Wire Electrochemical Etching of Superhydrophobic Nickel Surfaces with Enhanced Corrosion Protection
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Sample
2.3. Characterization
2.4. Electrochemical Impedance Spectroscopy Tests
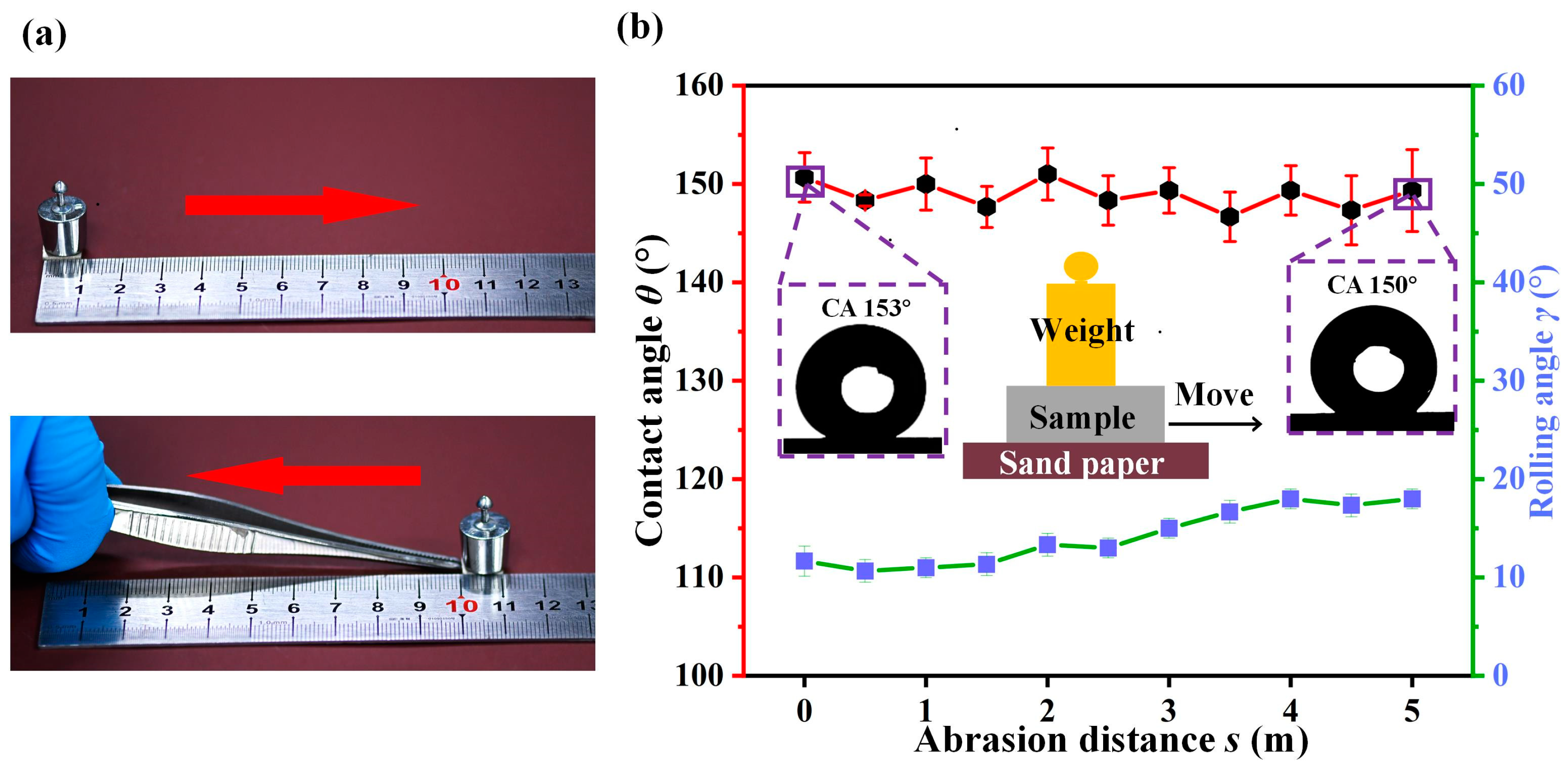
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palazzolo, A.; Poucin, C.; Freitas, A.P.; Ropp, A.; Bouillet, C.; Ersen, O.; Carenco, S. The delicate balance of phase speciation in bimetallic nickel cobalt nanoparticles. Nanoscale 2022, 14, 7547–7560. [Google Scholar] [CrossRef]
- Wu, F.; Kuenzel, M.; Diemant, T.; Mullaliu, A.; Fang, S.; Kim, J.-K.; Kim, H.W.; Kim, G.-T.; Passerini, S. Enabling High-Stability of Aqueous-Processed Nickel-Rich Positive Electrodes in Lithium Metal Batteries. Small 2022, 18, 2203874. [Google Scholar] [CrossRef] [PubMed]
- Pyo, C.; Jeong, S.-M.; Kim, J.; Park, M.; Shin, J.; Kim, Y.; Son, J.; Kim, J.-H.; Kim, M.-H. A Study on the Enhanced Process of Elaborate Heat Source Model Parameters for Flux Core Arc Welding of 9% Nickel Steel for Cryogenic Storage Tank. J. Mar. Sci. Eng. 2022, 10, 1810. [Google Scholar] [CrossRef]
- Miguel, F.L.; Mueller, R.; Rosenkranz, A.; Mathur, S.; Muecklich, F. Analysis and modelling of the dry-sliding friction and wear behaviour of electrodeposited Ni and Ni-matrix-nanocomposite films. Wear 2016, 346, 87–98. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Zhu, L.; He, L.; He, S.; Qin, X.; Zhao, C.; Kang, F.; Li, B. Synthesis design of interfacial nanostructure for nickel-rich layered cathodes. Nano Energy 2022, 97, 107119. [Google Scholar] [CrossRef]
- Nezhad, A.H.N.; Rahimi, E.; Arefinia, R.; Davoodi, A.; Hosseinpour, S. Effect of Substrate Grain Size on Structural and Corrosion Properties of Electrodeposited Nickel Layer Protected with Self-Assembled Film of Stearic Acid. Materials 2020, 13, 2052. [Google Scholar] [CrossRef]
- Wang, L.; Ye, M.; Wang, Y.; Tian, D.; Ye, Z.; Wang, C. Corrosion behavior of the nickel/nickel interface during the copper-sacrificial layer release process in micro-electroforming. Mater. Corros. 2022, 73, 961–970. [Google Scholar] [CrossRef]
- Krolikowska, A.; Komorowski, L.; Langer, E.; Zubielewicz, M. Promising Results of the Comparison of Coatings on Aged Bridges and of Same Coatings in Laboratory. Materials 2022, 15, 3064. [Google Scholar] [CrossRef]
- Kalendova, A.; Vesely, D.; Kalenda, P. Anticorrosion pigment based on calcium titanate with a perovskite structure. Pigment Resin Technol. 2007, 36, 123–133. [Google Scholar] [CrossRef]
- Shu, Z.; Axe, L.; Jahan, K.; Ramanujachary, K.; Kochersberger, C. Metal concentrations and distribution in paint waste generated during bridge rehabilitation in New York State. Sci. Total Environ. 2015, 526, 262–270. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Zhang, D.; Ou, J. Effect of surface nanostructure on enhanced atmospheric corrosion resistance of a superhydrophobic surface. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129058. [Google Scholar] [CrossRef]
- Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Preparation of transparent superhydrophobic boehmite and silica films by sublimation of aluminum acetylacetonate. Adv. Mater. 1999, 11, 1365–1368. [Google Scholar] [CrossRef]
- Li, D.; Ma, L.; Zhang, B.; Chen, S. Large-scale fabrication of a durable and self-healing super-hydrophobic coating with high thermal stability and long-term corrosion resistance. Nanoscale 2021, 13, 7810–7821. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Kumagai, S.; Tsunakawa, M.; Furukawa, T.; Nakamura, K. Ultrafast fabrication of superhydrophobic surfaces on engineering light metals by single-step immersion process. Mater. Lett. 2017, 193, 42–45. [Google Scholar] [CrossRef]
- Saji, V.S. Superhydrophobic surfaces and coatings by electrochemical anodic oxidation and plasma electrolytic oxidation. Adv. Colloid Interface Sci. 2020, 283, 102245. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, Y.; Liu, J.; Zhao, D.; Ming, P.; Song, J. Electrochemical 3D printing of superhydrophobic pillars with conical, cylindrical, and inverted conical shapes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126869. [Google Scholar] [CrossRef]
- Yang, X.; Zhuang, K.; Lu, Y.; Wang, X. Creation of Topological Ultraslippery Surfaces for Droplet Motion Control. ACS Nano 2020, 15, 2589–2599. [Google Scholar] [CrossRef]
- Yan, D.F.; Lu, Y.; Liu, J.M.; Chen, Y.; Sun, J.; Song, J.L. Enhanced water transportation on a superhydrophilic serial cycloid-shaped pattern. Nanoscale 2023, 15, 11473–11481. [Google Scholar] [CrossRef]
- Cho, H.; Kim, I.; Park, J.; Kim, D. A waterwheel hybrid generator with disk triboelectric nanogenerator and electromagnetic generator as a power source for an electrocoagulation system. Nano Energy 2022, 95, 107048. [Google Scholar] [CrossRef]
- Wu, S.; Yan, D.; Chen, Y.; Song, J. Self-driven oil/water separator with super-high separation rate. Nano Energy 2024, 119, 109066. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Z.; Luo, X.; Chen, C.; Jiang, G.; Hu, X.; Peng, R.; Zhang, H.; Zhong, M. Superomniphobic surfaces for easy-removals of environmental-related liquids after icing and melting. Nano Res. 2023, 16, 3267–3277. [Google Scholar] [CrossRef]
- Ivvala, J.; Mandal, P.; Arora, H.; Grewal, H. Facile approach for modulating wetting of hierarchically structured metallic surface. Surf. Innov. 2022, 10, 25–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Ming, P.; Xue, B.; Liu, H.; Yang, X.; Li, L.; Niu, S.; Yan, L.; Zheng, X.; Qin, G. Facilely fabricating large-area robust heterogeneous wettability surface by mask-patterned ultrafine anode scanning electrodeposition for efficient water collection. Surf. Interfaces 2023, 41, 103247. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, X.M.; Yang, X.D.; Lv, S.W.; Zhao, J.H.; Bie, L.; Liu, F. Fabrication of robust superhydrophobic-superoleophilic mesh for oil-water separation under harsh environment. Surf. Innov. 2023. [Google Scholar] [CrossRef]
- Fan, Q.; Ji, X.; Lan, Q.; Zhang, H.; Li, Q.; Zhang, S.; Yang, B. An anti-icing copper-based superhydrophobic layer prepared by one-step electrodeposition in both cathode and anode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 637, 128220. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Proverbio, E. Anticorrosion Superhydrophobic Surfaces on AA6082 Aluminum Alloy by HF/HCl Texturing and Self-Assembling of Silane Monolayer. Materials 2022, 15, 8549. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Z.S.; Chen, M.J.; Li, Z.; Luo, X.W.; Lu, W.Z.; Gu, Z.L. Fabrication and Characterization of Superhydrophobic Al-Based Surface Used for Finned-Tube Heat Exchangers. Materials 2022, 15, 3060. [Google Scholar] [CrossRef]
- Doskocil, L.; Somanova, P.; Masilko, J.; Buchtik, M.; Hasonova, M.; Kalina, L.; Wasserbauer, J. Characterization of Prepared Superhydrophobic Surfaces on AZ31 and AZ91 Alloys Etched with ZnCl2 and SnCl2. Coatings 2022, 12, 1414. [Google Scholar] [CrossRef]
- Ma, N.; Cheng, D.; Zhang, J.; Zhao, S.; Lu, Y. A simple, inexpensive and environmental-friendly electrochemical etching method to fabricate superhydrophobic GH4169 surfaces. Surf. Coat. Technol. 2020, 399, 126180. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Ming, P.; Zhao, D.; Song, J. Wire electrochemical etching of superhydrophobic 304 stainless steel surfaces based on high local current density with neutral electrolyte. Appl. Surf. Sci. 2021, 571, 151269. [Google Scholar] [CrossRef]
- Yan, D.; Chen, Y.; Liu, J.; Song, J. Super-Fast Fog Collector Based on Self-Driven Jet of Mini Fog Droplets. Small 2023, 19, 2301745. [Google Scholar] [CrossRef]
- Skoczypiec, S. Discussion of ultrashort voltage pulses electrochemical micromachining: A review. Int. J. Adv. Manuf. Technol. 2016, 87, 177–187. [Google Scholar] [CrossRef]
- Nanev, C.; Dicheva, K. Electrochemical Etching. Electrochim. Acta 1983, 28, 933–940. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y. Electrochemical machining of complex components of aero-engines: Developments, trends, and technological advances. Chin. J. Aeronaut. 2021, 34, 28–53. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, N.; Das, A.K. Micro-machining through electrochemical discharge processes: A review. Mater. Manuf. Process. 2020, 35, 363–404. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, G.; Tian, Z.; Chen, C.; Hu, X.; Peng, R.; Zhang, H.; Fan, P.; Zhong, M. Superhydrophobic microstructures for better anti-icing performances: Open-cell or closed-cell? Mater. Horiz. 2023, 10, 209–220. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Liu, J.; Zhang, Y.; Cao, X.; Wang, S.; Duan, Z.; Yuan, Z.; Chen, Y.; Meng, Y.; et al. Antiadhesion Superhydrophobic Bipolar Electrocoagulation Tweezers with High Conductivity and Stability. Langmuir 2023, 39, 10593–10600. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Xue, Y.; Sui, X.; Wang, F.; Liang, W.; Dong, Q. Multifunctional electro-thermal superhydrophobic shape memory film with in situ reversible wettability and anti-icing/deicing properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129960. [Google Scholar] [CrossRef]
- Tan, S.F.; Reidy, K.; Klein, J.; Pinkowitz, A.; Wang, B.; Ross, F.M. Real-time imaging of nanoscale electrochemical Ni etching under thermal conditions. Chem. Sci. 2021, 12, 5259–5268. [Google Scholar] [CrossRef]
- Shin, H.S. Deep Electrochemical Etching of Stainless Steel Using a Deposited Copper Layer. Appl. Sci. 2022, 12, 12473. [Google Scholar] [CrossRef]
- Khan, S.; Abu Jarad, N.; Ladouceur, L.; Rachwalski, K.; Bot, V.; Shakeri, A.; Maclachlan, R.; Sakib, S.; Weitz, J.; Brown, E.; et al. Transparent and Highly Flexible Hierarchically Structured Polydimethylsiloxane Surfaces Suppress Bacterial Attachment and Thrombosis Under Static and Dynamic Conditions. Small 2022, 18, 2108112. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, Q.; Ma, J.; Xu, W.; Sun, J.; Liu, X.; Song, J. Solid-Like Slippery Coating with Highly Comprehensive Performance. Adv. Funct. Mater. 2023, 33, 2302311. [Google Scholar] [CrossRef]
- Xie, H.; Xu, W.-H.; Du, Y.; Gong, J.; Niu, R.; Wu, T.; Qu, J.-P. Cost-Effective Fabrication of Micro-Nanostructured Superhydrophobic Polyethylene/Graphene Foam with Self-Floating, Optical Trapping, Acid-/Alkali Resistance for Efficient Photothermal Deicing and Interfacial Evaporation. Small 2022, 18, 2200175. [Google Scholar] [CrossRef] [PubMed]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Pan, W.; Ma, J.; Yan, D.; Xu, W.; Chen, Y.; Huang, L.; Song, J. A facile and high-efficient method to fabricate slippery liquid-infused porous surface with enhanced functionality. Surf. Coat. Technol. 2023, 472, 129897. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Sun, Y.; Sui, X.; Wang, Y.; Liang, W.; Wang, Y.; Zhu, D.; Zhao, H. Flexible superhydrophobic films with the electrothermal and photothermal response for enhanced passive anti-icing and active de-icing. Surf. Interfaces 2023, 42, 103430. [Google Scholar] [CrossRef]
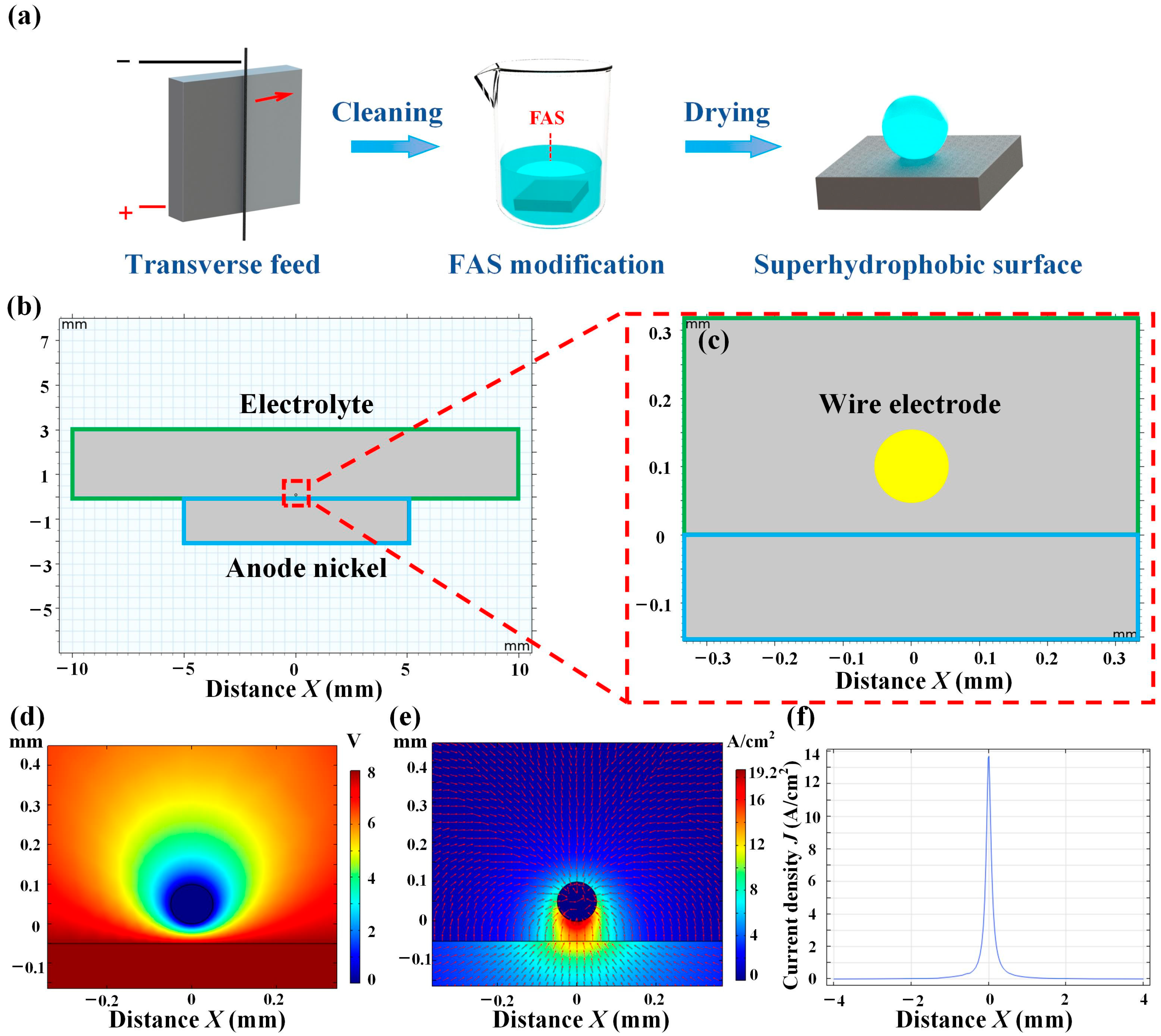
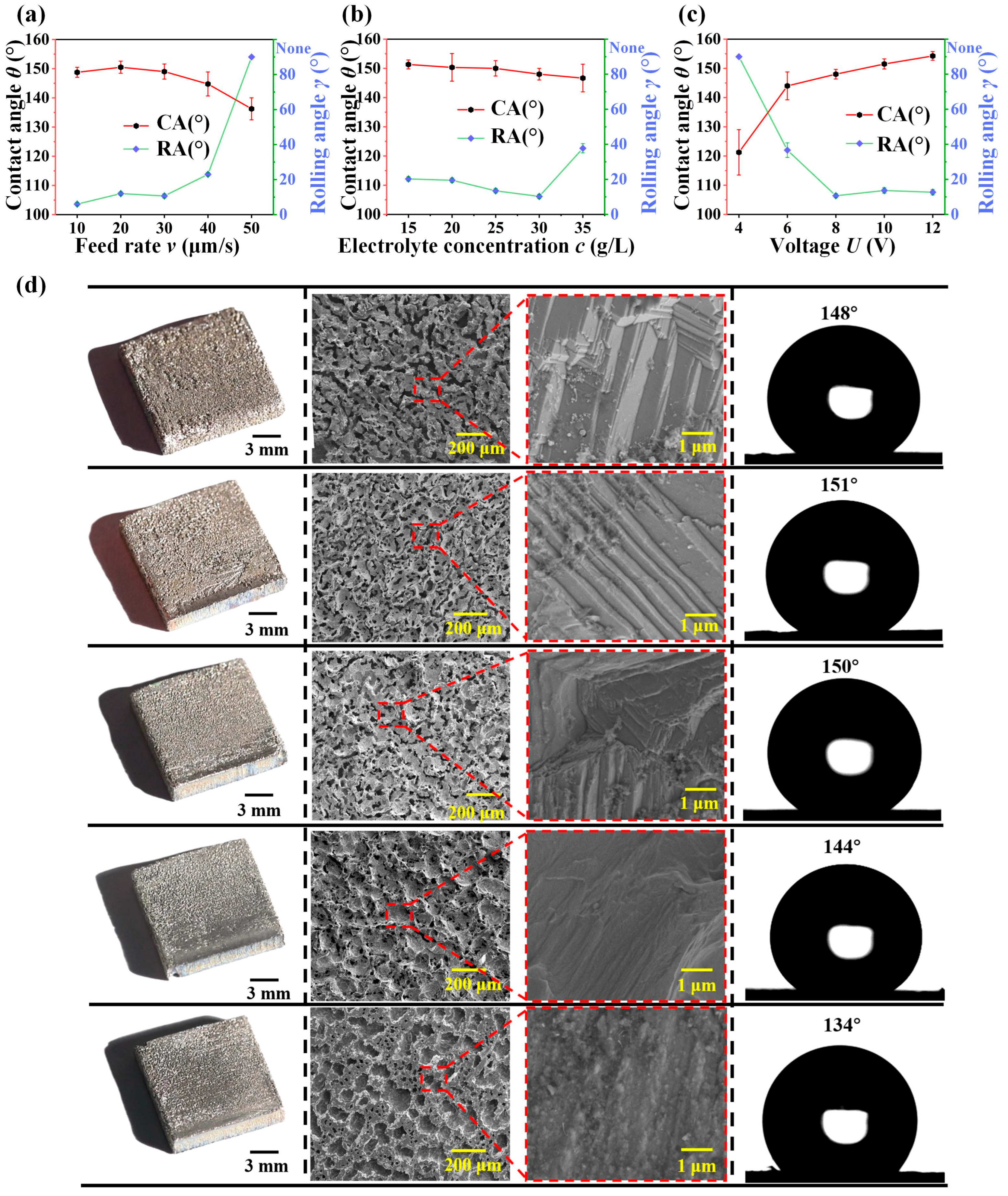
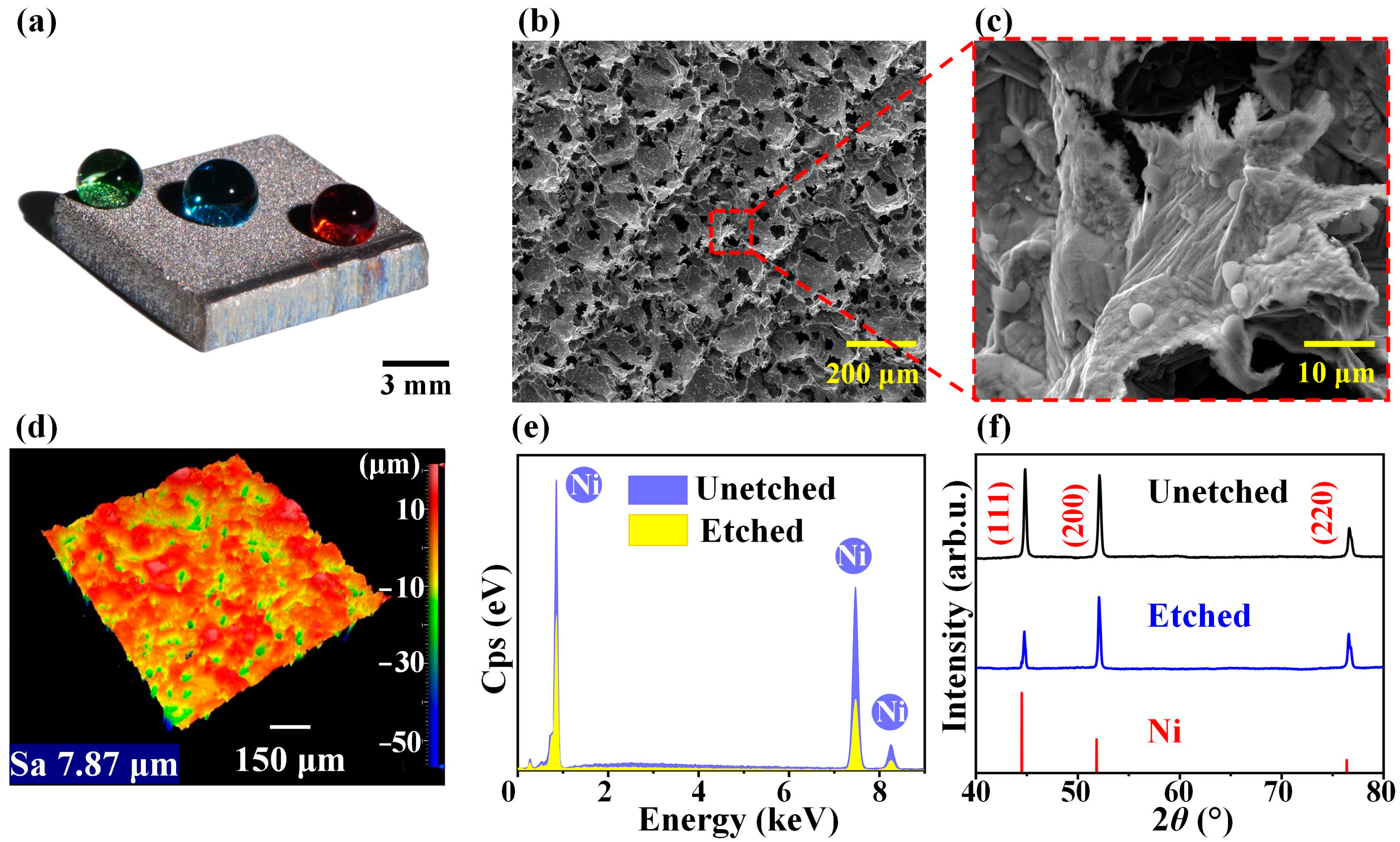


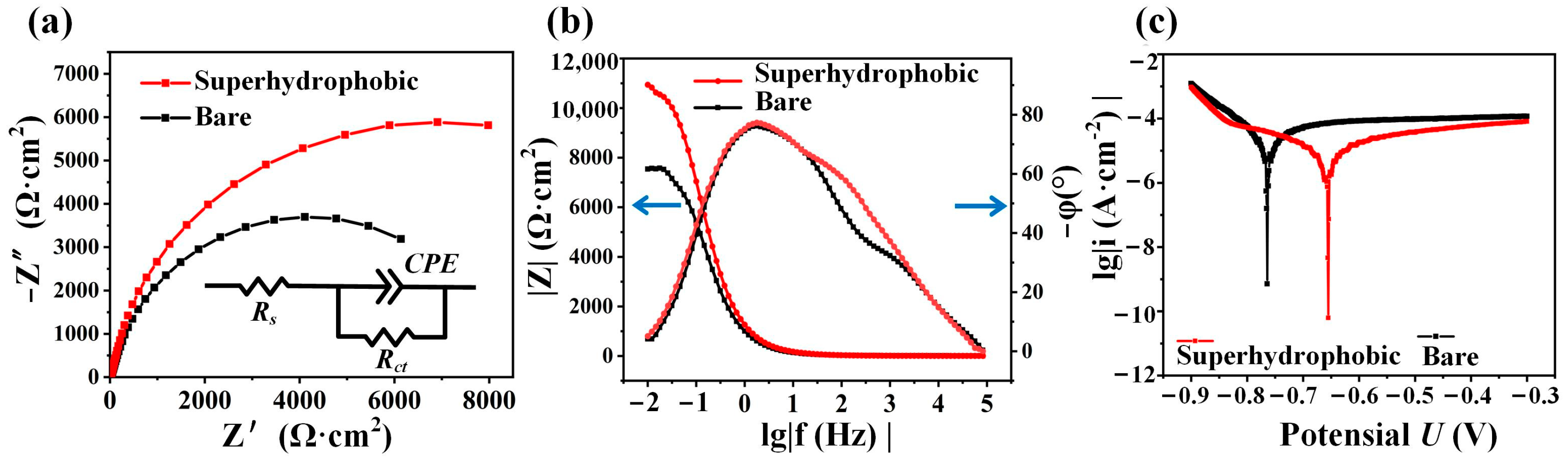
| Plate Cathode | Wire Cathode | |
|---|---|---|
| Anode length | 10 mm | 10 mm |
| Cathode length (diameter) | 10 mm | 100 μm |
| Gap between the anode and cathode | 20 mm | 30 μm |
| Initial potential | 8 V | 8 V |
| Anode size | 10 mm × 10 mm | 10 mm × 10 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Yan, D.; Lin, J.; Song, J. Wire Electrochemical Etching of Superhydrophobic Nickel Surfaces with Enhanced Corrosion Protection. Materials 2023, 16, 7472. https://doi.org/10.3390/ma16237472
Wu B, Yan D, Lin J, Song J. Wire Electrochemical Etching of Superhydrophobic Nickel Surfaces with Enhanced Corrosion Protection. Materials. 2023; 16(23):7472. https://doi.org/10.3390/ma16237472
Chicago/Turabian StyleWu, Binghan, Defeng Yan, Junyi Lin, and Jinlong Song. 2023. "Wire Electrochemical Etching of Superhydrophobic Nickel Surfaces with Enhanced Corrosion Protection" Materials 16, no. 23: 7472. https://doi.org/10.3390/ma16237472





