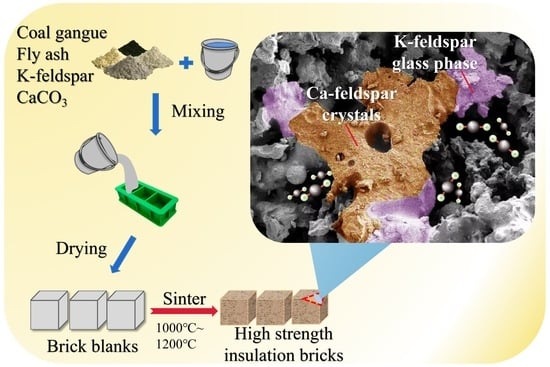
Newly Generated Ca-Feldspar during Sintering Processes Enhances the Mechanical Strength of Coal Gangue-Based Insulation Bricks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Coal-Gangue-Based Thermal Insulation Bricks
2.2. Performance Testing of Coal-Gangue-Based Thermal Insulation Bricks
2.3. Characterization of Pore Structure and the Solid Phase Composition of Thermal Insulation Bricks
2.4. Statistical Analysis
3. Result and Discussions
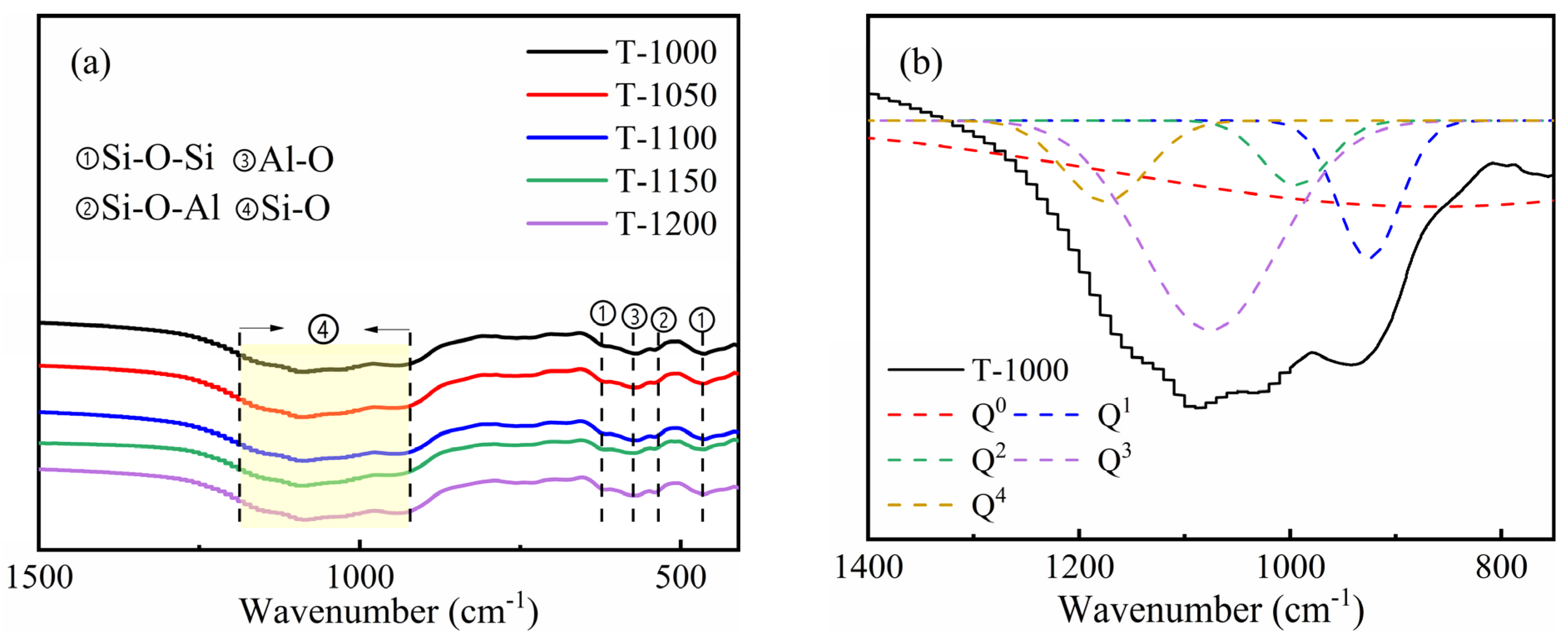
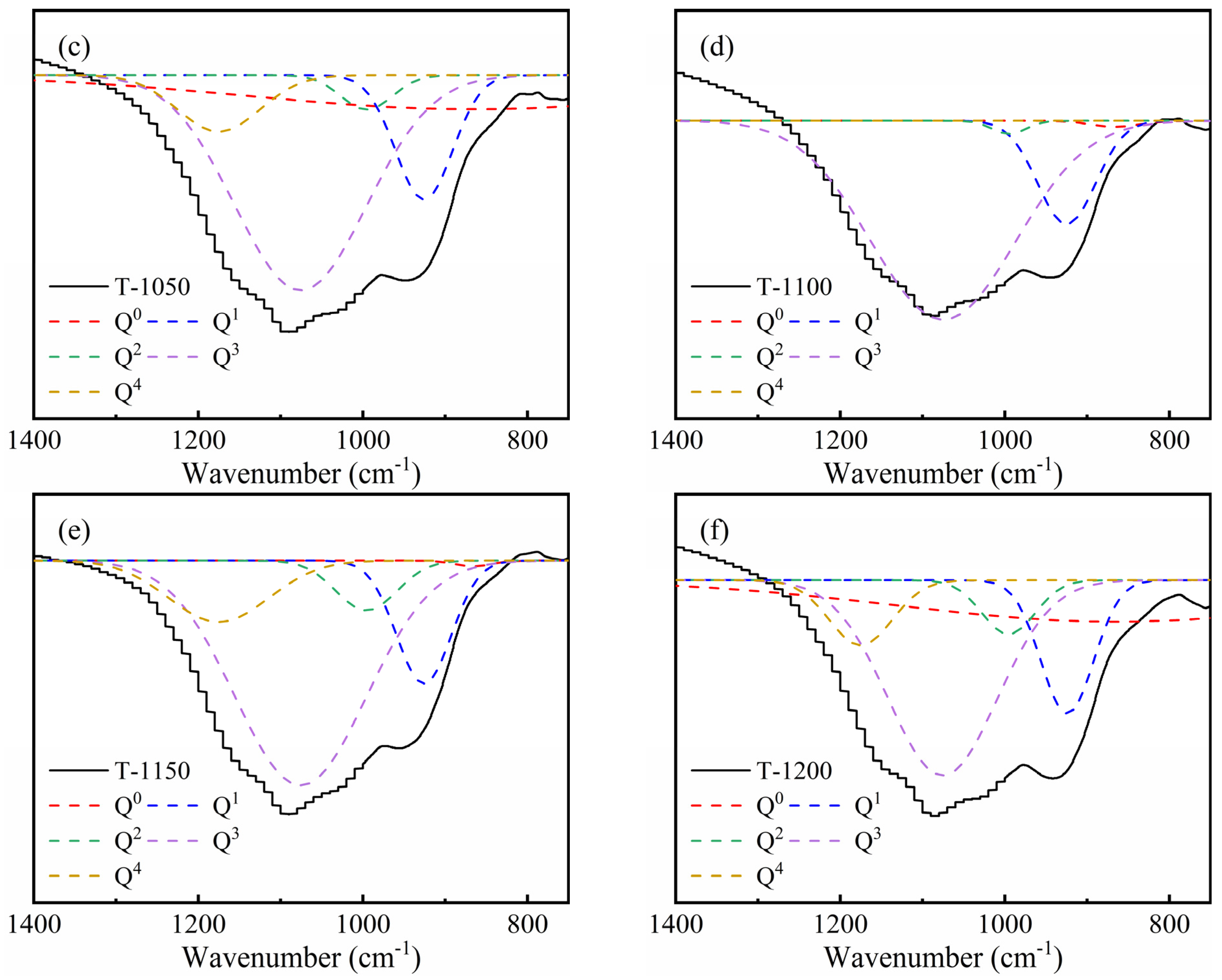
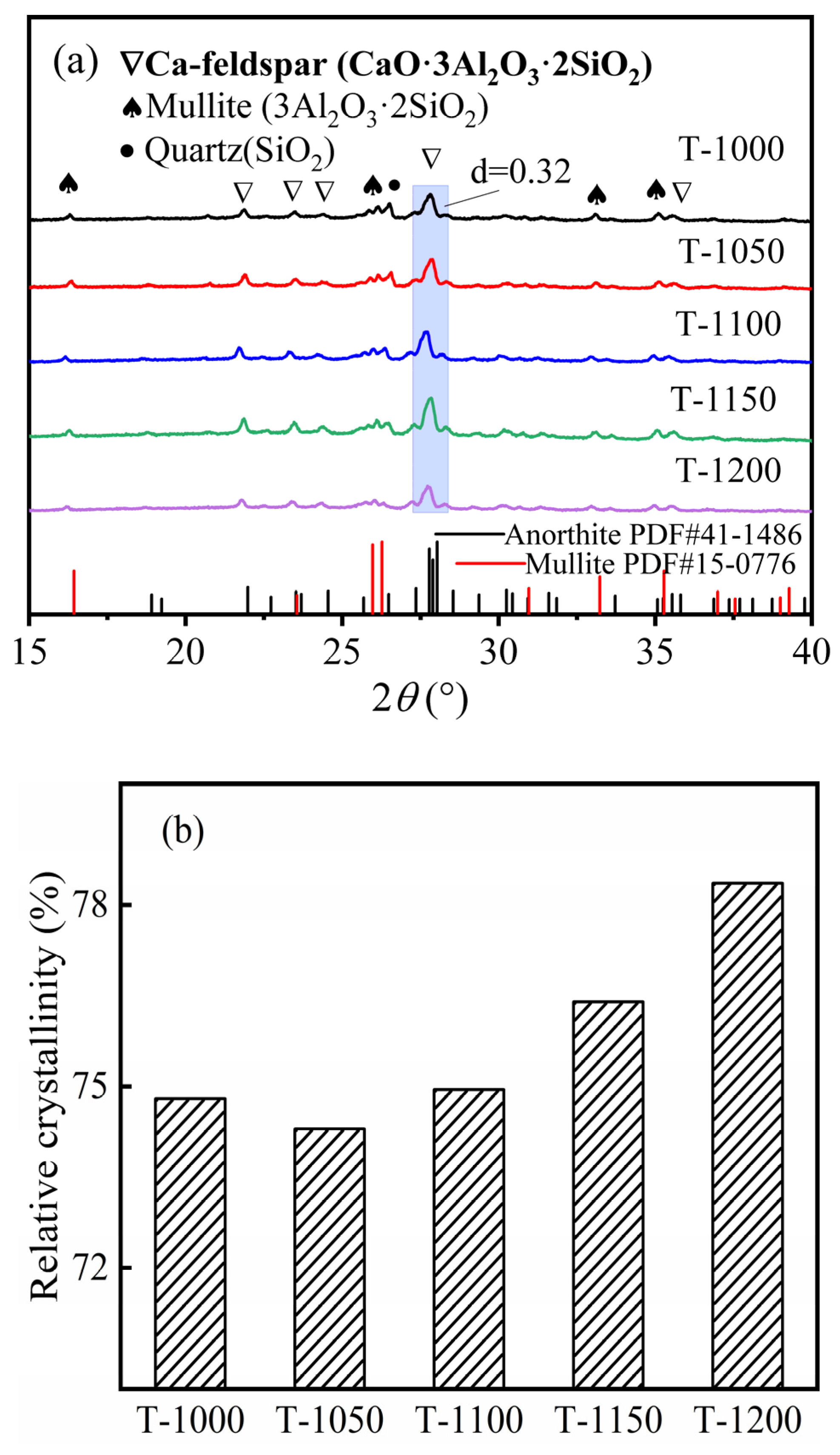
3.1. K-Feldspar Promotes the Conversion of CaCO3 to Ca-Feldspar
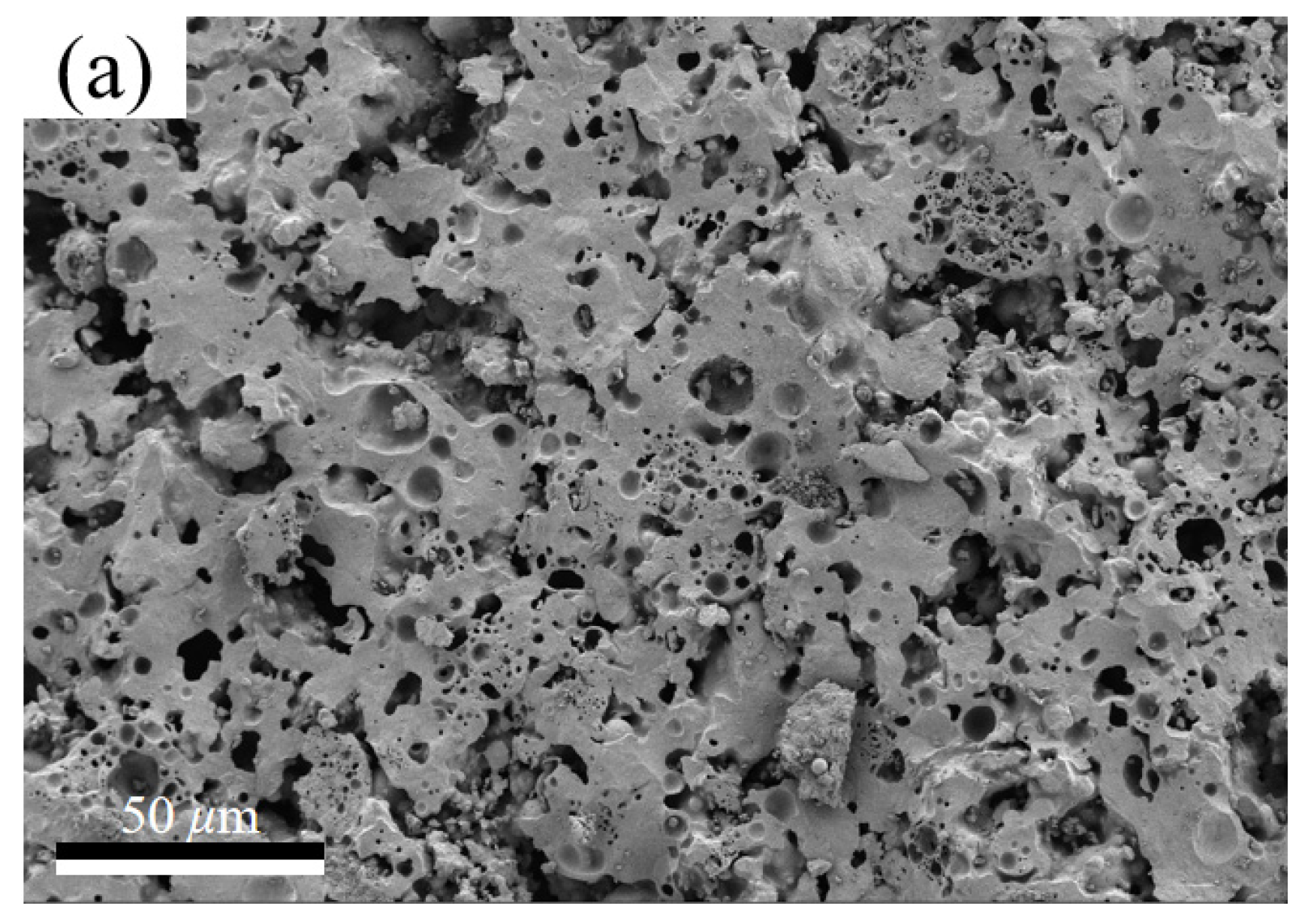
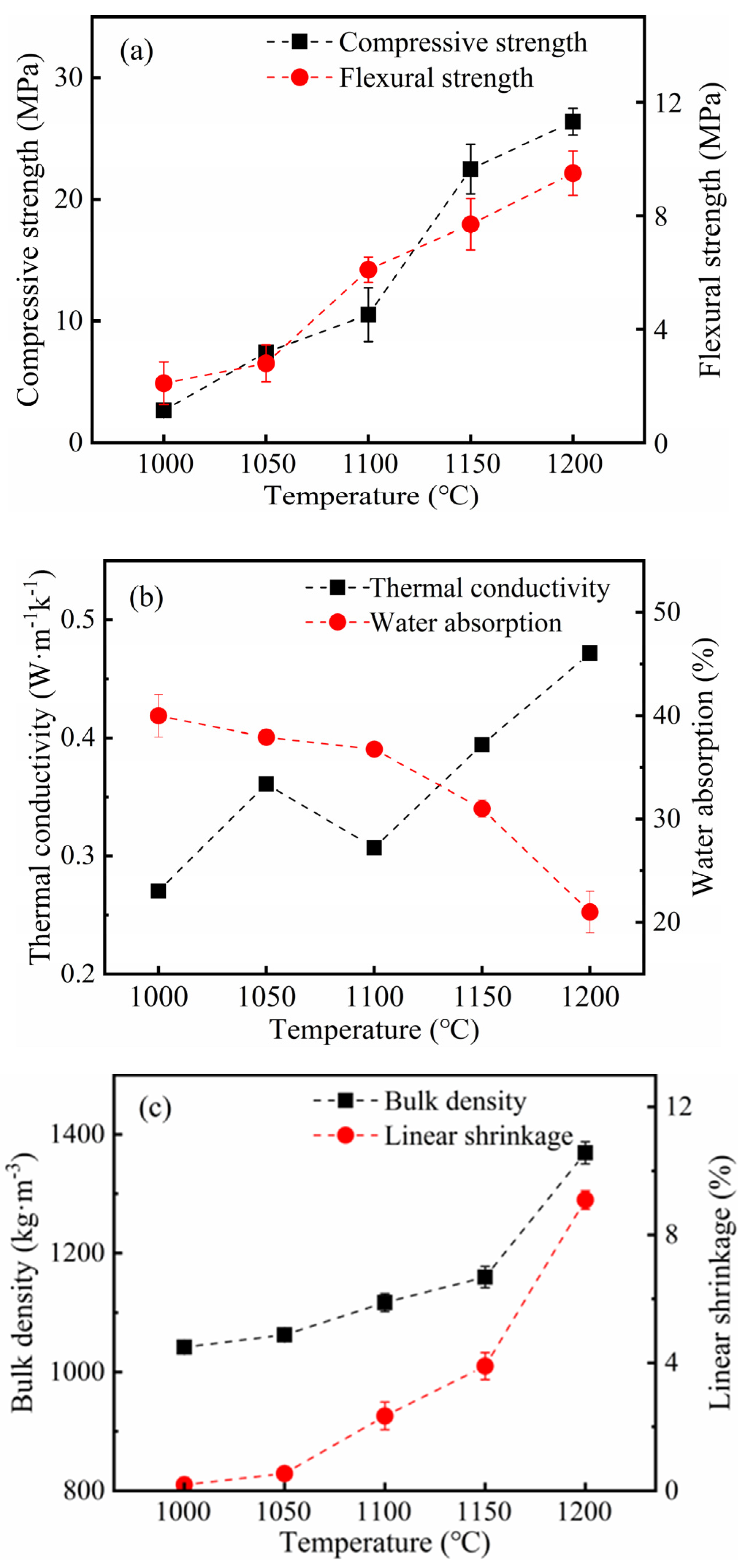
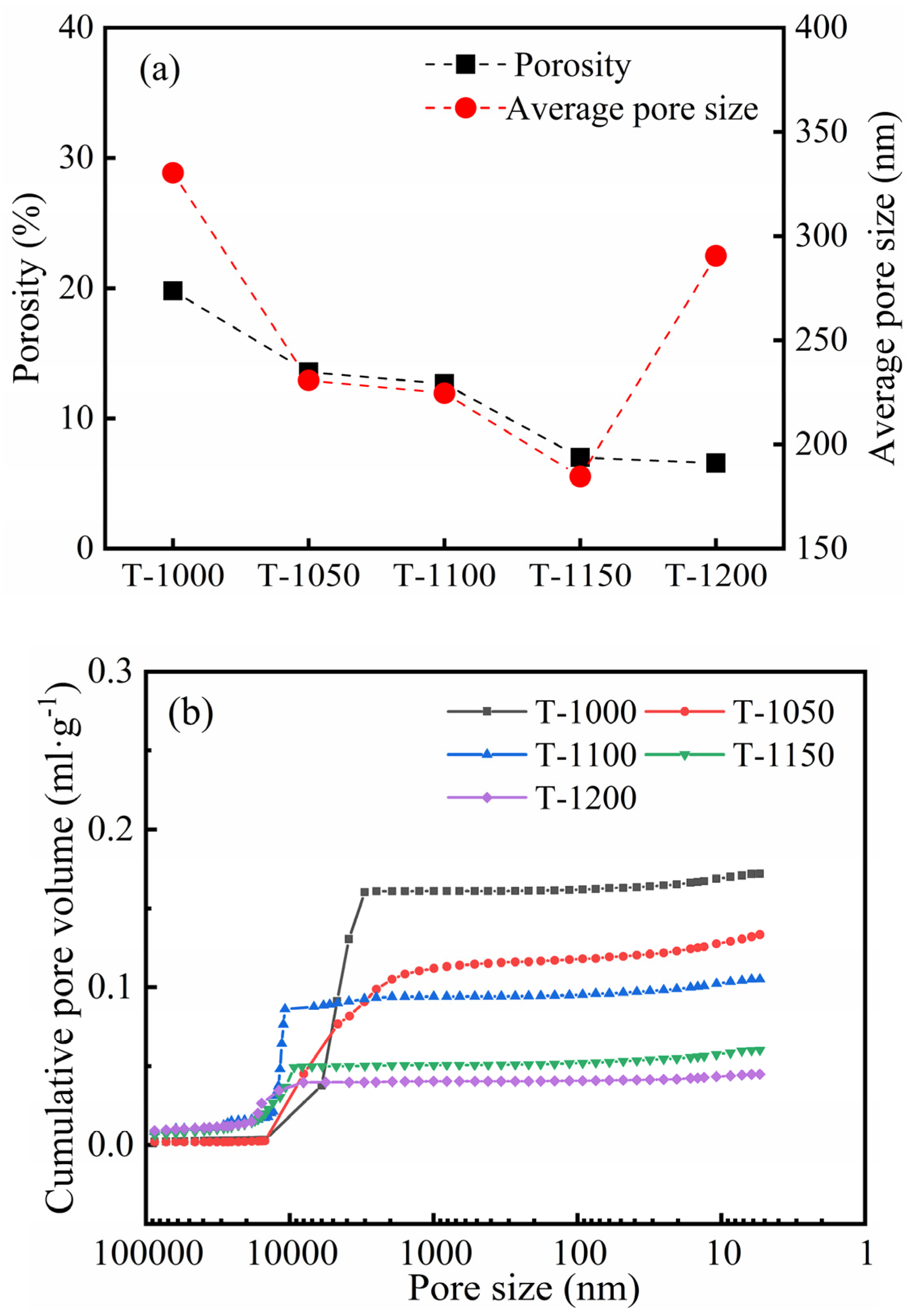
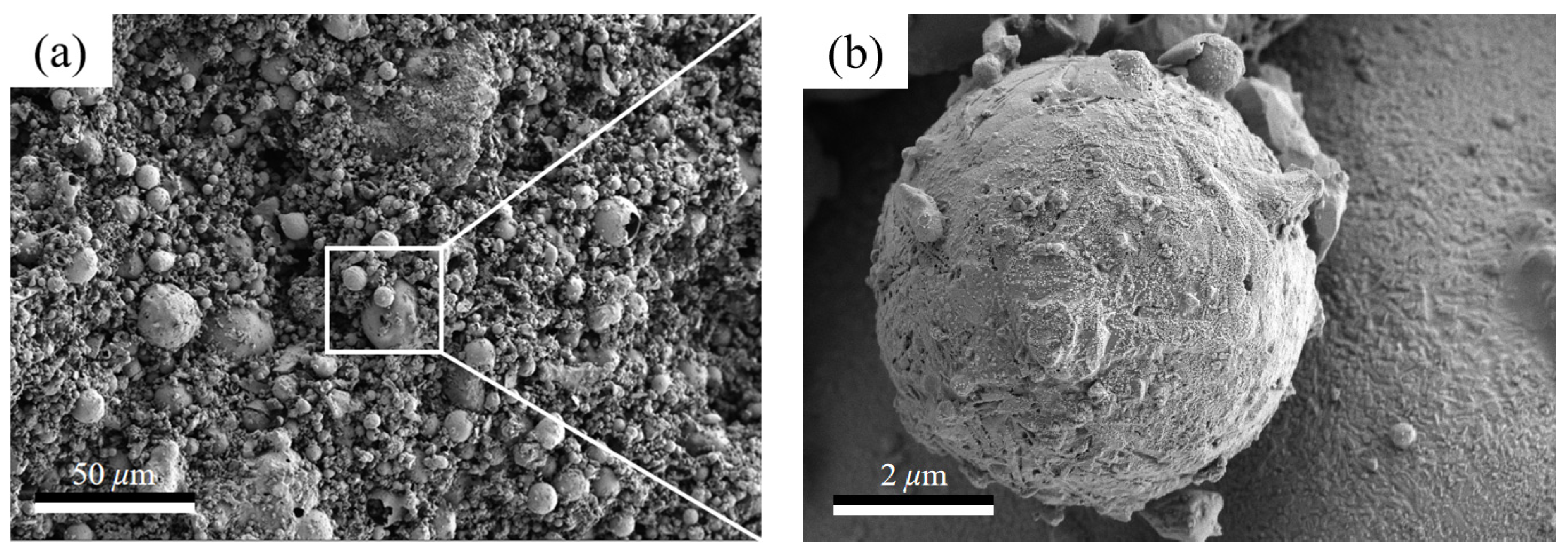
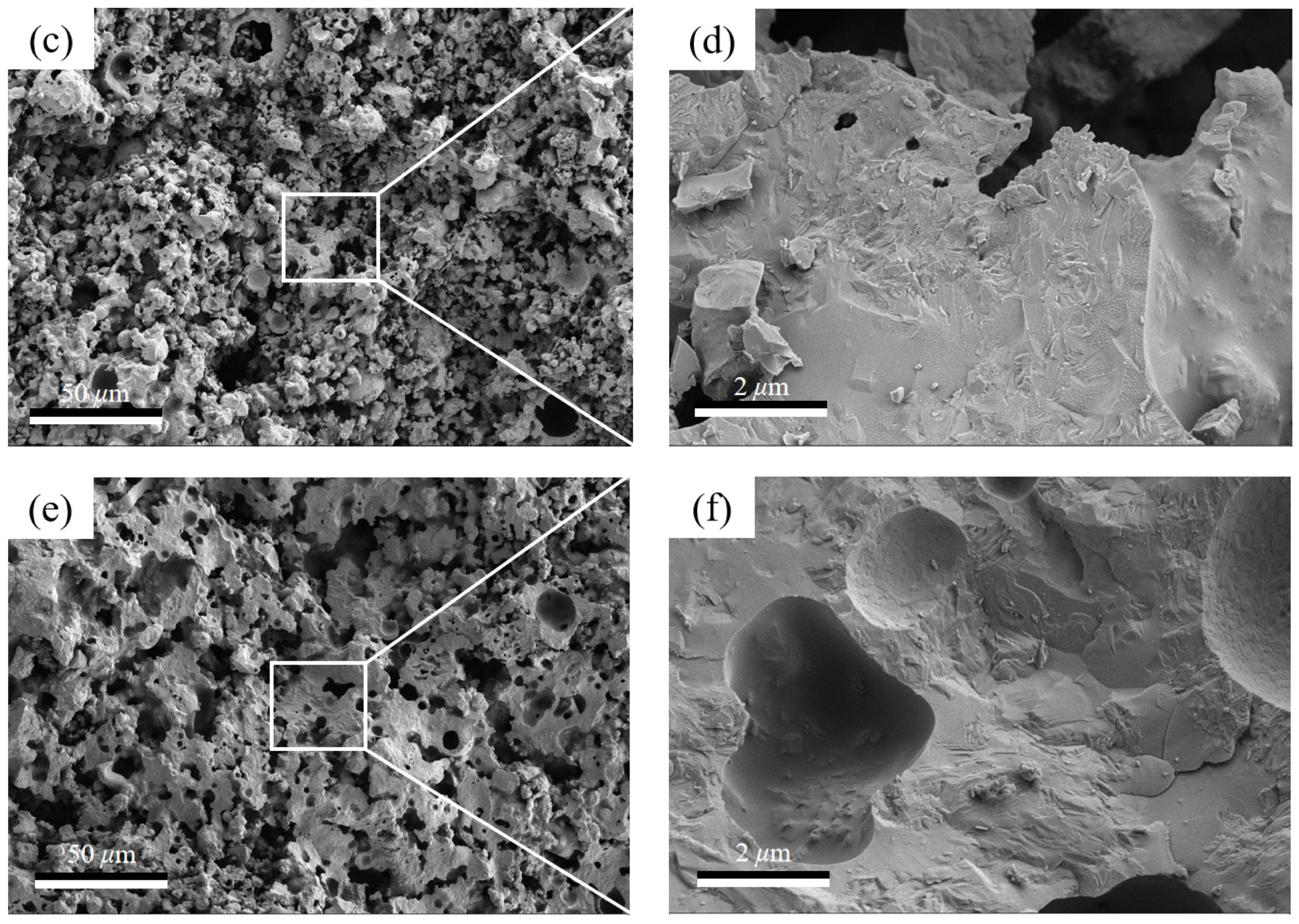
3.2. Sintering Temperature Affects the Silicate Polyhedral and Porous Structure of Thermal Insulation Bricks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Song, X.; Gong, C.; Pan, Z. Research on cementitious behavior and mechanism of pozzolanic cement with coal gangue. Cem. Concr. Res. 2006, 36, 1752–1759. [Google Scholar] [CrossRef]
- Cheng, Y.; Hongqiang, M.; Hongyu, C.; Jiaxin, W.; Jing, S.; Zonghui, L.; Mingkai, Y. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T.-C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Wang, S.; Du, Z.; Guo, Y.; Cheng, F. Synthesis and optimization of a novel poly-silicic metal coagulant from coal gangue for tertiary-treatment of coking wastewater. J. Chem. Technol. Biotechnol. 2018, 93, 365–371. [Google Scholar] [CrossRef]
- Xu, H.; Song, W.; Cao, W.; Shao, G.; Lu, H.; Yang, D.; Chen, D.; Zhang, R. Utilization of coal gangue for the production of brick. J. Mater. Cycles Waste Manag. 2017, 19, 1270–1278. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, C.; Wang, Y.; Cai, J. Development of N-doped carbons from zeolite-templating route as potential electrode materials for symmetric supercapacitors. Int. J. Min. Met. Mater. 2018, 25, 1482–1492. [Google Scholar] [CrossRef]
- Wei, Q.; Gao, W.; Sui, X. Synthesis of low-temperature, fast, single-firing body for porcelain stoneware tiles with coal gangue. Waste Manag. Res. 2010, 28, 944–950. [Google Scholar] [CrossRef]
- Bilgin, N.; Yeprem, H.A.; Arslan, S.; Bilgin, A.; Günay, E.; Marşoglu, M. Use of waste marble powder in brick industry. Constr. Build. Mater. 2012, 29, 449–457. [Google Scholar] [CrossRef]
- Xu, Z.F.; Zhang, M.X.; Zhu, J.B. Application of fly ash and coal gangue for preparing high-class solid insulating brick by roasting at low temperature conditions. Adv. Mater. Res. 2012, 446–449, 883–889. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Gualtieri, A.F.; Gagliardi, S.; Ruffini, P.; Ferrari, R.; Hanuskova, M. Thermal conductivity of fired clays: Effects of mineralogical and physical properties of the raw materials. Appl. Clay Sci. 2010, 49, 269–275. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.; Pérez-Villarejo, L.; Garzón, E.; Sánchez-Soto, P.J. Influence of firing temperature on the ceramic properties of illite-chlorite-calcitic clays. Ceram. Int. 2023, 49, 24541–24557. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Iglesias-Godino, F.J.; Martínez-García, C.; Corpas-Iglesias, F.A. Valorization of biodiesel production residues in making porous clay brick. Fuel Process. Technol. 2012, 103, 166–173. [Google Scholar] [CrossRef]
- Mao, L.; Zeng, M.; Peng, M.; Hu, L.; Zhang, W. A comparison study of three fluxing agents in promoting properties and environmental safety of bricks incorporating hazardous waste. J. Clean. Prod. 2021, 292, 126086. [Google Scholar] [CrossRef]
- Cheng, L.; Yu, Y.Z.; Zhao, Y.H. The preparation of pavement brick and the research on compression resistance. Appl. Mech. Mater. 2013, 295–298, 1789–1792. [Google Scholar] [CrossRef]
- Mandal, A.K.; Verma, H.R.; Sinha, O.P. Utilization of aluminum plant’s waste for production of insulation bricks. J. Clean. Prod. 2017, 162, 949–957. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Corpas-Iglesias, F.A.; Pérez-Villarejo, L.; Iglesias-Godino, F.J. Recycling of sawdust, spent earth from oil filtration, compost and marble residues for brick manufacturing. Constr. Build. Mater. 2012, 34, 275–284. [Google Scholar] [CrossRef]
- Cultrone, G.; Aurrekoetxea, I.; Casado, C.; Arizzi, A. Sawdust recycling in the production of lightweight bricks: How the amount of additive and the firing temperature influence the physical properties of the bricks. Constr. Build. Mater. 2020, 235, 117436. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liang, S.; Zhang, Y.; Ye, Q.; Zhang, L.; Pan, C.; Lv, R.; Li, Q.; Xiao, K.; et al. Preparation of water-permeable bricks derived from fly ash by autoclaving. Constr. Build. Mater. 2021, 271, 121556. [Google Scholar] [CrossRef]
- Dai, Z.; Zhou, H.; Zhang, W.; Hu, L.; Huang, Q.; Mao, L. The improvement in properties and environmental safety of fired clay bricks containing hazardous waste electroplating sludge: The role of Na2SiO3. J. Clean. Prod. 2019, 228, 1455–1463. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, B.; Ding, Y.; Zhang, J.; Wen, Q.; Ekberg, C.; Zhang, S. Study on glass-ceramics made from MSWI fly ash, pickling sludge and waste glass by one-step process. J. Clean. Prod. 2020, 271, 122674. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Mansori, M.; Hakkou, R. Recycling feasibility of glass wastes and calamine processing tailings in fired bricks making. Waste Biomass Valoriz. 2017, 8, 1479–1489. [Google Scholar] [CrossRef]
- Geng, R.; Xia, J.; Chen, Z.; Yang, J.; Zheng, S.; Liu, H. Effects of Potassium Feldspar on Slagging and Fluxing in Phosphorus Produced via Electric Furnace. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 475–480. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, F.; Zhu, R.; Dai, G.; Wang, W.; Wang, W.; Guo, X.; Jiang, J.; Wang, Z. Mechanism analysis of pore structure and crystalline phase of thermal insulation bricks with high municipal sewage sludge content. Constr. Build. Mater. 2020, 263, 120021. [Google Scholar] [CrossRef]
- Adesoji Adediran, A.; Abiodun Balogun, O.; Adewale Akinwande, A.; Seun Adesina, O.; Samson Bello, O. Influence of waste glass and particulate coconut shells as reinforcement materials in the production of masonry bricks. J. Mater. Civ. Eng. 2021, 33, 4021276. [Google Scholar] [CrossRef]
- Kairakbaev, A.K.; Abdrakhimov, V.Z.; Abdrakhimova, E.S. Phase composition and physico-mechanical properties at different firing temperatures of ceramic earthquake-resistant bricks with the use of ferro-dust. Glass Ceram. 2021, 77, 478–482. [Google Scholar] [CrossRef]
- Zouaoui, H.; Bouaziz, J. Physical and mechanical properties improvement of a porous clay ceramic. Appl. Clay Sci. 2017, 150, 131–137. [Google Scholar] [CrossRef]
- Barnabas, A.A.; Balogun, O.A.; Akinwande, A.A.; Ogbodo, J.F.; Ademati, A.O.; Dongo, E.I.; Romanovski, V. Reuse of walnut shell waste in the development of fired ceramic bricks. Environ. Sci. Pollut. Res. 2023, 30, 11823–11837. [Google Scholar] [CrossRef]
- Wu, K.; Hu, Y.; Xu, L.; Zhang, L.; Zhang, X.; Su, Y.; Yang, Z. Recycling of sewage sludge in clay-free thermal insulation brick: Assessment of microstructure, performance, and environment impact. Environ. Sci. Pollut. Res. 2022, 29, 89184–89197. [Google Scholar] [CrossRef]
- Abjaghou, H.; Bourret, J.; Tessier-Doyen, N.; Fassier, M.; Bruneaux, M.A.; Lacanilao, A.; Quint, V.; Smith, A.; Smith, D.S.; Peyratout, C. Incorporation of wooden furniture wastes in fired clay bricks for improved thermal insulation: A feasability study. Waste Biomass Valoriz. 2020, 11, 6943–6951. [Google Scholar] [CrossRef]
- GB/T 26538-2011; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (2011), Fired Heat Preservation Brick and Block. China Building Materials Federation (CBMF): Beijing, China, 2011.
- Luo, L.; Li, K.; Fu, W.; Liu, C.; Yang, S. Preparation, characteristics and mechanisms of the composite sintered bricks Produced from shale, sewage sludge, coal gangue powder and iron ore tailings. Constr. Build. Mater. 2020, 232, 117250. [Google Scholar] [CrossRef]
- Vizcayno, C.; de Gutiérrez, R.M.; Castello, R.; Rodriguez, E.; Guerrero, C.E. Pozzolan Obtained by Mechanochemical and Thermal Treatments of Kaolin. Appl. Clay Sci. 2010, 49, 405–413. [Google Scholar] [CrossRef]
- Pang, Z.; Lv, X.; Yan, Z.; Liang, D.; Dang, J. Transition of blast furnace slag from silicate based to aluminate based: Electrical conductivity. Metall. Mater. Trans. B 2019, 50, 385–394. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Gao, J.; Qu, Z.; Zhang, Z. Effects of Cr2O3 and CaF2 on the Structure, Crystal growth behavior, and properties of augite-based glass ceramics. J. Eur. Ceram. Soc. 2019, 39, 4283–4291. [Google Scholar] [CrossRef]
- McMillan, P. Structural studies of silicate glasses and melts-applications and limitations of raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Sutcu, M.; Akkurt, S. The use of recycled paper processing residues in making porous brick with reduced thermal conductivity. Ceram. Int. 2009, 35, 2625–2631. [Google Scholar] [CrossRef]
- Ahmad, S.; Iqbal, Y.; Muhammad, R. Effects of coal and wheat husk additives on the physical, thermal and mechanical properties of clay bricks. Bol. Soc. Esp. Ceram. Vidrio 2017, 56, 131–138. [Google Scholar] [CrossRef]


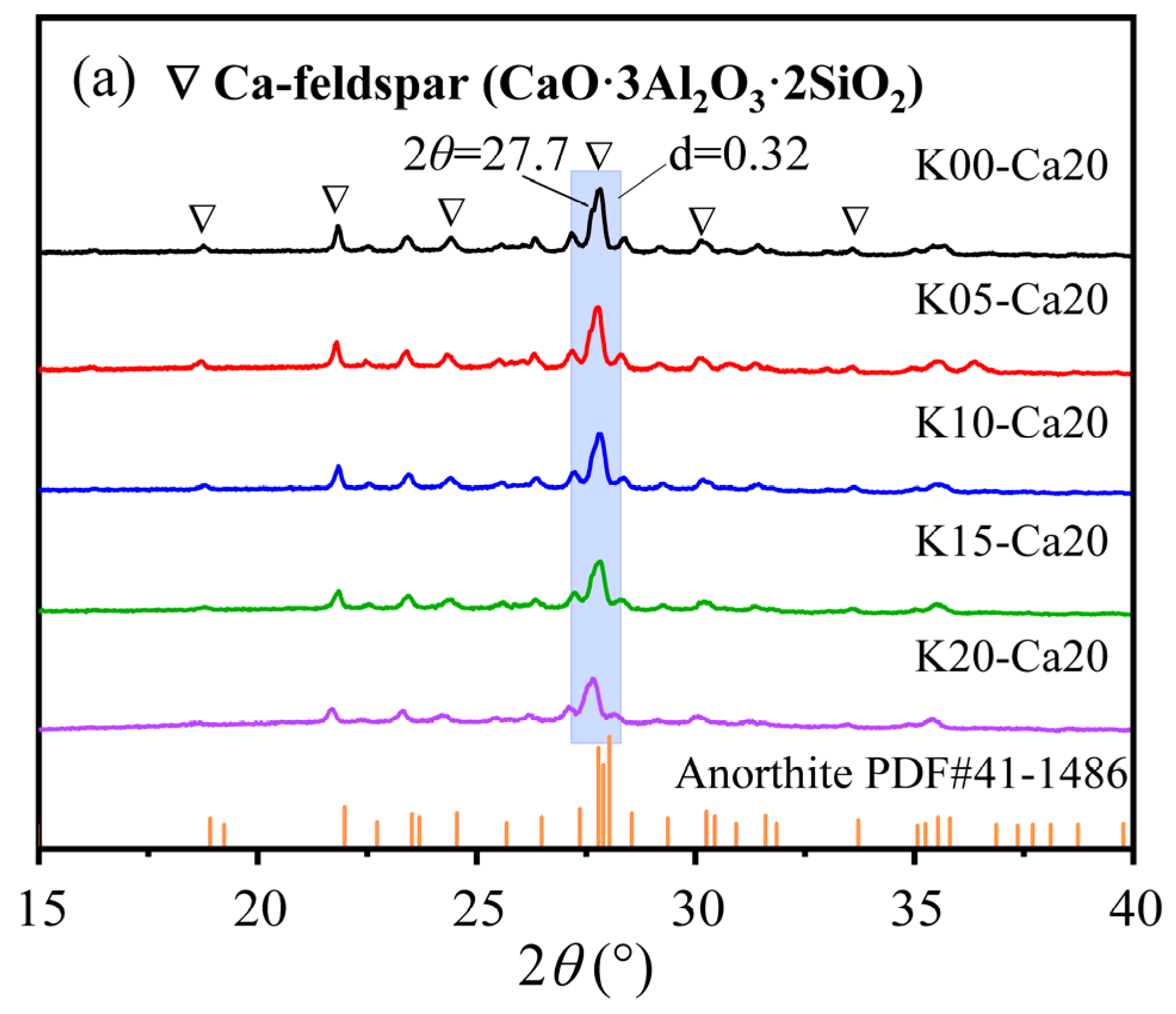
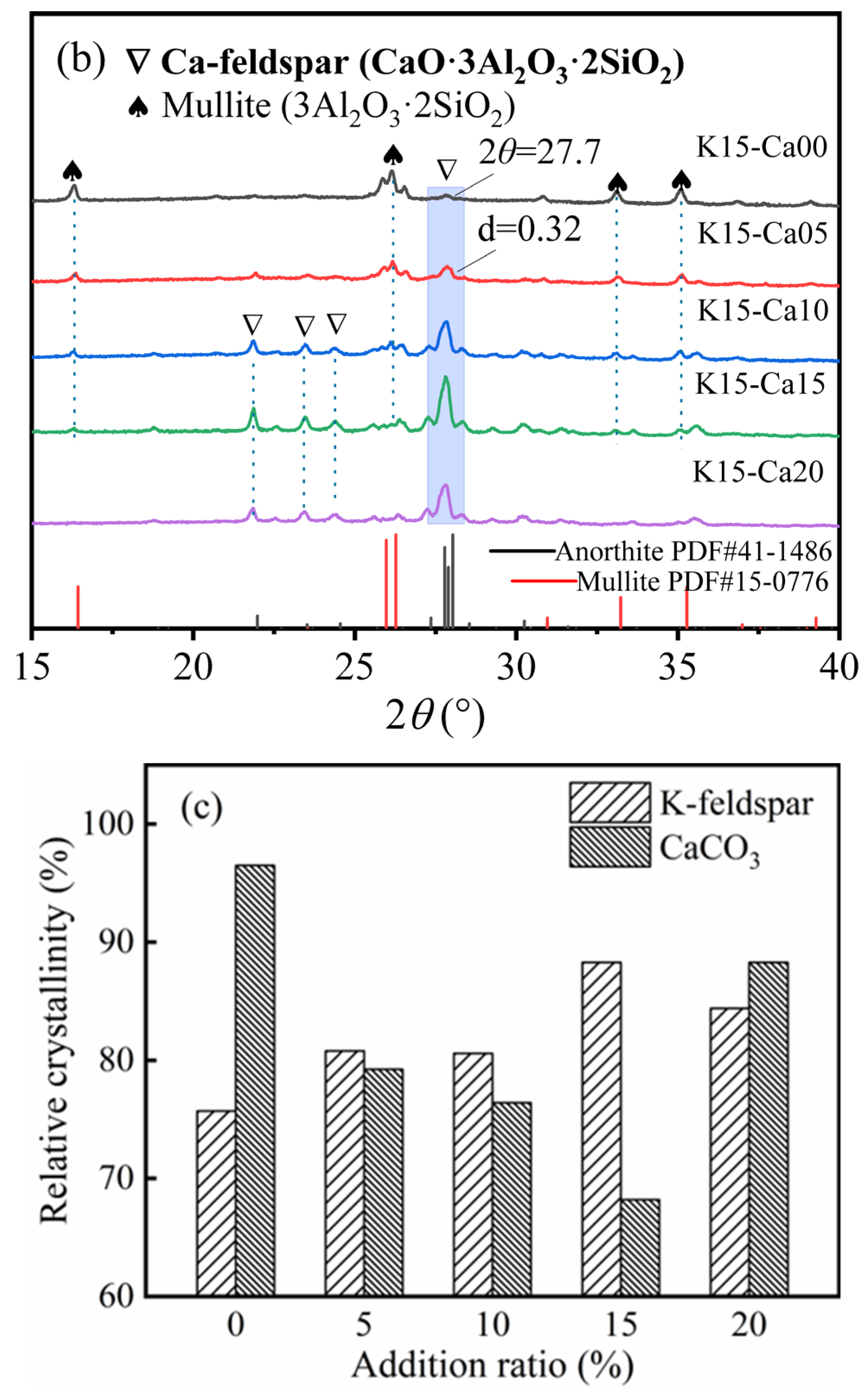
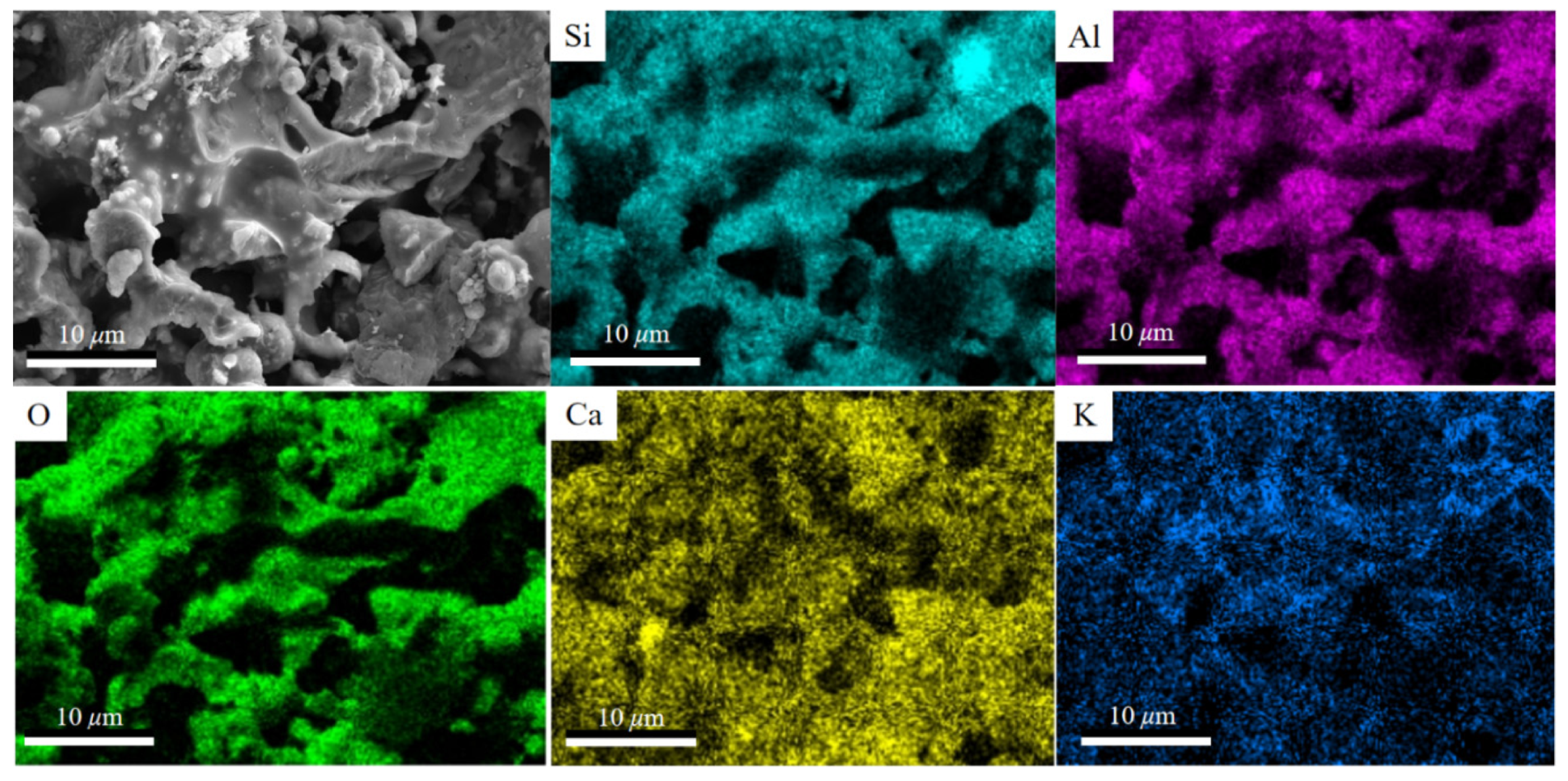



| K-Feldspar (%) | Fly Ash (%) | Coal Gangue (%) | CaCO3 (%) | |
|---|---|---|---|---|
| K00-Ca20 | 0 | 10 | 70 | 20 |
| K05-Ca20 | 5 | 10 | 65 | 20 |
| K10-Ca20 | 10 | 10 | 60 | 20 |
| K15-Ca20 | 15 | 10 | 55 | 20 |
| K20-Ca20 | 20 | 10 | 50 | 20 |
| K15-Ca00 | 15 | 10 | 75 | 0 |
| K15-Ca05 | 15 | 10 | 70 | 5 |
| K15-Ca10 | 15 | 10 | 65 | 10 |
| K15-Ca15 | 15 | 10 | 60 | 15 |
| K15-Ca20 | 15 | 10 | 55 | 20 |
| Coal Gangue | K-Feldspar | Fly Ash | |
|---|---|---|---|
| SiO2 | 39.7 | 64.1 | 45.4 |
| Al2O3 | 43.0 | 17.6 | 33.5 |
| Fe2O3 | 6.16 | 2.64 | 8.80 |
| CaO | 4.06 | 2.23 | 5.75 |
| K2O | 1.78 | 8.86 | 1.84 |
| ZnO | 0.04 | / | 0.05 |
| MgO | 0.49 | 0.37 | 0.63 |
| Na2O | 0.31 | 3.59 | 0.30 |
| TiO2 | 2.70 | 0.16 | / |
| MnO | 0.08 | 0.06 | 0.07 |
| P2O5 | 0.30 | 0.03 | 0.39 |
| SO3 | 1.08 | 0.03 | 1.07 |
| Sample | Q0 | Q1 | Q2 | Q3 | NBO/T |
|---|---|---|---|---|---|
| T-1000 | 51.4 | 8.61 | 4.36 | 28.9 | 2.69 |
| T-1050 | 26.1 | 12.5 | 3.16 | 50.0 | 1.98 |
| T-1100 | 0.82 | 16.8 | 1.25 | 81.1 | 1.37 |
| T-1150 | 0.46 | 14.8 | 6.71 | 64.6 | 1.24 |
| T-1200 | 33.1 | 13.3 | 5.47 | 40.4 | 2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Cui, J.; Gao, P.; Lv, J.; Chi, L.; Nan, H.; Huang, Y.; Yang, F. Newly Generated Ca-Feldspar during Sintering Processes Enhances the Mechanical Strength of Coal Gangue-Based Insulation Bricks. Materials 2023, 16, 7193. https://doi.org/10.3390/ma16227193
Zheng Y, Cui J, Gao P, Lv J, Chi L, Nan H, Huang Y, Yang F. Newly Generated Ca-Feldspar during Sintering Processes Enhances the Mechanical Strength of Coal Gangue-Based Insulation Bricks. Materials. 2023; 16(22):7193. https://doi.org/10.3390/ma16227193
Chicago/Turabian StyleZheng, Yangfan, Jiayan Cui, Pengxiao Gao, Junfan Lv, Lin Chi, Hongyan Nan, Yuandong Huang, and Fan Yang. 2023. "Newly Generated Ca-Feldspar during Sintering Processes Enhances the Mechanical Strength of Coal Gangue-Based Insulation Bricks" Materials 16, no. 22: 7193. https://doi.org/10.3390/ma16227193