Exploring the Detection of Cl− Penetration in Portland Cement Mortars via Surface Electrical Resistivity
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Method
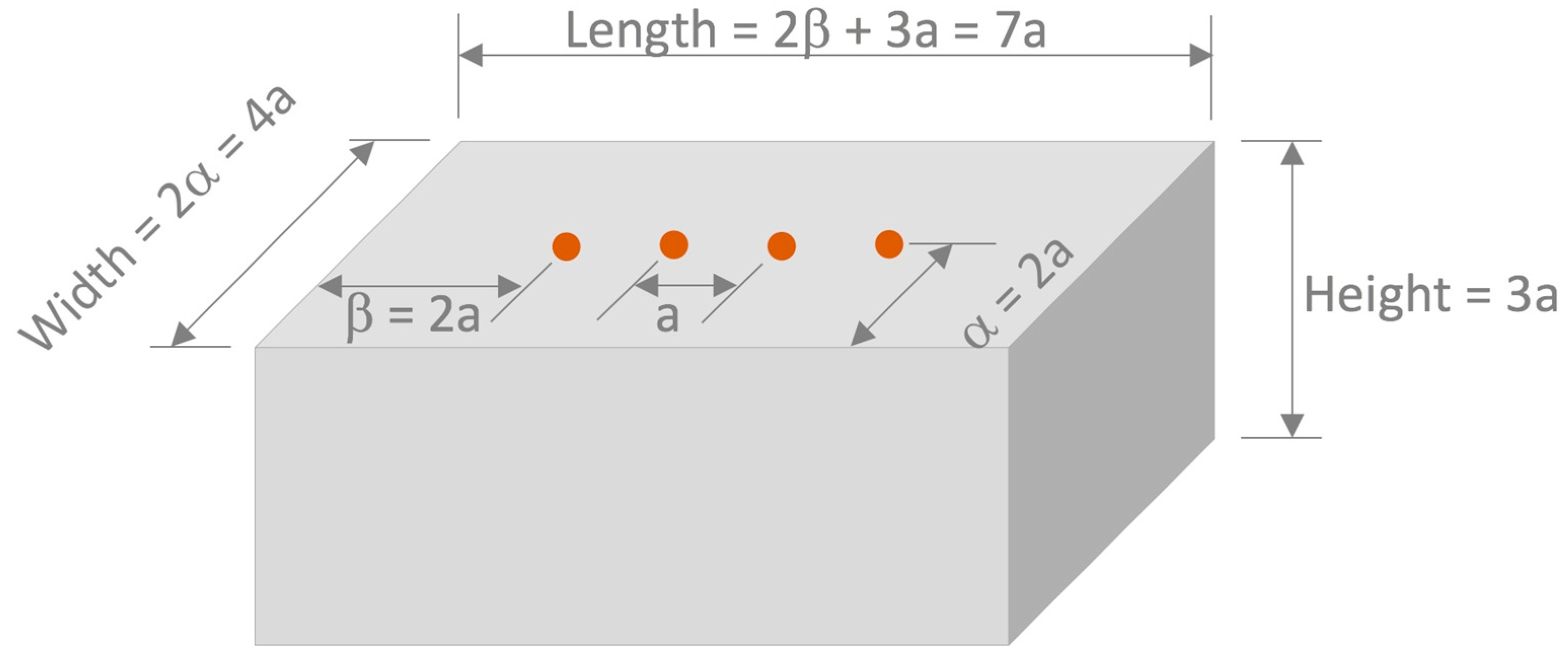
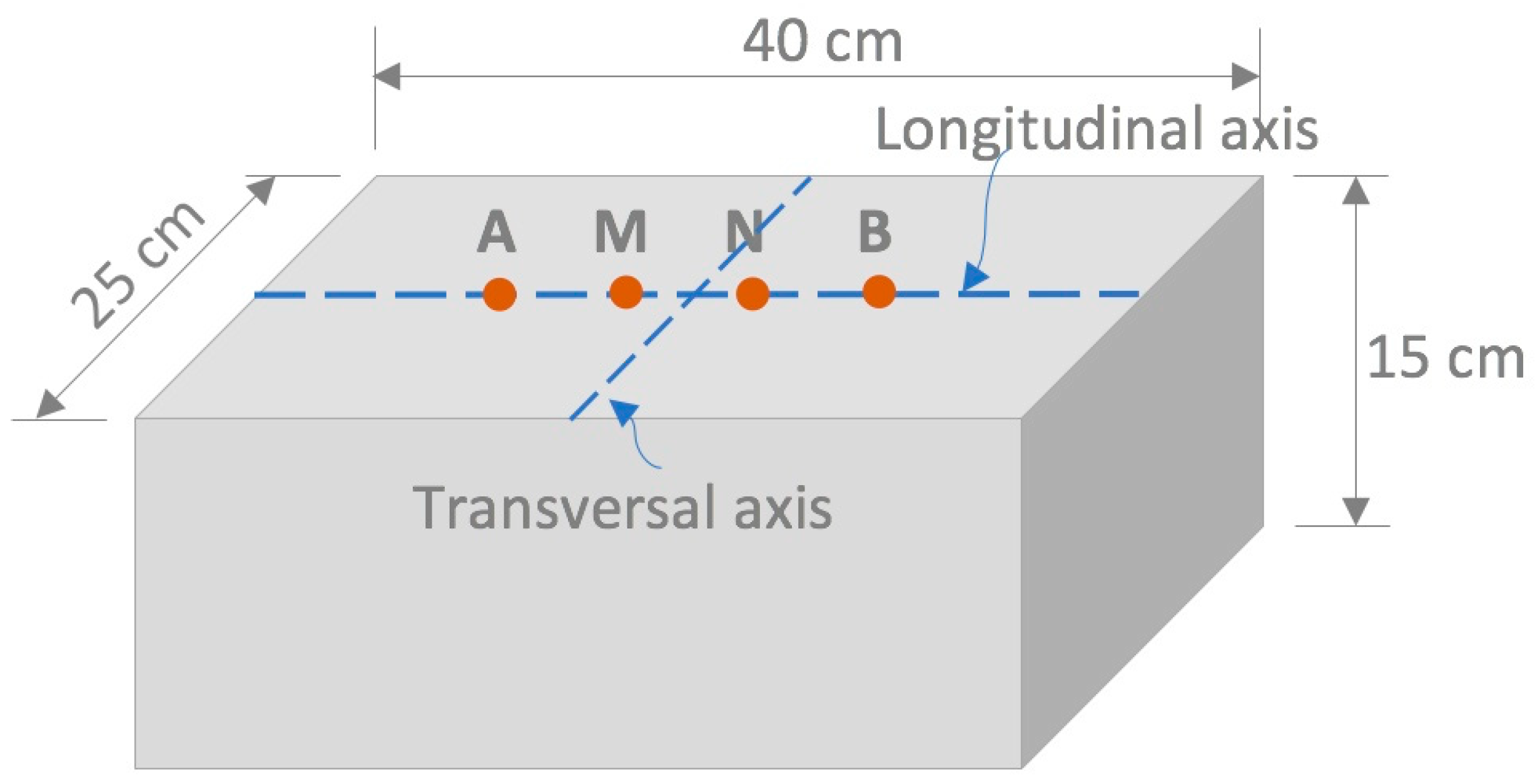
2.2.1. Preparation and Conditioning of Mortar Specimens
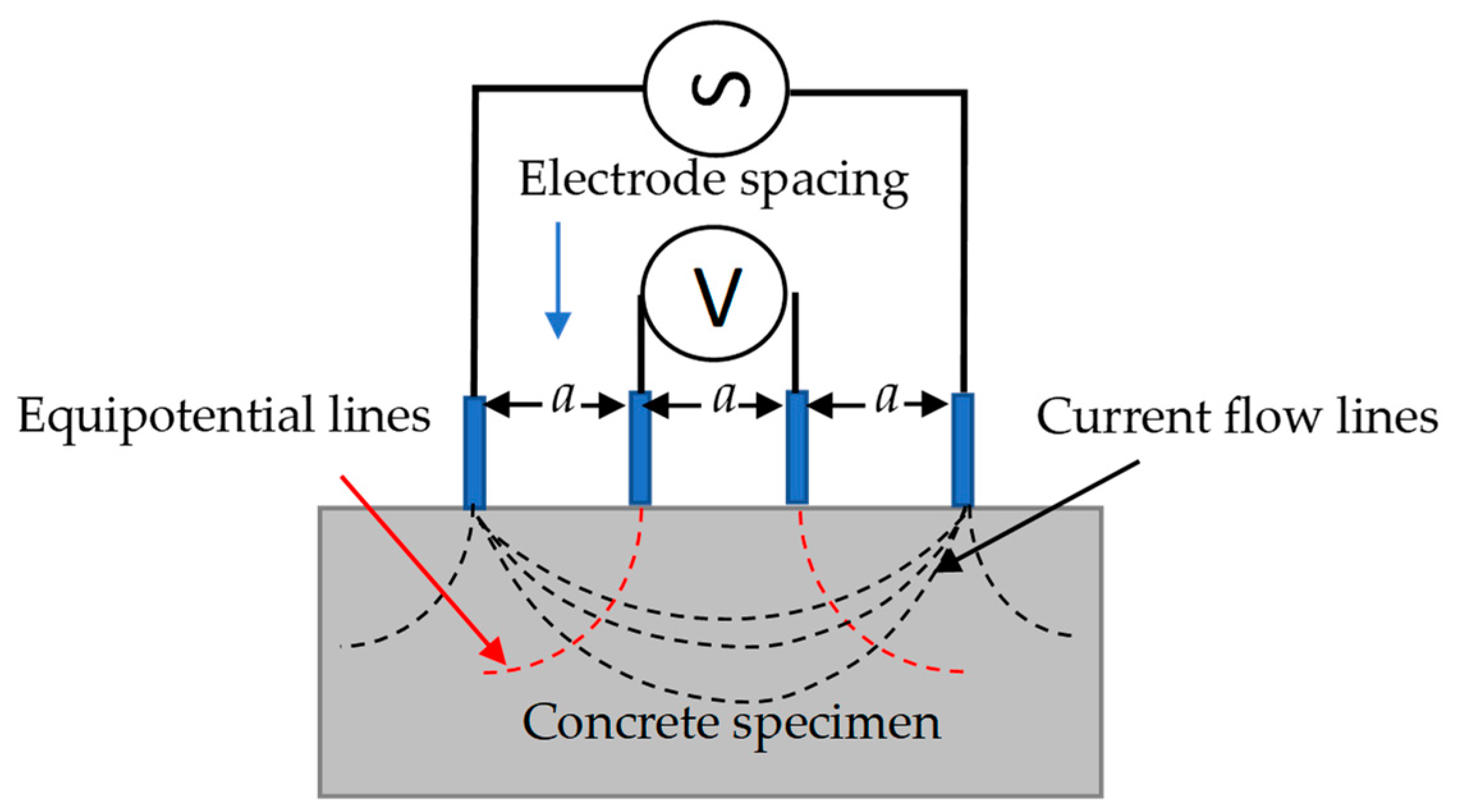
2.2.2. Surface Electrical Resistivity Measurements
2.2.3. Data Inversion Using the RES1D Free Software
2.2.4. Chloride Penetration Depth Using the Colorimetric Technique
2.2.5. Chloride Content Profiles
3. Results and Discussion
3.1. Electrical Resistivity Measurements
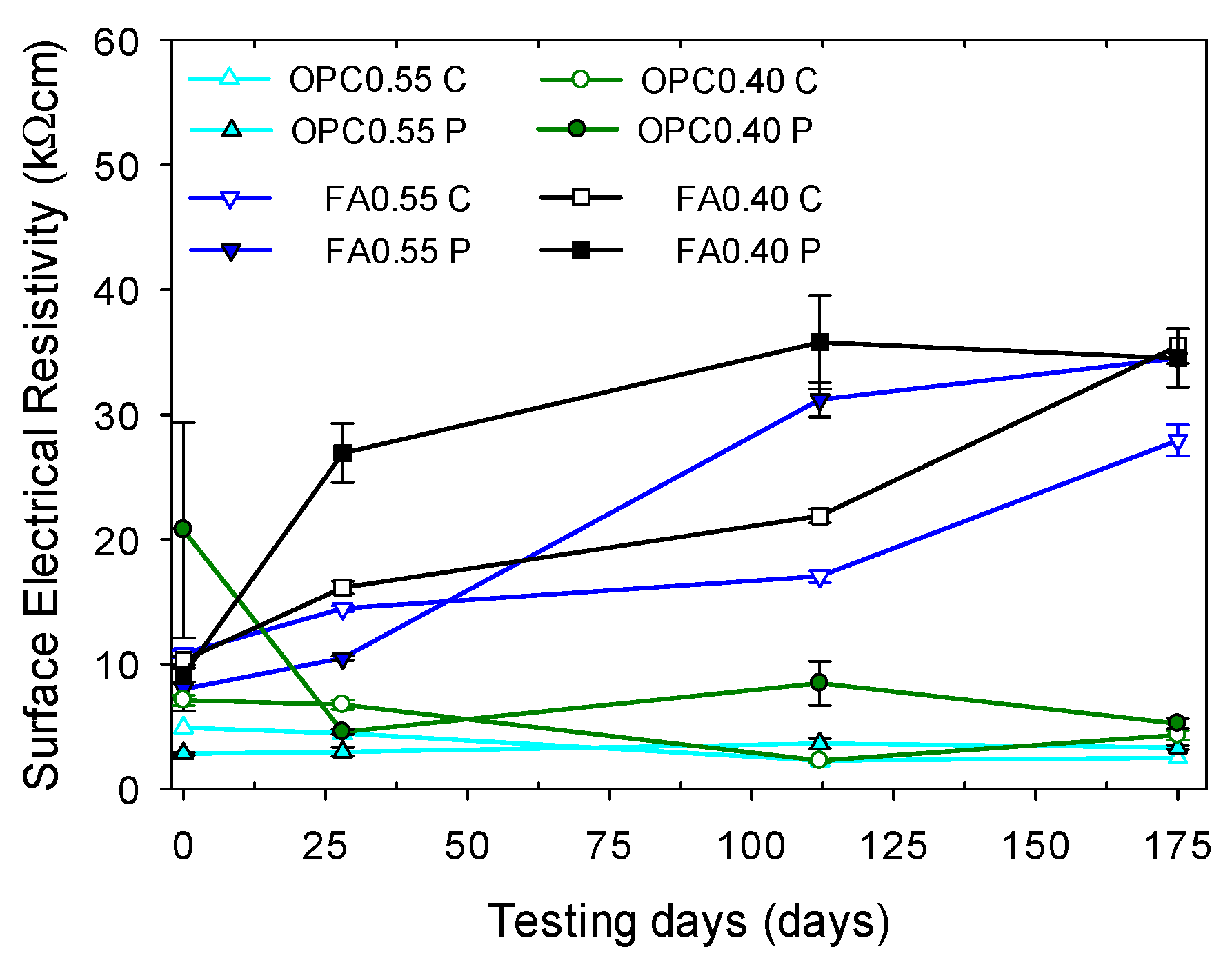
3.1.1. Electrical Resistivity of Chloride-Free Specimens
3.1.2. Electrical Resistivity of Mortars without Hydration Effects Exposed to Chlorides
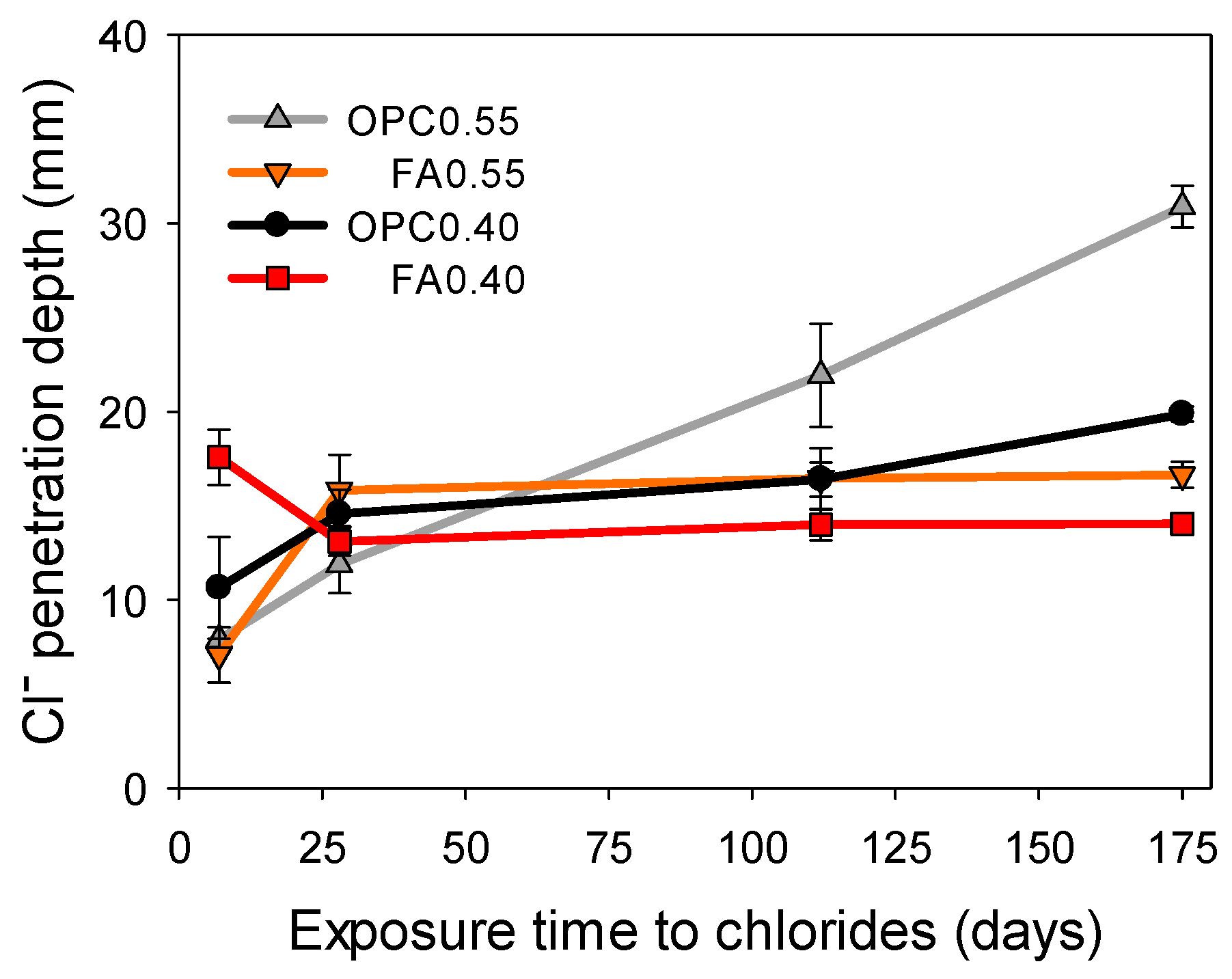
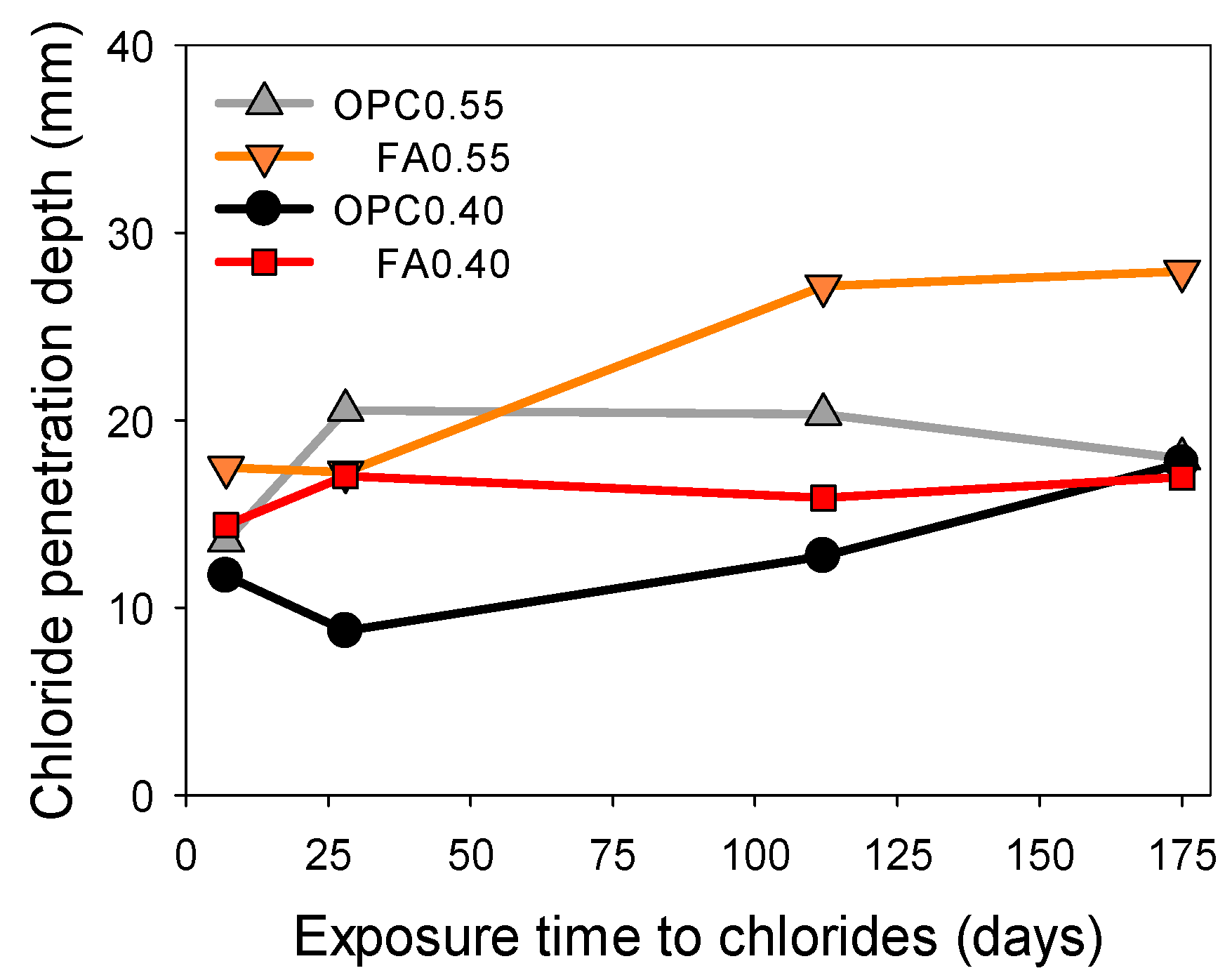
3.2. Chloride Penetration Depth Using Three Different Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neville, A. Chloride Attack of Reinforced Concrete: An Overview. Mater. Struct. 1995, 28, 63–70. [Google Scholar] [CrossRef]
- Spragg, R.; Bu, Y.; Snyder, K.; Bentz, D.; Weiss, J. Electrical Testing of Cement-Based Materials: Role of Testing Techniques, Sample Conditioning, and Accelerated Curing; Indiana Department of Transportation and Purdue University: West Lafayette, IN, USA, 2013. [Google Scholar]
- Spragg, R.; Villani, C.; Snyder, K.; Bentz, D.; Bullard, J.; Weiss, J. Factors That Influence Electrical Resistivity Measurements in Cementitious Systems. Transp. Res. Rec. J. Transp. Res. Board 2013, 2342, 90–98. [Google Scholar] [CrossRef]
- Balonis, M.; Lothenbach, B.; Le Saout, G.; Glasser, F.P. Impact of Chloride on the Mineralogy of Hydrated Portland Cement Systems. Cem. Concr. Res. 2010, 40, 1009–1022. [Google Scholar] [CrossRef]
- Marvila, M.T.; de Azevedo, A.R.G.; Ferreira, R.L.S.; Vieira, C.M.F.; de Brito, J.; Adesina, A. Validation of Alternative Methodologies by Using Capillarity in the Determination of Porosity Parameters of Cement-Lime Mortars. Mater. Struct. Constr. 2022, 55, 19. [Google Scholar] [CrossRef]
- Coates, G.R.; Xiao, L.; Prammer, M.G. NMR Logging Principles and Applications; Halliburton Energy Services: Houston, TX, USA, 1999; ISBN 9780080438801. [Google Scholar]
- ASTM C1202; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM International: West Conshohocken, PA, USA, 2012.
- NT BUILD 492; Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady-State Migration Experiments. NORDTEST: Espoo, Finland, 1999.
- AASHTO T259; Standard Method of Test for Resistance of Concrete to Chloride Ion Penetration. American Association of State Highway and Transportation Officials: Washington, DC, USA, 2009.
- NT BUILD 443; Concrete, Hardened: Accelerated Chloride Penetration. NORDTEST: Espoo, Finland, 2010.
- ASTM C1556; Standard Test Method for Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion. ASTM International: West Conshohocken, PA, USA, 2003.
- Jenkins, A. Surface Resistivity as an Alternative for Rapid Chloride Permeability Test of Hardened Concrete (FHWA-KS-14-15); Kansas Department of Transportation Bureau of Research: Kansas City, MO, USA, 2015. [Google Scholar]
- ASTM C1876-19; Standard Test Method for Electrical Resistivity or Conductivity of Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- AASHTO TP 119; Standard Method of Test for Electrical Resistivity of a Concrete Cylinder Tested in a Uniaxial Resistance Test. American Association of State Highway and Transportation Officials: Washington, DC, USA, 2017.
- Morris, W.; Moreno, E.I.; Sagués, A.A. Practical Evaluation of Resistivity of Concrete in Test Cylinders Using a Wenner Array Probe. Cem. Concr. Res. 1996, 551–556. [Google Scholar] [CrossRef]
- Cosoli, G.; Mobili, A.; Tittarelli, F.; Revel, G.; Chiariotti, P. Electrical Resistivity and Electrical Impedance Measurement in Mortar and Concrete Elements: A Systematic Review. Appl. Sci. 2020, 10, 9152. [Google Scholar] [CrossRef]
- Millard, S.G. Reinforced Concrete Resistivity Measurement Techniques. Proc.-Inst. Civ. Eng. Part 2 Res. Theory 1991, 91, 71–88. [Google Scholar] [CrossRef]
- Oleiwi, H.; Wang, Y.; Xiang, N.; Chen, X.; Shabalin, I. An Experimental Study of Concrete Resistivity and the Effects of Electrode Configuration and Current Frequency on Measurement. In Proceedings of the Sixth International Conference on Durability of Concrete Structures, Leeds, UK, 18–20 July 2018; pp. 592–599. [Google Scholar]
- AASHTO T358-15; Standard Method of Test for Surface Resistivity Indication of Concrete’s Ability to Resist Chloride Ion Penetration. American Association of State Highway and Transportation Officials: Washington, DC, USA, 2015.
- Cheytani, M.; Chan, S.L.I. The Applicability of the Wenner Method for Resistivity Measurement of Concrete in Atmospheric Conditions. Case Stud. Constr. Mater. 2021, 15, e00663. [Google Scholar] [CrossRef]
- Robles, K.P.V.; Yee, J.J.; Kee, S.H. Effect of the Geometrical Constraints to the Wenner Four-Point Electrical Resistivity Test of Reinforced Concrete Slabs. Sensors 2021, 21, 4622. [Google Scholar] [CrossRef]
- Polder, R.B. Test Methods for on Site Measurement of Resistivity of Concrete—A RILEM TC-154 Technical Recommendation. Constr. Build. Mater. 2001, 15, 125–131. [Google Scholar] [CrossRef]
- Qiao, C.; Moradllo, M.K.; Hall, H.; Tyler Ley, M.; Weiss, W.J. Electrical Resistivity and Formation Factor of Air-Entrained Concrete. ACI Mater. J. 2019, 116, 85–93. [Google Scholar] [CrossRef]
- Layssi, H.; Ghods, P.; Alizadeh, A.; Salehi, M. Electrical Resistivity of Concrete. Concr. Int. 2015, 37, 41–46. [Google Scholar]
- Fares, M.; Villain, G.; Bonnet, S.; Palma Lopes, S.; Thauvin, B.; Thiery, M. Determining Chloride Content Profiles in Concrete Using an Electrical Resistivity Tomography Device. Cem. Concr. Compos. 2018, 94, 315–326. [Google Scholar] [CrossRef]
- ASTM C128-01; Standard Test Method for Density, Relative Density (Specific Gravity), and Absorption. ASTM International: West Conshohocken, PA, USA, 2001.
- Ramírez Ortiz, A.E. Respuesta Ultrasónica de Pastas de Cemento Sujetas a Difusión y Ligado de Iones Cloruro. Ph.D. Thesis, Instituto Politécnico Nacional, Ciudad de México, Mexico, 2018. [Google Scholar]
- ASTM C192/C192M; Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM C109/C109M-02; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2002.
- Minagawa, H.; Miyamoio, S.; Hisada, M. Relationship of Apparent Electrical Resistivity Measured by Four Probe Method with Water Content Distribution in Concrete. J. Adv. Concr. Technol. 2017, 15, 278–289. [Google Scholar] [CrossRef]
- Gowers, K.R.; Millard, S.G. Measurement of Concrete Resistivity for Assessment of Corrosion Severity of Steel Using Wenner Technique. ACI Mater. J. 1999, 96, 536–541. [Google Scholar] [CrossRef]
- Chen, C.T.; Chang, J.J.; Yeih, W.C. The Effects of Specimen Parameters on the Resistivity of Concrete. Constr. Build. Mater. 2014, 71, 35–43. [Google Scholar] [CrossRef]
- Loke, M. 1-D Resistivity, IP & SIP Inversion and Forward Modeling Wenner and Schlumberger Arrays. Available online: https://www.geotomosoft.com/downloads.php (accessed on 20 June 2019).
- Nokken, M.; Boddy, A.; Hooton, R.D.; Thomas, M.D.A. Time Dependent Diffusion in Concrete-Three Laboratory Studies. Cem. Concr. Res. 2006, 36, 200–207. [Google Scholar] [CrossRef]
- Bentz, E.C.; Thomas, M. Life-365 Service Life Prediction Model and Computer Program for Predicting the Service Life and Life-Cycle Cost of Reinforced Concrete Exposed to Chlorides. Available online: http://www.life-365.org/download.html (accessed on 15 January 2019).
- Villagrán-Zaccardi, Y.A. Ingreso de Cloruro En Hormigones Con Cemento Pórtland Compuesto. Influencias Del Tiempo y de La Capacidad de Fijación. Ph.D. Thesis, Universidad Nacional del Centro de la Provincia de Buenos Aires, Tandil, Argentina, 2009. [Google Scholar]
- Mangat, P.S.; Molloy, B.T. Prediction of Long Term Chloride Concentration in Concrete. Mater. Struct. 1994, 27M, 338–346. [Google Scholar] [CrossRef]
- Park, K.B.; Lee, H.S.; Wang, X.Y. Prediction of Time-Dependent Chloride Diffusion Coefficients for Slag-Blended Concrete. Adv. Mater. Sci. Eng. 2017, 2017, 1901459. [Google Scholar] [CrossRef]
- Polder, R.; Andrade, C.; Elsener, B.; Vennesland, Ø.; Gulikers, J.; Weidert, R.; Raupach, M. RILEM TC 154-EMC: Electrochemical Techniques for Measuring Metallic Corrosion. Test Methods for on Site Measurement of Resistivity of Concrete. Mater. Struct. 2000, 33, 603–611. [Google Scholar]
- Meck, E.; Sirivivatnanon, V. Field Indicator of Chloride Penetration Depth. Cem. Concr. Res. 2003, 33, 1113–1117. [Google Scholar] [CrossRef]
- Wang, Y.; Nanukuttan, S.; Bai, Y.; Yang, K.; Basheer, M. Electrical Resistance to Monitor Carbonation and Chloride Ingress. ACI Mater. J. 2019, 116, 129–138. [Google Scholar] [CrossRef]
- Loche, J.M.; Ammar, A.; Dumargue, P. Influence of the Migration of Chloride Ions on the Electrochemical Impedance Spectroscopy of Mortar Paste. Cem. Concr. Res. 2005, 35, 1797–1803. [Google Scholar] [CrossRef]
- Tibbetts, C.M.; Paris, J.M.; Ferraro, C.C.; Riding, K.A.; Towsend, T.G. Relating Water Permeability to Electrical Resistivity and Chloride Penetrability of Concrete Containing Different Supplementary Cementitious Different Supplementary Cementitious Materials. Cem. Concr. Compos. 2020, 107, 103491. [Google Scholar] [CrossRef]
- de Grazia, M.T.; Deda, H.; Sanchez, L.F.M. The Influence of the Binder Type & Aggregate Nature on the Electrical Resistivity of Conventional Concrete. J. Build. Eng. 2021, 43, 102540. [Google Scholar] [CrossRef]
- Medeiros, R.A.; Lima, M.G. Electrical Resistivity of Unsaturated Concrete Using Different Types of Cement. Constr. Build. Mater. 2016, 107, 11–16. [Google Scholar] [CrossRef]
- Kang, S.; Tyler Ley, M.; Lloyd, Z.; Kim, T. Using the Particle Model to Predict Electrical Resistivity Performance of Fly Ash in Concrete. Constr. Build. Mater. 2020, 261, 119975. [Google Scholar] [CrossRef]
- Hou, D.; Li, T.; Wang, P. Molecular Dynamics Study on the Structure and Dynamics of NaCl Solution Transport in the Nanometer Channel of CASH Gel. ACS Sustain. Chem. Eng. 2018, 6, 9498–9509. [Google Scholar] [CrossRef]
- du Plooy, R.; Villain, G.; Palma Lopes, S.; Ihamouten, A.; Dérobert, X.; Thauvin, B. Electromagnetic Non-Destructive Evaluation Techniques for the Monitoring of Water and Chloride Ingress into Concrete: A Comparative Study. Mater. Struct. Constr. 2015, 48, 369–386. [Google Scholar] [CrossRef]
- Samouëlian, A.; Cousin, I.; Tabbagh, A.; Bruand, A.; Richard, G. Electrical Resistivity Survey in Soil Science: A Review. Soil Tillage Res. 2005, 83, 173–193. [Google Scholar] [CrossRef]
- Horňáková, M.; Lehner, P. Relationship of Surface and Bulk Resistivity in the Case of Mechanically Damaged Fibre Reinforced Red Ceramic Waste Aggregate Concrete. Materials 2020, 23, 5501. [Google Scholar] [CrossRef]
- He, F.; Shi, C.; Yuan, Q.; Chen, C.; Zheng, K. AgNO3 Based Colorimetric Methods for Measurement of Chloride Penetration in Concrete. Constr. Build. Mater. 2012, 26, 1–8. [Google Scholar] [CrossRef]
- De Weerdt, K. Chloride Binding in Concrete: Recent Investigations and Recognised Knowledge Gaps: RILEM Robert L’Hermite Medal Paper 2021. Mater. Struct. 2021, 54, 1–16. [Google Scholar] [CrossRef]







| Main Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | P2O5 | MgO | Na2O | K2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 21.07 | 3.69 | 4.50 | 61.93 | 0.97 | 0.10 | 1.83 | 0.09 | 0.30 | 2.54 | 4.38 |
| FA | 62.28 | 20.38 | 4.09 | 4.68 | 0.94 | 0.38 | 0.98 | 0.31 | 0.99 | - | 3.43 |
| Material | Mix | |||
|---|---|---|---|---|
| (kg) | OPC0.55 | FA0.55 | OPC0.40 | FA0.40 |
| Cement | 518 | 301 | 562 | 326 |
| Fly ash | - | 200 | - | 217 |
| Sand | 1425 | 1381 | 1544 | 1493 |
| Water | 285 | 276 | 225 | 217 |
| Test | Type | Dimensions | Quantity | |||
|---|---|---|---|---|---|---|
| w (cm) | l (cm) | h (cm) | Ø (cm) | |||
| Surface electrical resistivity | Control prisms | 25 | 40 | 15 | 2 | |
| * Prisms | 25 | 40 | 15 | 2 | ||
| Control cylinders | 20 | 10 | 4 | |||
| Colorimetry/chloride content profiles | Control cubes * Cubes | 15 | 15 | 15 | 2 | |
| 15 | 15 | 15 | 8 | |||
| Exposure Time | OPC0.55 | FA0.55 | OPC0.40 | FA0.40 | ||||
|---|---|---|---|---|---|---|---|---|
| D | Cs | D | Cs | D | Cs | D | Cs | |
| (Days) | (m2/s) | (%) | (m2/s) | (%) | (m2/s) | (%) | (m2/s) | (%) |
| 7 | 2.6 × 10−11 | 1.09 | 4.1 × 10−11 | 0.98 | 3.3 × 10−11 | 1.00 | 1.2 × 10−10 | 1.85 |
| 28 | 6.8 × 10−11 | 0.79 | 5.0 × 10−11 | 1.08 | 7.0 × 10−11 | 0.70 | 2.9 × 10−11 | 0.96 |
| 112 | 1.4 × 10−11 | 1.62 | 5.6 × 10−12 | 3.00 | 1.8 × 10−11 | 1.04 | 5.0 × 10−12 | 2.12 |
| 175 | 2.6 × 10−11 | 1.41 | 5.6 × 10−12 | 2.46 | 1.4 × 10−11 | 1.00 | 4.0 × 10−12 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pablo-Calderón, M.A.; Cano-Barrita, P.F.d.J.; León-Martínez, F.M. Exploring the Detection of Cl− Penetration in Portland Cement Mortars via Surface Electrical Resistivity. Materials 2023, 16, 7123. https://doi.org/10.3390/ma16227123
Pablo-Calderón MA, Cano-Barrita PFdJ, León-Martínez FM. Exploring the Detection of Cl− Penetration in Portland Cement Mortars via Surface Electrical Resistivity. Materials. 2023; 16(22):7123. https://doi.org/10.3390/ma16227123
Chicago/Turabian StylePablo-Calderón, Miguel Alberto, Prisciliano Felipe de Jesús Cano-Barrita, and Frank Manuel León-Martínez. 2023. "Exploring the Detection of Cl− Penetration in Portland Cement Mortars via Surface Electrical Resistivity" Materials 16, no. 22: 7123. https://doi.org/10.3390/ma16227123





