaces. Opt. Laser Technol. 2019, 117, 293–298. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, H.; Wang, F.S.; Ai, S.F.; Liao, D.S.; Wen, T. Laser decontamination microscopic process study on radioactive contaminations with Cs+ ion of 304 stainless steel surface. Appl. Radiat. Isotopes 2022, 182, 110112. [Google Scholar] [CrossRef]
- Anthofer, A.; Lippmann, W.; Hurtado, A. Laser decontamination of epoxy painted concrete surfaces in nuclear plants. Opt. Laser Technol. 2014, 57, 119–128. [Google Scholar] [CrossRef]
- ASTM B166-2008; Standard Specification for Nickel-Chromium-Iron Alloys (UNS N06600, N06601, N06603, N06690, N06693, N06025, N06045, and N06696)* and NickelChromium-Cobalt-Molybdenum Alloy (UNS N06617) Rod, Bar, and Wire. ASTM International: West Conshohocken, PA, USA, 2008.
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Chen, G.; Wang, G.; Shan, Z. Effect of single pulsed picosecond and 100 nanosecond laser cleaning on surface morphology and welding quality of aluminium alloy. Opt. Laser Technol. 2020, 127, 106197. [Google Scholar] [CrossRef]
- Li, X.H.; Wang, J.Q.; Han, E.H.; Ke, W. Corrosion behavior of nuclear grade alloys 690 and 800 in simulated high temperature and high pressure primary water of pressurized water reactor. Acta Metall. Sin. 2012, 48, 941–950. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.H.; Rao, J. The mechanism of oxide film formation on Alloy 690 in oxygenated high temperature water. Corros. Sci. 2011, 53, 3853–3860. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Han, E.H. Electrochemical properties and growth mechanism of passive films on Alloy 690 in high-temperature alkaline environments. Corros. Sci. 2010, 52, 3444–3452. [Google Scholar] [CrossRef]
- Song, K.H.; Shin, J.S. Surface removal of stainless steel using a single-mode continuous wave fiber laser to decontaminate primary circuits. Nucl. Eng. Technol. 2022, 54, 3293–3298. [Google Scholar] [CrossRef]
- Kong, D.J.; Feng, A.X.; Zhang, Y.K.; Lu, J.Z.; Tang, C.P. Experiment study on CO2 laser cleaning rubber sulfuring mould. In ICO20: Lasers Laser Technologies; SPIE: Bellingham, DC, USA, 2005; pp. 371–376. [Google Scholar]
- Veiko, V.P.; Shakhno, E.A. Physical mechanisms of laser cleaning. In Laser Cleaning; World Scientific: Singapore, 2002; pp. 311–340. [Google Scholar]
- Kumar, A.; Biswas, D.J. Particulate size and shape effects in laser cleaning of heavy metal oxide loose contamination off clad surface. Opt. Laser Technol. 2018, 106, 286–293. [Google Scholar] [CrossRef]
- Bertasa, M.; Korenberg, C. Successes and challenges in laser cleaning metal artefacts: A review. J. Cult. Heritage 2022, 53, 100–117. [Google Scholar] [CrossRef]
- Veiko, V.P.; Mutin, T.Y.; Smirnov, V.N.; Shakhno, E.A. Laser decontamination of radioactive nuclides polluted surfaces. Laser Phys. 2011, 21, 608–613. [Google Scholar] [CrossRef]
- Lu, Y.F.; Song, W.D.; Hong, M.H.; Teo, B.S.; Chong, T.C.; Low, T.S. Laser removal of particles from magnetic head sliders. J. Appl. Phys. 1996, 80, 499–504. [Google Scholar] [CrossRef]





| Element | Ni | Cr | Fe | Cu | Mn | Si | C | S | P |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 62.9 | 28.2 | 8.4 | 0.20 | 0.17 | 0.12 | 0.02 | 0.005 | 0.003 |
| Technical Parameter | Unit | Indicators |
|---|---|---|
| Product type | YFPN-200-GMC | |
| Maximum power | W | 200 |
| Power adjustment range | % | 10–100 |
| Pulse width | ns | 10–500 |
| Center wavelength | nm | 1060–1080 |
| Focusing spot diameter | μm | 50 |
| Cooling method | Air cooling | |
| Working temperature | °C | 0–40 |
| Power (W) | Laser Fluence/(J·cm−2) |
|---|---|
| 20 | 50.93 |
| 40 | 101.86 |
| 60 | 152.79 |
| 80 | 203.72 |
| 100 | 254.65 |
| 120 | 305.58 |
| 140 | 356.51 |
| 160 | 407.44 |
| 180 | 458.37 |
| 200 | 509.30 |
| Elemental Mass Percentage % | O | Fe | Cr | Ni |
|---|---|---|---|---|
| Alloy 690 | 0 | 8.40 | 28.20 | 63.30 |
| 960 h | 22.51 | 9.68 | 19.69 | 45.32 |
| Elemental Atomic Percentage % | O | Fe | Cr | Ni |
|---|---|---|---|---|
| Polyhedral 1 | 60.19 | 4.92 | 13.45 | 21.44 |
| Acicular 2 | 55.35 | 6.25 | 8.64 | 29.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Liu, C.; Li, K.; Cheng, J.; Zhang, Z.; Han, E. An Efficient Laser Decontamination Process Based on Non-Radioactive Specimens of Nuclear Power Materials. Materials 2023, 16, 7643. https://doi.org/10.3390/ma16247643
Hu Y, Liu C, Li K, Cheng J, Zhang Z, Han E. An Efficient Laser Decontamination Process Based on Non-Radioactive Specimens of Nuclear Power Materials. Materials. 2023; 16(24):7643. https://doi.org/10.3390/ma16247643
Chicago/Turabian StyleHu, Yang, Changsheng Liu, Kangte Li, Jian Cheng, Zhiming Zhang, and Enhou Han. 2023. "An Efficient Laser Decontamination Process Based on Non-Radioactive Specimens of Nuclear Power Materials" Materials 16, no. 24: 7643. https://doi.org/10.3390/ma16247643






