Preparation of Sr2CeZrO6 Refractory and Its Interaction with TiAl Alloy
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
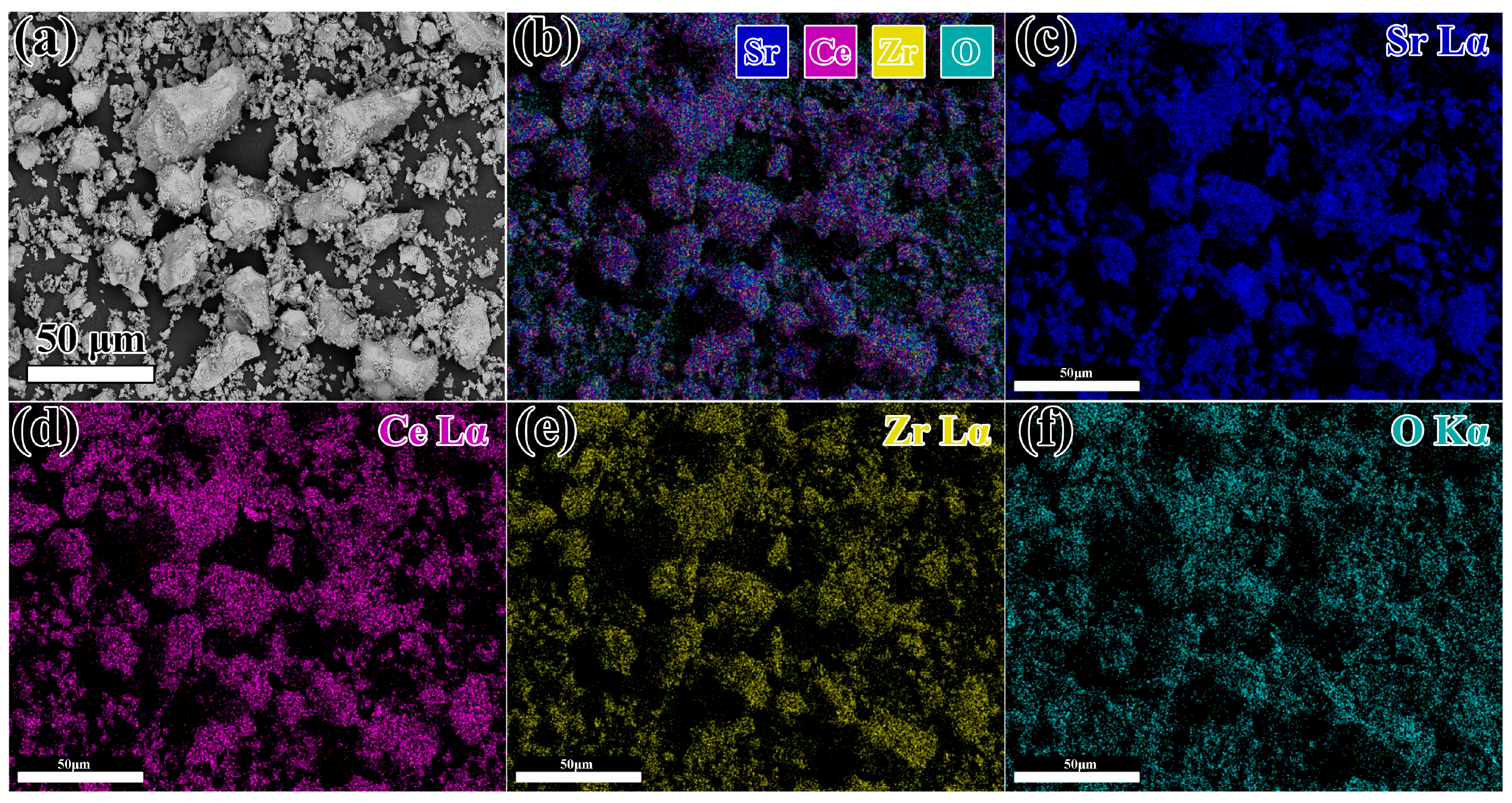
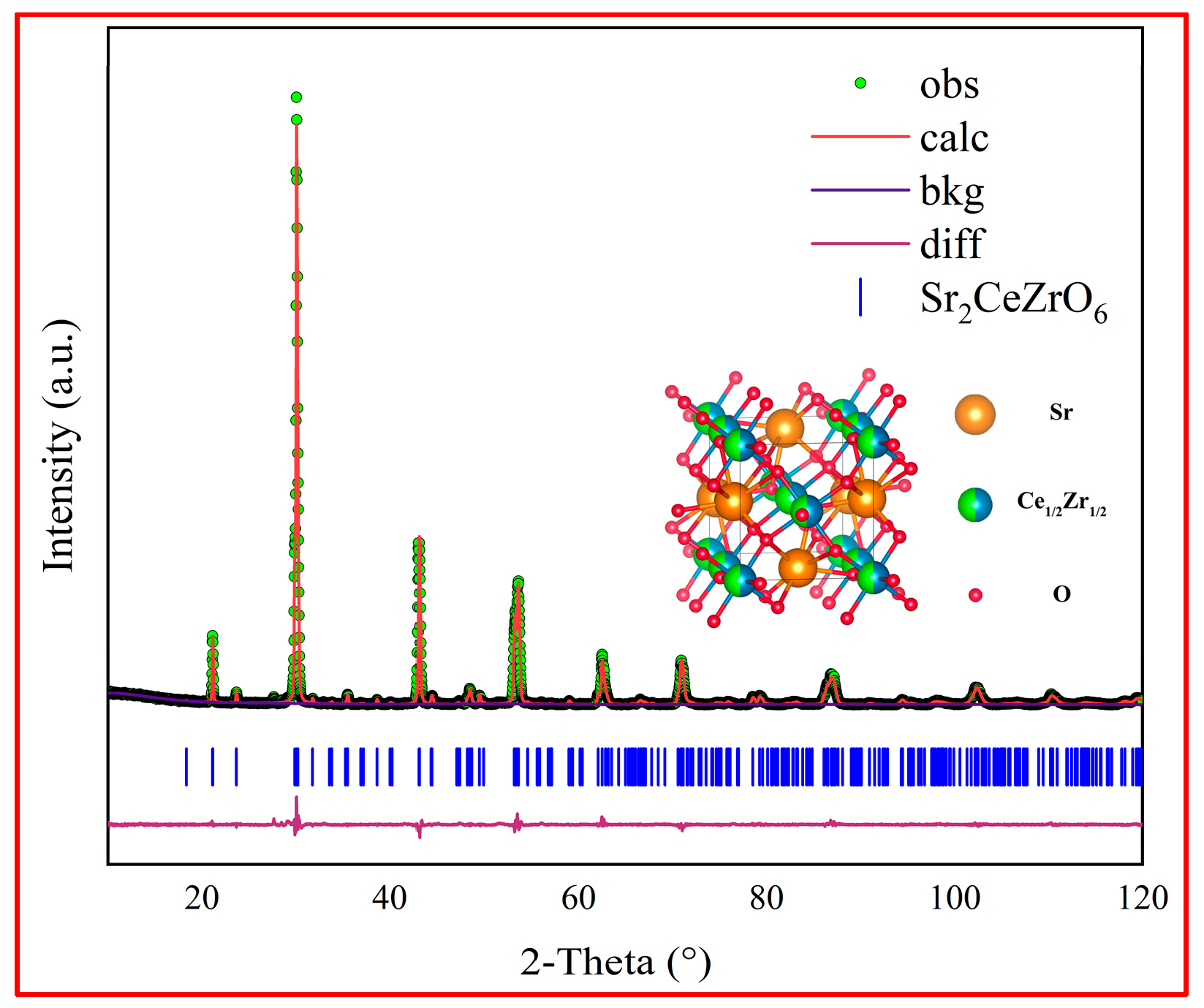
3.1. Synthesis of Sr2CeZrO6 Powder
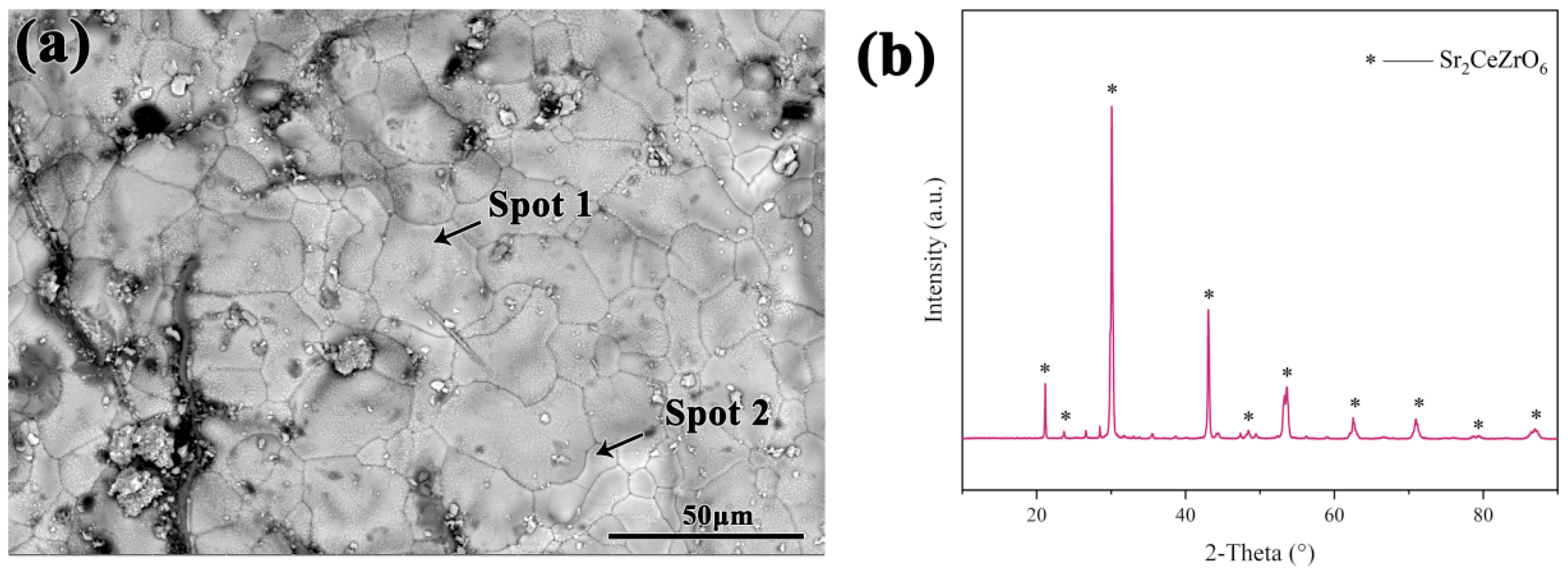
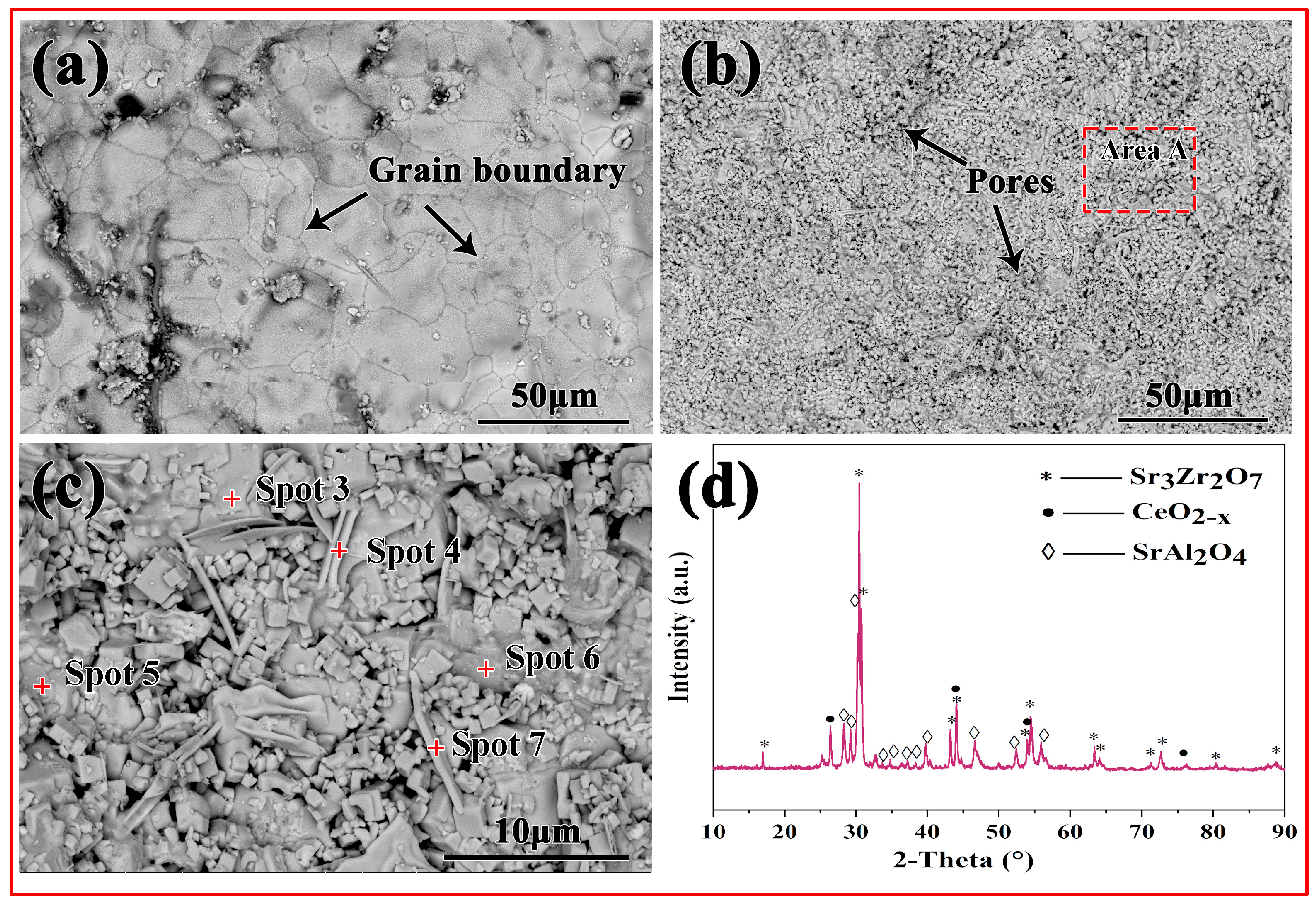
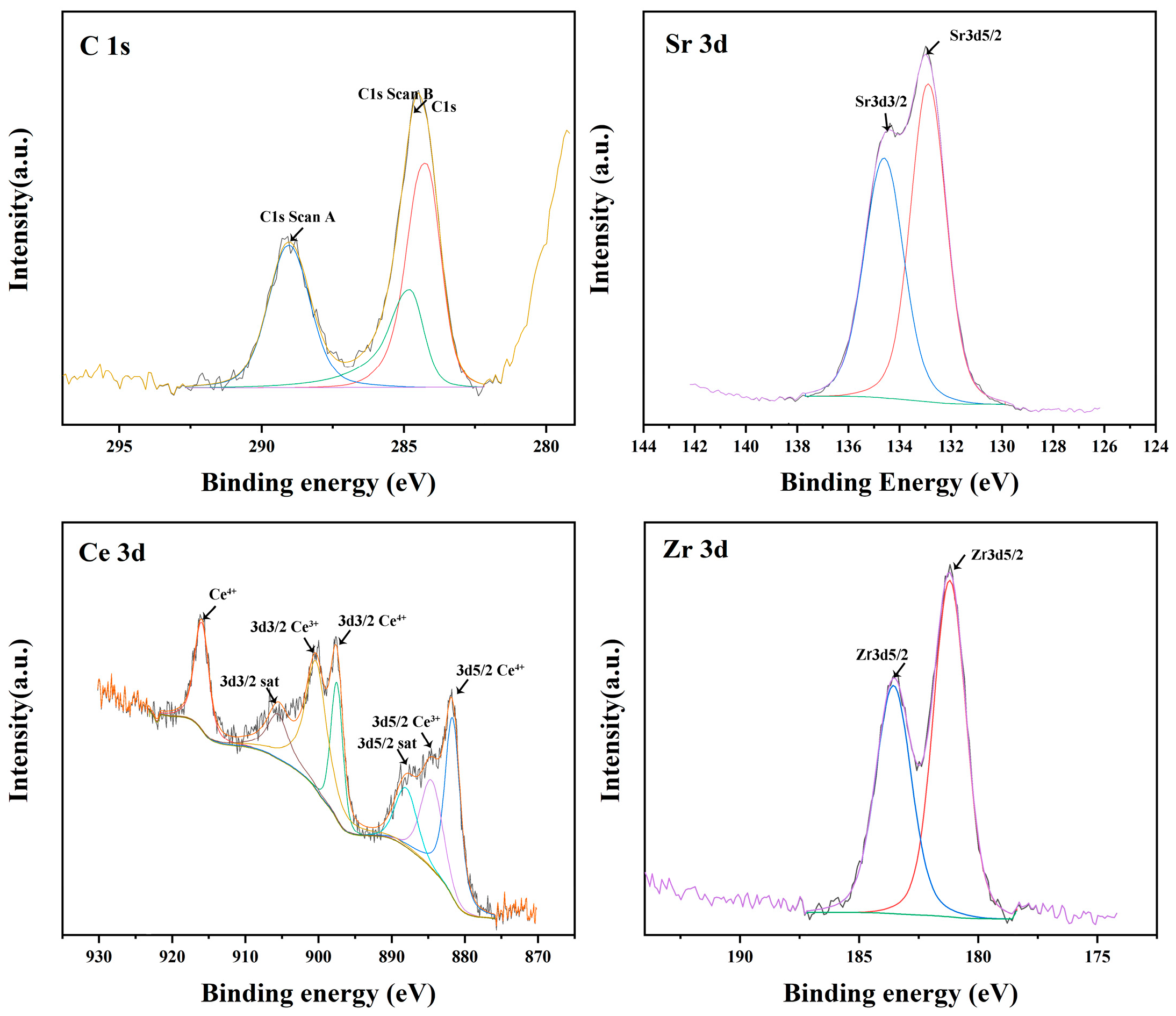
3.2. Phase Constitution of Sr2CeZrO6 Crucible
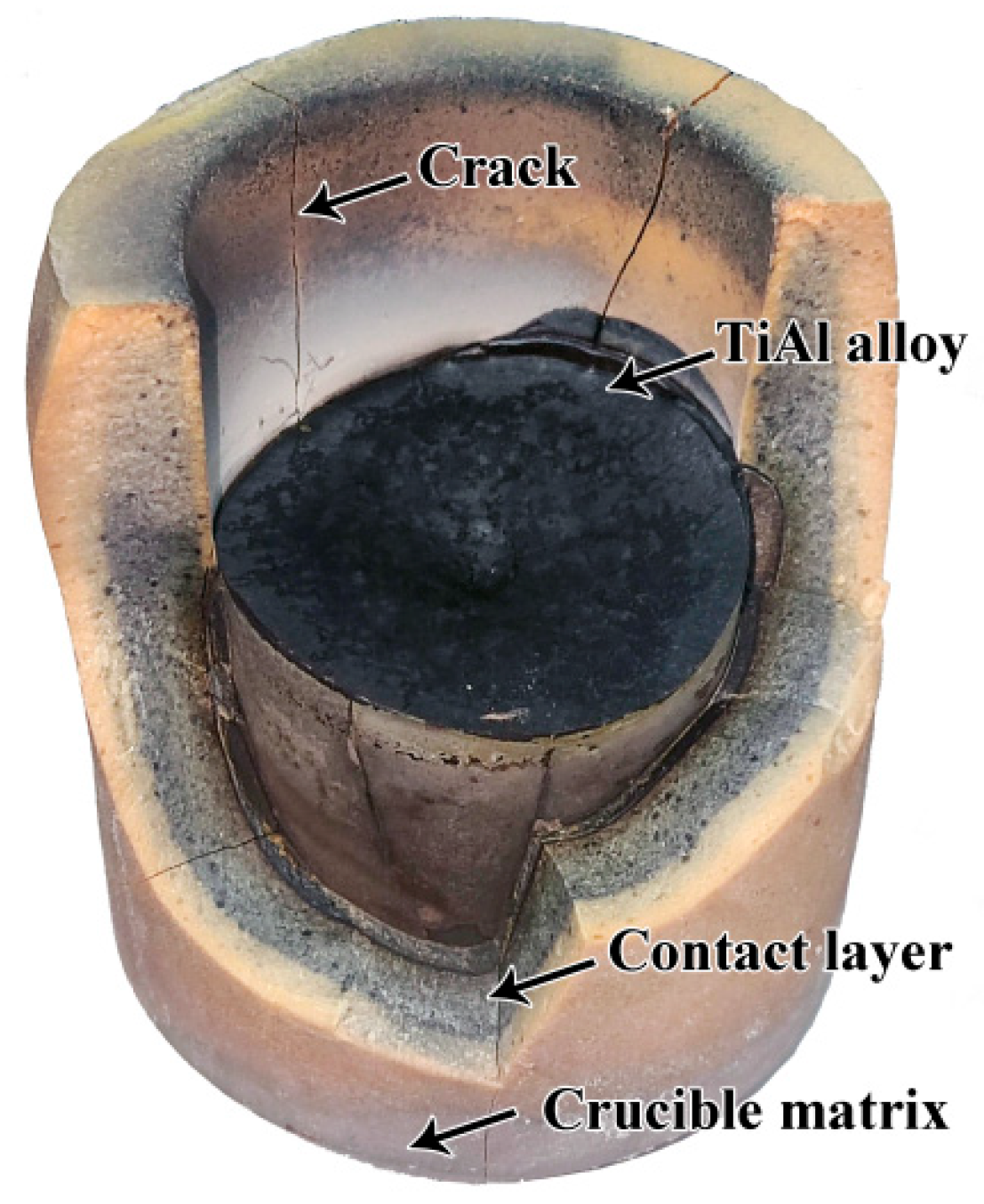
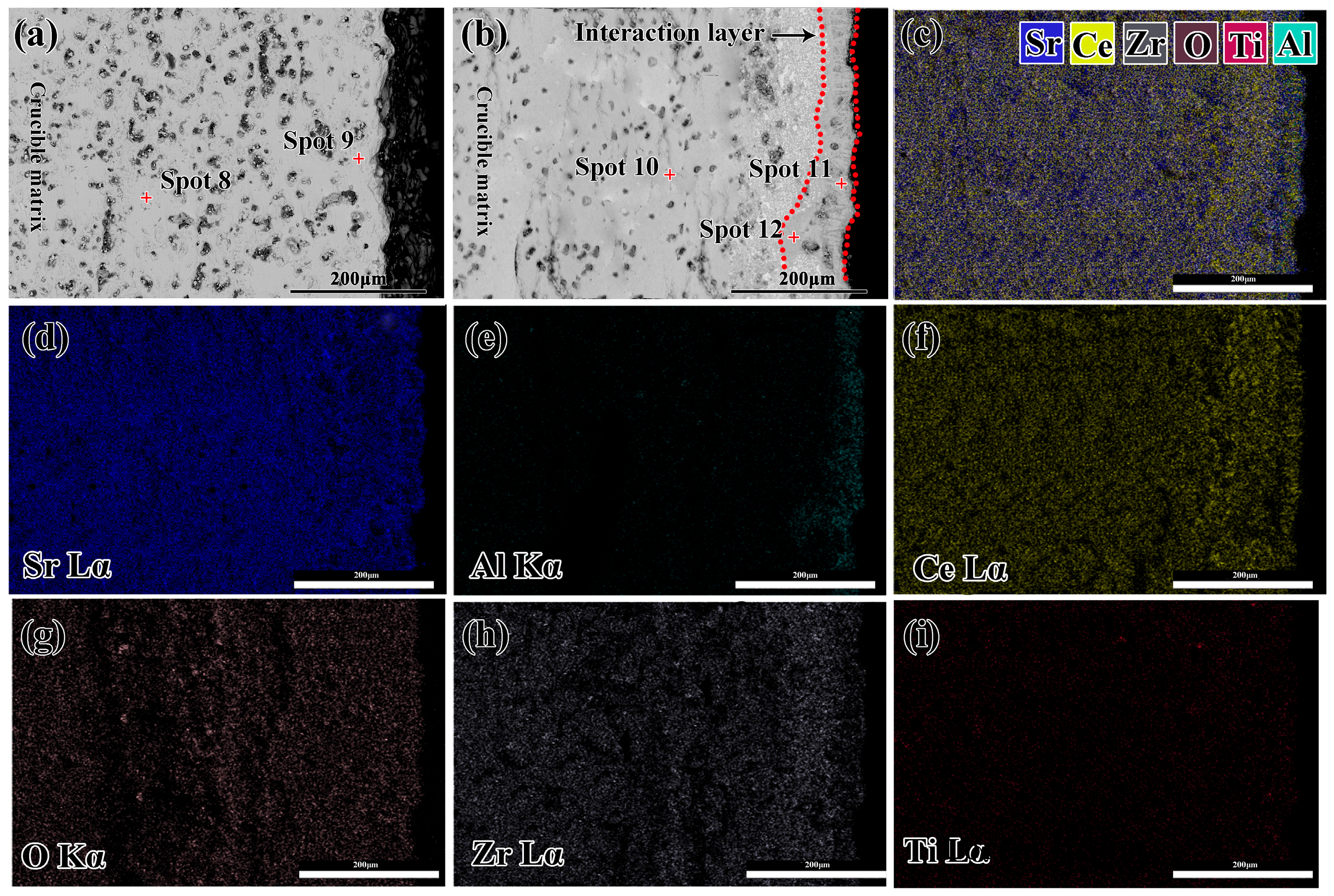
3.3. Interfacial Interaction
4. Conclusions
- (1)
- The pure compound Sr2CeZrO6 was successfully synthesized by a solid-state reaction after sintering at 1400 °C for 12 h. The Sr2CeZrO6 phase showed Pnma space group symmetry with a = 5.9742(3) Å, b = 8.3910(5) Å, c = 5.9069(5) Å.
- (2)
- The Sr2CeZrO6 refractory crucible maintained an intact shape after contacting with the TiAl alloy melt, and the thickness of the interaction layer was about 40 μm.
- (3)
- The Sr2CeZrO6 refractory decomposed into Sr3Zr2O7, SrAl2O4 and CeO2−x. The products were generated by the dissolution of the Sr2CeZrO6 refractory in the alloy melt, which was the main factor responsible for the interaction mechanism between them. This study provides some theoretical guidance for the development of new refractory materials for melting titanium alloys in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2007, 14, 1114–1122. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, Processing, Microstructure, Properties, and Applications of Advanced Intermetallic TiAl Alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Chen, S.H.; Schumacher, G. The application of transformation matrices on the determination of γ/γ interface types in a γ-TiAl alloy. Scr. Mater. 2004, 50, 31–34. [Google Scholar] [CrossRef]
- Kim, Y.W. Trends in the development of gamma TiAl alloys. In Gamma Titanium Aluminides; Kim, Y.W., Ed.; Minerals, Metals and Materials Society: Warrendale, PA, USA, 1995; pp. 637–652. [Google Scholar]
- Yamaguchi, M. High temperature intermetallics—With particular emphasis on TiAl. Mater. Sci. Technol. 1992, 8, 299–307. [Google Scholar] [CrossRef]
- Noda, T. Application of cast gamma TiAl for automobiles. Intermetallics 1998, 6, 709–713. [Google Scholar] [CrossRef]
- Liu, K.; Ma, Y.C.; Gao, M.; Rao, G.B.; Li, Y.Y.; Wei, K.; Wu, X.H.; Loretto, M.H. Single step centrifugal casting TiAl automotive valves. Intermetallics 2005, 13, 925–928. [Google Scholar] [CrossRef]
- Sung, S.Y.; Kim, Y.J. Economic net-shape forming of TiAl alloys for automotive parts. Intermetallics 2006, 14, 1163–1167. [Google Scholar] [CrossRef]
- Lan, L.; Xin, R.Y.; Jin, X.Y.; Gao, S.; He, B.; Rong, Y.H.; Min, N. Effects of Laser Shock Peening on Microstructure and Properties of Ti–6Al–4V Titanium Alloy Fabricated via Selective Laser Melting. Materials 2020, 13, 3261. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, N.D.; Craciun, E.M.; Soós, E. Mechanics of Elastic Composites; Chapman and Hall/CRC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Cui, R.J.; Tang, X.X.; Gao, M.; Zhang, H.; Gong, S.K. Interactions between TiAl alloys and ytrria refractory material in casting process. J. Mater. Process. Technol. 2010, 210, 1190–1196. [Google Scholar]
- Barbosa, J.; Ribeiro, C.S.; Monteiro, A.C. Influence of superheating on casting of γ-TiAl. Intermetallics 2007, 15, 945–955. [Google Scholar] [CrossRef]
- Tetsui, T.; Kobayashi, T.; Mori, T.; Kishimoto, T.; Harada, H. Evaluation of Ytrria Applicability as a Crucible for Induction Melting of TiAl Alloy. Mater. Trans. 2010, 51, 1656–1662. [Google Scholar] [CrossRef]
- Zhang, H.R.; Tang, X.X.; Zhou, C.G.; Zhang, H.; Zhang, S.W. Comparison of directional solidification of TiAl alloys in conventional Al2O3 and novel Y2O3-coated Al2O3 crucibles. J. Eur. Ceram. Soc. 2013, 33, 925–934. [Google Scholar] [CrossRef]
- Tetsui, T.; Kobayashi, T.; Ueno, T.; Hiroshi, H. Consideration of the influence of contamination from oxide crucibles on TiAl cast material, and the possibility of achieving low-purity TiAl precision cast turbine wheels. Intermetallics 2012, 31, 274–281. [Google Scholar] [CrossRef]
- Sakamoto, K.; Yoshikawa, K.; Kusamichi, T.; Onoye, T. Changes in Oxygen Contents of Titanium Aluminides by Vacuum Induction, Cold Crucible Induction and Electron Beam Melting. Trans. Iron Steel Inst. Jpn. 1992, 32, 616–624. [Google Scholar] [CrossRef]
- Tetsui, T.; Kobayashi, T.; Kishimoto, A.; Harada, H. Structural optimization of an yttria crucible for melting TiAl alloy. Intermetallics 2012, 20, 16–23. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Y.; Xu, K.; Yang, J.Z.; Niu, S.B.; Yao, H. Effect of steam on CaO regeneration, carbonation and hydration resistance for CO2 capture. Fuel Process. Technol. 2016, 151, 101–106. [Google Scholar] [CrossRef]
- Uwanyuze, R.S.; Yavas, B.; Zhang, J.Y.; Kanyo, J.E.; Frame, L.D.; Hebert, R.J.; Schaffoner, S.; Al, S.P. High-temperature interactions between titanium alloys and strontium zirconate refractories. J. Mater. Eng. Perform. 2023, 32, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.J. Modeling oxygen ionic conductivities of ABO3 Perovskites through machine learning. Chem. Phys. 2022, 558, 111511. [Google Scholar] [CrossRef]
- Xiao, Y.B.; Chen, G.Y.; Yu, F.H.; Hou, X.; Zhang, Y.; Zou, X.L.; Lu, X.G.; Li, C.H. Preparation of a novel Sr-Zr oxide refractory for induction melting of high-activity alloy. J. Eur. Ceram. Soc. 2021, 41, 6738–6743. [Google Scholar] [CrossRef]
- Zhou, B.; Ke, Q.L.; Wen, M.J.; Ying, T.B.; Cui, G.K.; Gu, Z.Y.; Lu, H.F. Catalytic combustion of toluene on Pt/Al2O3 and Pd/Al2O3 catalysts with CeO2, CeO2-Y2O3 and La2O3 as coatings. J. Rare Earths 2023, 41, 1171–1178. [Google Scholar] [CrossRef]



| Atom | Wyckoff Position | x | y | z | Site Occ | Biso |
|---|---|---|---|---|---|---|
| Sr | 4c | 0.46569 | 0.25000 | −0.00795 | 1.0000 | 0.1780(7) |
| Ce | 4a | 0.00000 | 0.00000 | 0.00000 | 0.5000 | 0.00805(2) |
| Zr | 4a | 0.00000 | 0.00000 | 0.00000 | 0.5000 | 0.00810(2) |
| O | 4c | 0.02400 | 0.25000 | 0.09200 | 1.00000 | 0.03900(2) |
| O | 8d | 0.02874 | −0.04360 | 0.20730 | 1.00000 | 0.02960(3) |
| Element at% | ||||
|---|---|---|---|---|
| Sr | Ce | Zr | O | |
| Spot 1 | 13.99 | 8.56 | 7.53 | 69.92 |
| Spot 2 | 17.36 | 6.97 | 7.15 | 68.52 |
| Element at% | ||||||
|---|---|---|---|---|---|---|
| Sr | Ce | Zr | O | Al | Ti | |
| Spot 3 | 19.50 | 9.30 | 1.95 | 40.12 | 26.02 | 3.11 |
| Spot 4 | 13.84 | 13.94 | 2.61 | 57.14 | 11.99 | 0.48 |
| Spot 5 | 24.34 | 1.32 | 16.07 | 55.03 | 3.12 | 0.12 |
| Spot 6 | 10.04 | 6.97 | 2.05 | 65.72 | 14.10 | 1.12 |
| Spot 7 | 11.28 | 7.81 | 2.16 | 62.75 | 13.84 | 2.16 |
| Element at% | ||||||
|---|---|---|---|---|---|---|
| Sr | Ce | Zr | O | Al | Ti | |
| Spot 8 | 19.86 | 11.31 | 10.44 | 58.39 | / | / |
| Spot 9 | 20.27 | 10.83 | 10.86 | 58.04 | / | / |
| Spot 10 | 21.61 | 10.57 | 11.49 | 56.33 | / | / |
| Spot 11 | 10.53 | 26.40 | 3.68 | 59.39 | / | / |
| Spot 12 | 20.46 | 3.94 | 14.48 | 57.02 | 4.10 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, F.; Cai, Z.; Liu, J.; Liu, Y.; Zhang, M.; Fu, Y.; Zhu, K.; Chen, G.; Li, C. Preparation of Sr2CeZrO6 Refractory and Its Interaction with TiAl Alloy. Materials 2023, 16, 7298. https://doi.org/10.3390/ma16237298
Bian F, Cai Z, Liu J, Liu Y, Zhang M, Fu Y, Zhu K, Chen G, Li C. Preparation of Sr2CeZrO6 Refractory and Its Interaction with TiAl Alloy. Materials. 2023; 16(23):7298. https://doi.org/10.3390/ma16237298
Chicago/Turabian StyleBian, Fuli, Zheyu Cai, Jian Liu, Yu Liu, Man Zhang, Yixin Fu, Kailiang Zhu, Guangyao Chen, and Chonghe Li. 2023. "Preparation of Sr2CeZrO6 Refractory and Its Interaction with TiAl Alloy" Materials 16, no. 23: 7298. https://doi.org/10.3390/ma16237298





