MOF-808 as an Efficient Catalyst for Valorization of Biodiesel Waste Production: Glycerol Acetalization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Preparations of the Materials
2.3. Catalytic Studies
3. Results and Discussion
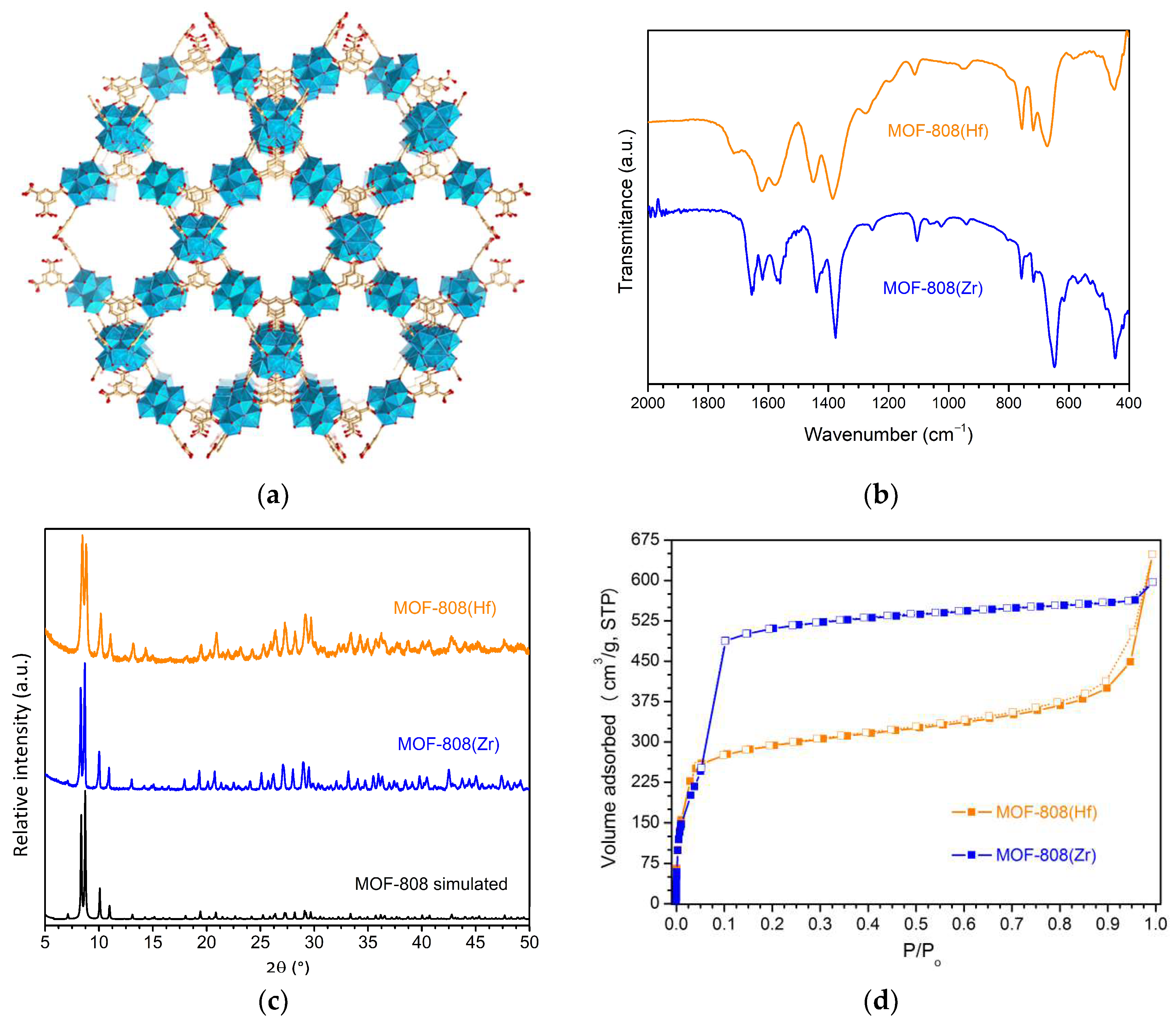
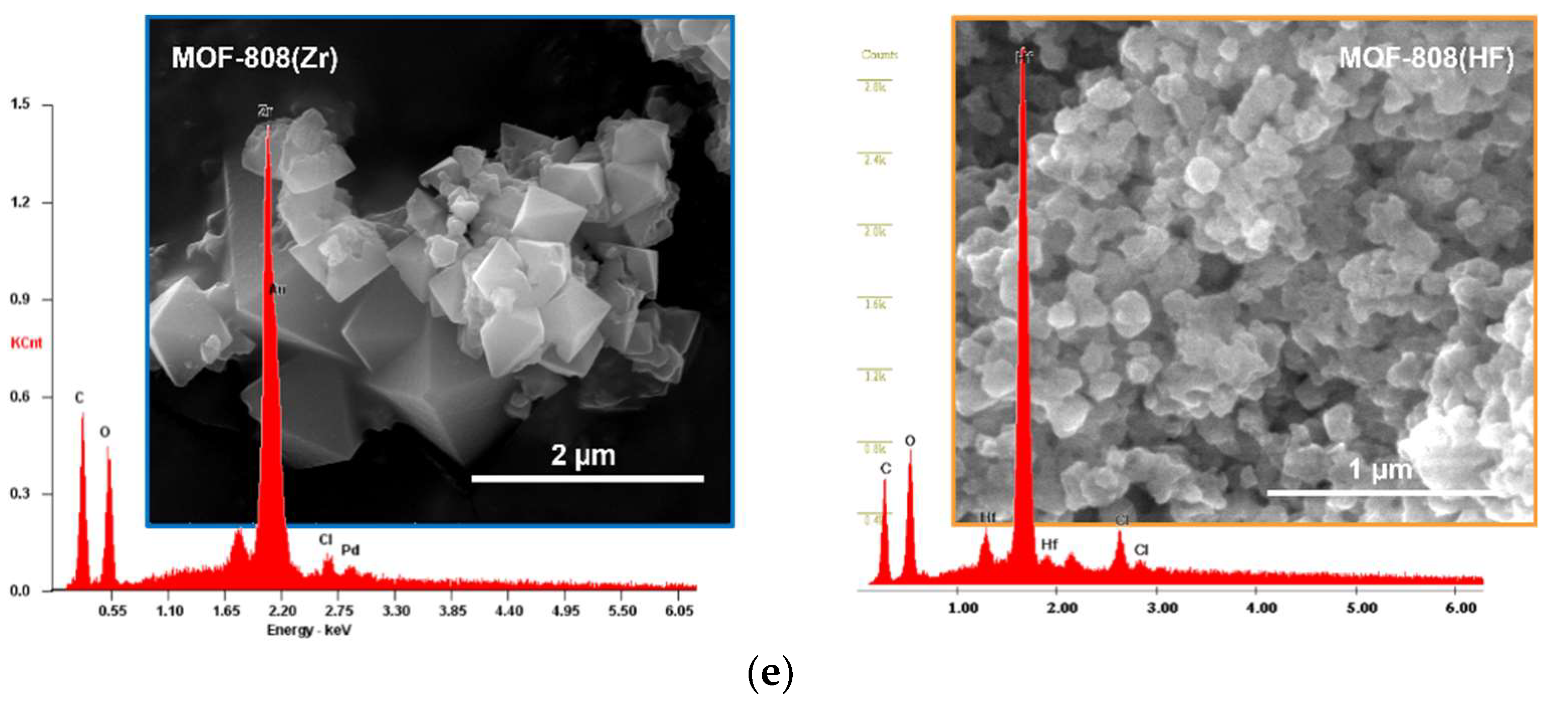
3.1. Catalysts Characterization
3.2. Acidity Characterization
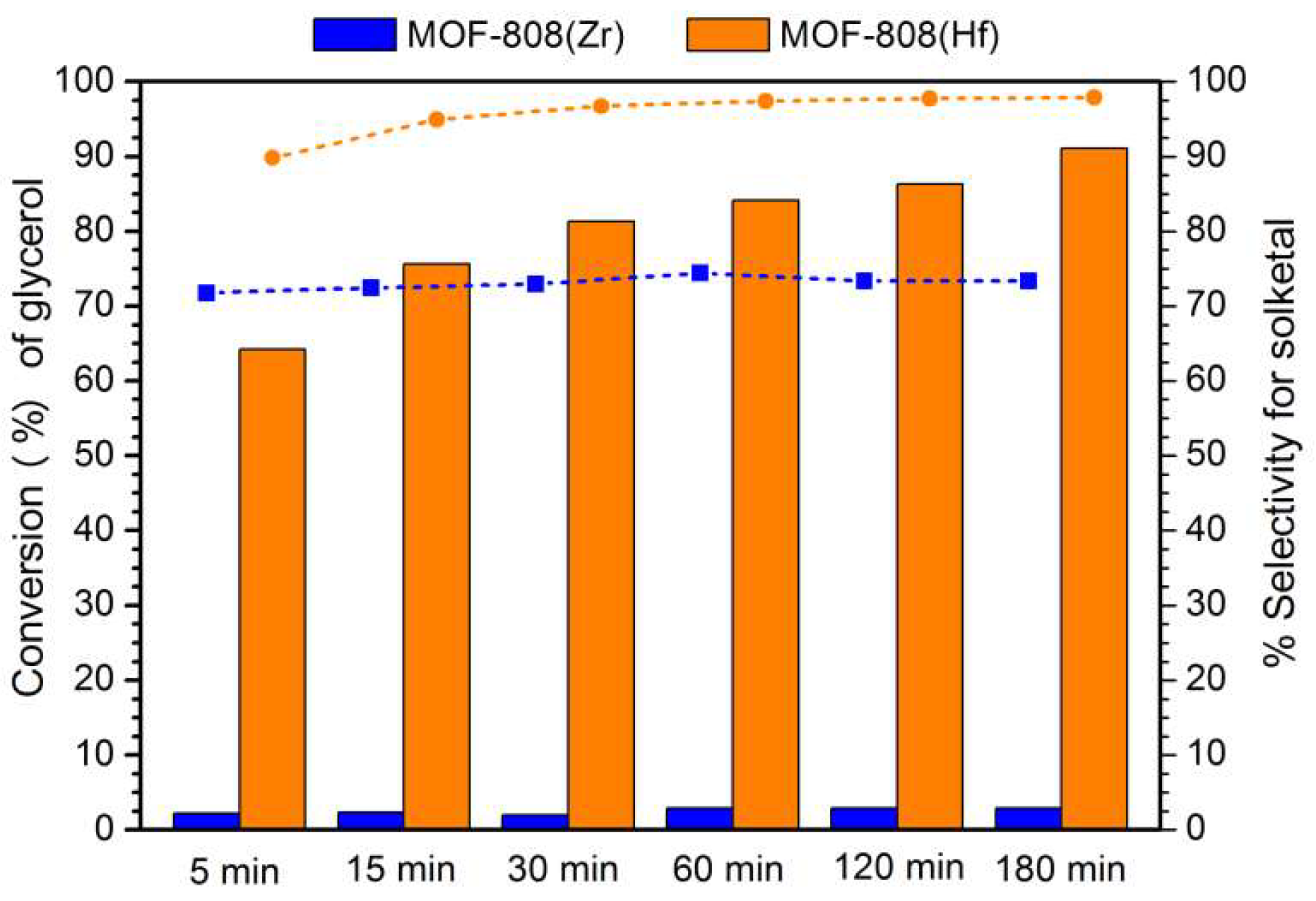
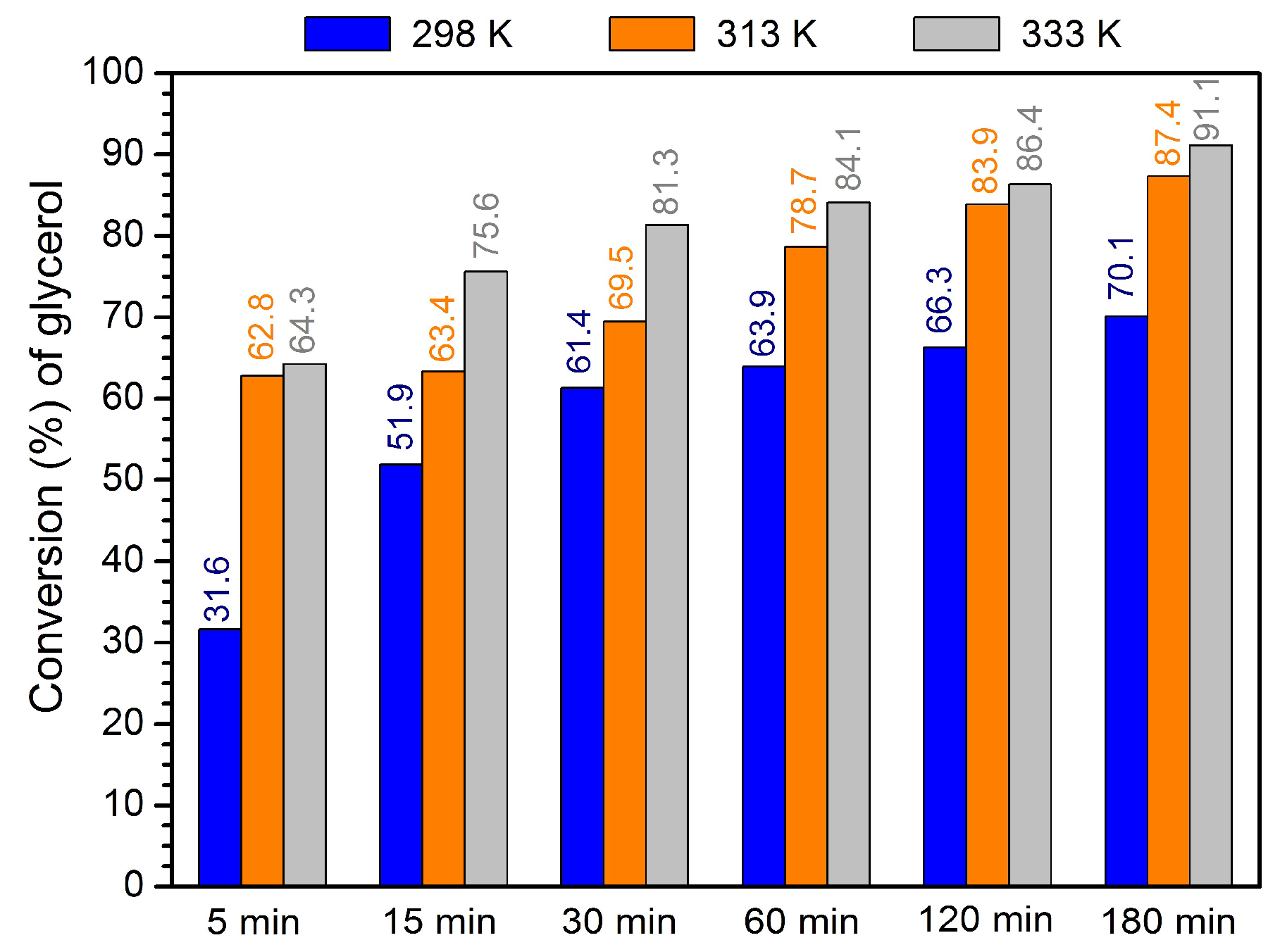
3.3. Evaluation of Catalytic Activity
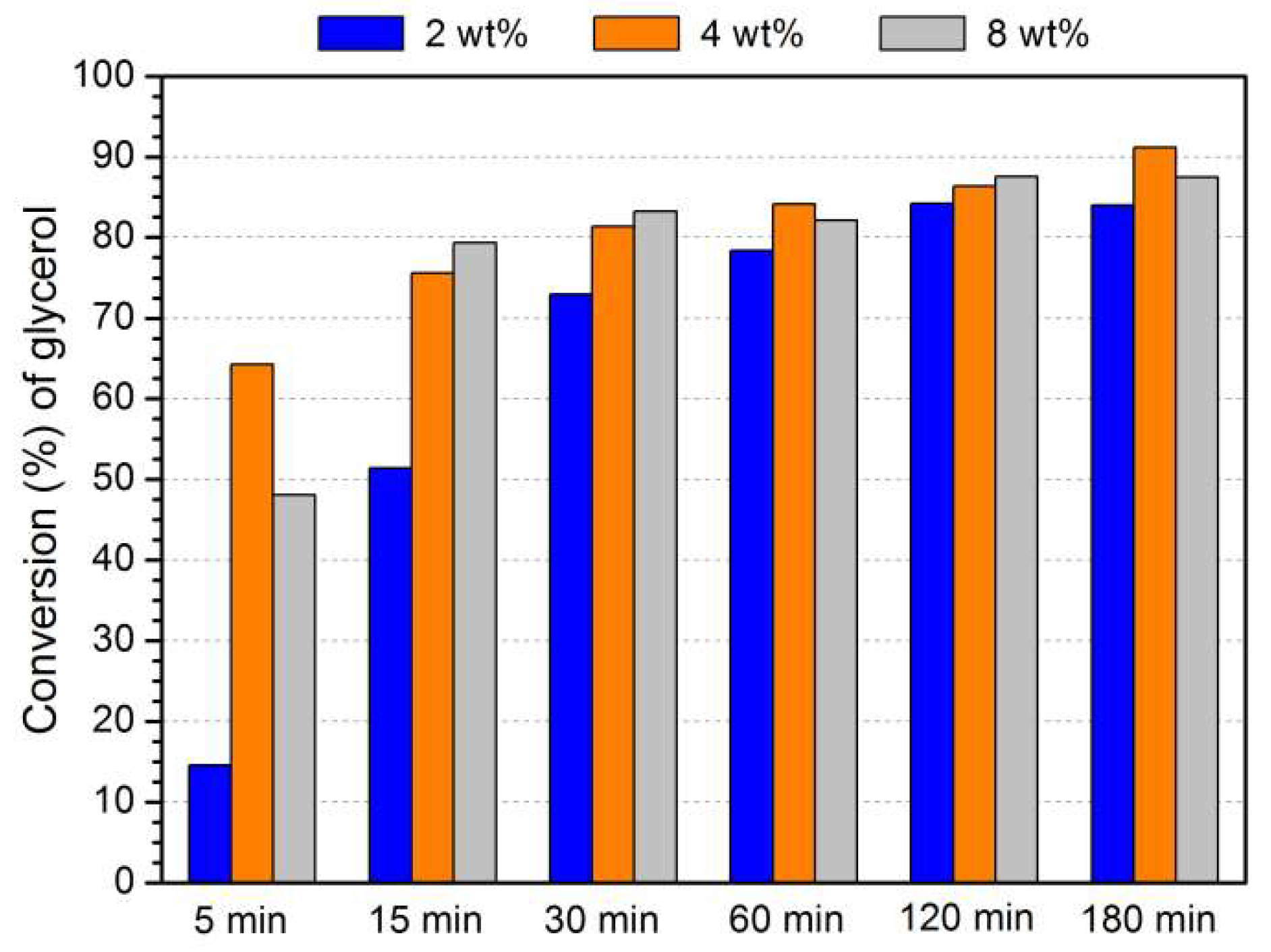
3.4. Optimization of Acetalization Reaction
3.5. Reutilization of MOF-808(Hf) Catalyst
3.6. Comparison with Reported Catalysts
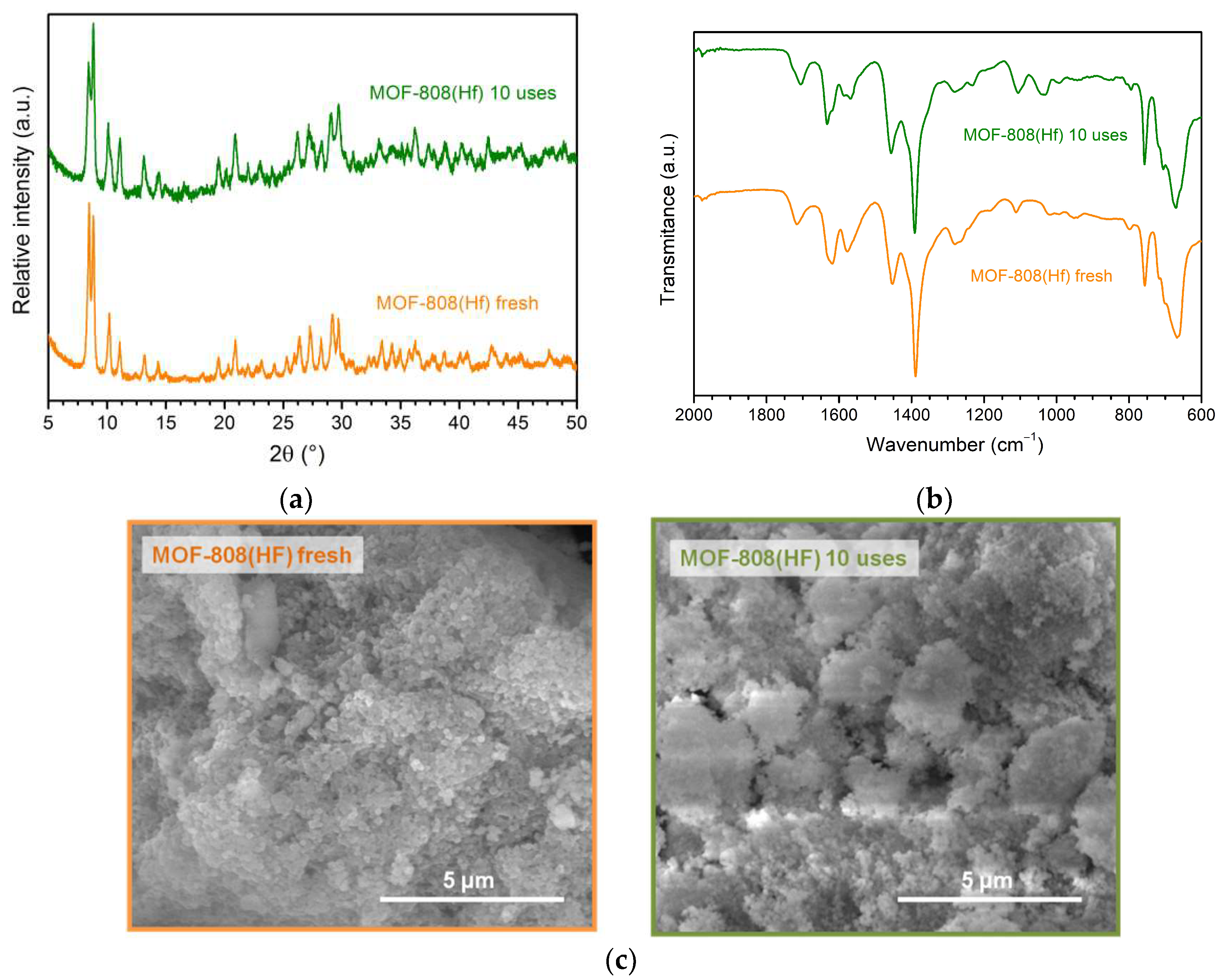
3.7. Catalyst Stability
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, X.-L.; Gao, Q.-X. Contributions of natural systems and human activity to greenhouse gas emissions. Adv. Clim. Chang. Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Moreira, M.N.; Corrêa, I.; Ribeiro, A.M.; Rodrigues, A.E.; Faria, R.P.V. Solketal Production in a Fixed Bed Adsorptive Reactor through the Ketalization of Glycerol. Ind. Eng. Chem. Res. 2020, 59, 2805–2816. [Google Scholar] [CrossRef]
- Research, M. Glycerol Market Size, Share & Trends Analysis Report by Source (Biodiesel, Fatty Acids, Fatty Alcohols, Soap), by Type (Crude, Refined) by End Use (Food & Beverage, Pharmaceutical), by Region, and Segment Forecasts, 2020–2027; GlobeNewswire: San Francisco, CA, USA, 2020; p. 148. [Google Scholar]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An Overview of Recent Research in the Conversion of Glycerol into Biofuels, Fuel Additives and other Bio-Based Chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, I.; Faria, R.P.V.; Rodrigues, A.E. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustain. Chem. 2021, 2, 286–324. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Al-Saadi, L.S.; Eze, V.C.; Harvey, A.P. Techno-economic analysis of glycerol valorization via catalytic applications of sulphonic acid-functionalized copolymer beads. Front. Chem. 2020, 7, 882. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Menezes, F.D.L.; Guimaraes, M.D.O.; da Silva, M.J. Highly Selective SnCl2-Catalyzed Solketal Synthesis at Room Temperature. Ind. Eng. Chem. Res. 2013, 52, 16709–16713. [Google Scholar] [CrossRef]
- Clarkson, J.S.; Walker, A.J.; Wood, M.A. Continuous reactor technology for ketal formation: An improved synthesis of solketal. Org. Process Res. Dev. 2001, 5, 630–635. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl. Catal. B Environ. 2010, 98, 94–99. [Google Scholar] [CrossRef]
- Vicente, G.; Melero, J.A.; Morales, G.; Paniagua, M.; Martín, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12, 899–907. [Google Scholar] [CrossRef]
- da Silva, C.X.A.; Gonçalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Guo, J.; Qin, Y.; Zhu, Y.; Zhang, X.; Long, C.; Zhao, M.; Tang, Z. Metal–organic frameworks as catalytic selectivity regulators for organic transformations. Chem. Soc. Rev. 2021, 50, 5366–5396. [Google Scholar] [CrossRef]
- Rui, K.; Zhao, G.; Chen, Y.; Lin, Y.; Zhou, Q.; Chen, J.; Zhu, J.; Sun, W.; Huang, W.; Dou, S.X. Hybrid 2D Dual-Metal–Organic Frameworks for Enhanced Water Oxidation Catalysis. Adv. Funct. Mater. 2018, 28, 1801554. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Khan, N.A.; Hasan, Z.; Prosvirin, I.P.; Tsybulya, S.V.; Jhung, S.H. Isostructural metal-carboxylates MIL-100(M) and MIL-53(M) (M: V, Al, Fe and Cr) as catalysts for condensation of glycerol with acetone. Appl. Catal. A Gen. 2017, 529, 167–174. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Churipard, S.R.; Maradur, S.P.; Kalidindi, S.B. Exploring the Brønsted acidity of UiO-66 (Zr, Ce, Hf) metal–organic frameworks for efficient solketal synthesis from glycerol acetalization. Dalton Trans. 2019, 48, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Santos-Vieira, I.C.M.S.; Mendes, R.F.; Almeida Paz, F.A.; Rocha, J.; Simões, M.M.Q. Solketal Production via Solvent-Free Acetalization of Glycerol over Triphosphonic-Lanthanide Coordination Polymers. Catalysts 2021, 11, 598. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, R.; Ye, B.; Hou, Z. Acetalization of glycerol over sulfated UiO-66 under mild condition. J. Ind. Eng. Chem. 2022, 110, 357–366. [Google Scholar] [CrossRef]
- Dashtipour, B.; Dehghanpour, S.; Sharbatdaran, M. Improvement of the acidic properties of MOF by doped SnO2 quantum dots for the production of solketal. J. Chem. Sci. 2022, 134, 106. [Google Scholar] [CrossRef]
- Melchiorre, M.; Lentini, D.; Cucciolito, M.E.; Taddeo, F.; Hmoudah, M.; Di Serio, M.; Ruffo, F.; Russo, V.; Esposito, R. Sustainable Ketalization of Glycerol with Ethyl Levulinate Catalyzed by the Iron(III)-Based Metal-Organic Framework MIL-88A. Molecules 2022, 27, 7229. [Google Scholar] [CrossRef] [PubMed]
- Plessers, E.; Fu, G.; Tan, C.Y.; De Vos, D.E.; Roeffaers, M.B.J. Zr-Based MOF-808 as Meerwein–Ponndorf–Verley Reduction Catalyst for Challenging Carbonyl Compounds. Catalysts 2016, 6, 104. [Google Scholar] [CrossRef]
- Liu, Y.; Klet, R.C.; Hupp, J.T.; Farha, O. Probing the correlations between the defects in metal–organic frameworks and their catalytic activity by an epoxide ring-opening reaction. Chem. Commun. 2016, 52, 7806–7809. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Liu, Y.; Hupp, J.T.; Farha, O.K. Instantaneous Hydrolysis of Nerve-Agent Simulants with a Six-Connected Zirconium-Based Metal–Organic Framework. Angew. Chem. Int. Ed. 2015, 54, 6795–6799. [Google Scholar] [CrossRef]
- Wang, B.; Jin, C.; Shao, S.; Yue, Y.; Zhang, Y.; Wang, S.; Chang, R.; Zhang, H.; Zhao, J.; Li, X. Electron-deficient Cu site catalyzed acetylene hydrochlorination. Green Energy Environ. 2023, 8, 1128–1140. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Silva, P.; Saini, V.K.; Paz, F.A.A.; Pires, J.; Cunha-Silva, L.; Balula, S.S. Novel heterogeneous catalysts based on lanthanopolyoxometalates supported on MIL-101(Cr). Catal. Today 2013, 218, 35–42. [Google Scholar] [CrossRef]
- Balula, S.S.; Granadeiro, C.M.; Barbosa, A.D.S.; Santos, I.; Cunha-Silva, L. Multifunctional catalyst based on sandwich-type polyoxotungstate and MIL-101 for liquid phase oxidations. Catal. Today 2013, 210, 142–148. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Lanthanopolyoxometalates: From the structure of polyanions to the design of functional materials. Polyhedron 2013, 52, 10–24. [Google Scholar] [CrossRef]
- Juliao, D.; Gomes, A.C.; Pillinger, M.; Valenca, R.; Ribeiro, J.C.; de Castro, B.; Goncalves, I.S.; Cunha Silva, L.; Balula, S.S. Zinc-Substituted Polyoxotungstate@amino-MIL-101(Al)—An Efficient Catalyst for the Sustainable Desulfurization of Model and Real Diesels. Eur. J. Inorg. Chem. 2016, 2016, 5114–5122. [Google Scholar] [CrossRef]
- Viana, A.M.; Juliao, D.; Mirante, F.; Faria, R.G.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Straightforward activation of metal-organic framework UiO-66 for oxidative desulfurization processes. Catal. Today 2021, 362, 28–34. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jagiello, J.; Thommes, M. Comparison of DFT characterization methods based on N2, Ar, CO2, and H2 adsorption applied to carbons with various pore size distributions. Carbon 2004, 42, 1227–1232. [Google Scholar] [CrossRef]
- Peixoto, A.F.; Soliman, M.M.A.; Pinto, T.V.; Silva, S.M.; Costa, P.; Alegria, E.C.B.A.; Freire, C. Highly active organosulfonic aryl-silica nanoparticles as efficient catalysts for biomass derived biodiesel and fuel additives. Biomass Bioenergy 2021, 145, 105936. [Google Scholar] [CrossRef]
- Hu, Z.; Kundu, T.; Wang, Y.; Sun, Y.; Zeng, K.; Zhao, D. Modulated Hydrothermal Synthesis of Highly Stable MOF-808(Hf) for Methane Storage. ACS Sustain. Chem. Eng. 2020, 8, 17042–17053. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Viana, A.M.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Synergistic combination of the nanoporous system of MOF-808 with a polyoxomolybdate to design an effective catalyst: Simultaneous oxidative desulfurization and denitrogenation processes. Sustain. Energy Fuels 2021, 5, 4032–4040. [Google Scholar] [CrossRef]
- Bohigues, B.; Rojas-Buzo, S.; Moliner, M.; Corma, A. Coordinatively Unsaturated Hf-MOF-808 Prepared via Hydrothermal Synthesis as a Bifunctional Catalyst for the Tandem N-Alkylation of Amines with Benzyl Alcohol. ACS Sustain. Chem. Eng. 2021, 9, 15793–15806. [Google Scholar] [CrossRef]
- Julião, D.; Mirante, F.; Balula, S.S. Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids. Molecules 2022, 27, 6573. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.A.; Selishcheva, S.A.; Yakovlev, V.A. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595. [Google Scholar] [CrossRef]
- Phan, D.-P.; Le, V.N.; Nguyen, T.H.; Kim, H.B.; Park, E.D.; Kim, J.; Lee, E.Y. Effect of amino-defective-MOF materials on the selective hydrodeoxygenation of fatty acid over Pt-based catalysts. J. Catal. 2021, 400, 283–293. [Google Scholar] [CrossRef]


| MOF | pH (Before Titration) | Acidity (mmol g−1) |
|---|---|---|
| MOF-808(Hf) | 3.61 | 0.7505 |
| MOF-808(Zr) | 4.35 | 0.4292 |
| Catalyst | Temperature (K) | Glycerol/Acetone | Time (h) | Conversion (%) | Reference |
|---|---|---|---|---|---|
| MIL-100(V) | 298 | 1:5 | 1.5 | 83 (98) | [21] |
| MIL-47(V) | 298 | 1:5 | 1.5 | 73 (87) | [21] |
| UiO-66-Zr | r.t. | 1:4 | 1 | 1.5 (73) | [22] |
| UiO-66-Hf | r.t. | 1:4 | 1 | 94 (97) | [22] |
| UiO-66-SO3H | 333 | 1:10 | 1 | 60 (99) | [24] |
| Mil-118-SnO2 | reflux | 1:10 | 4 | 76 (97) | [25] |
| UAV-63 | 328 | 1:10 | 6 | 84(96) | [23] |
| MOF-808(Zr) | 333 | 1:6 | 3 | 6 (100) | This work |
| MOF-808(Hf) | 333 | 1:6 | 3 | 91 (98) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirante, F.; Leo, P.; Dias, C.N.; Cunha-Silva, L.; Balula, S.S. MOF-808 as an Efficient Catalyst for Valorization of Biodiesel Waste Production: Glycerol Acetalization. Materials 2023, 16, 7023. https://doi.org/10.3390/ma16217023
Mirante F, Leo P, Dias CN, Cunha-Silva L, Balula SS. MOF-808 as an Efficient Catalyst for Valorization of Biodiesel Waste Production: Glycerol Acetalization. Materials. 2023; 16(21):7023. https://doi.org/10.3390/ma16217023
Chicago/Turabian StyleMirante, Fátima, Pedro Leo, Catarina N. Dias, Luís Cunha-Silva, and Salete S. Balula. 2023. "MOF-808 as an Efficient Catalyst for Valorization of Biodiesel Waste Production: Glycerol Acetalization" Materials 16, no. 21: 7023. https://doi.org/10.3390/ma16217023








