Optimization of Pore Characteristics of Graphite-Based Anode for Li-Ion Batteries by Control of the Particle Size Distribution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
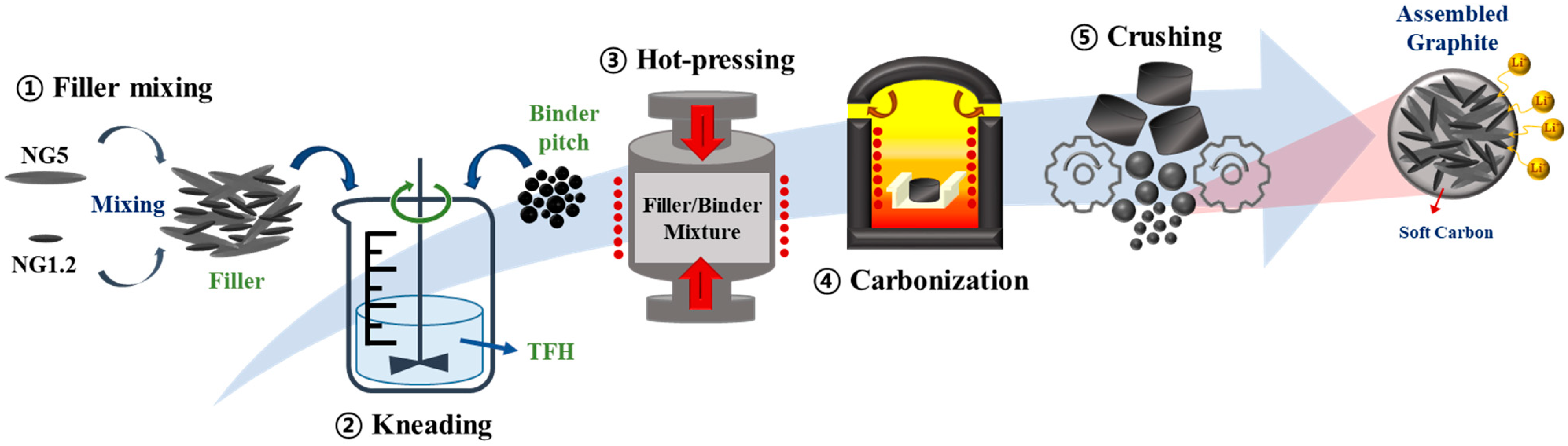
2.2. Experiments
2.3. Characterization of Assembled Anode
2.4. Electrochemical Characterization of Assembled Anode
3. Results and Discussion
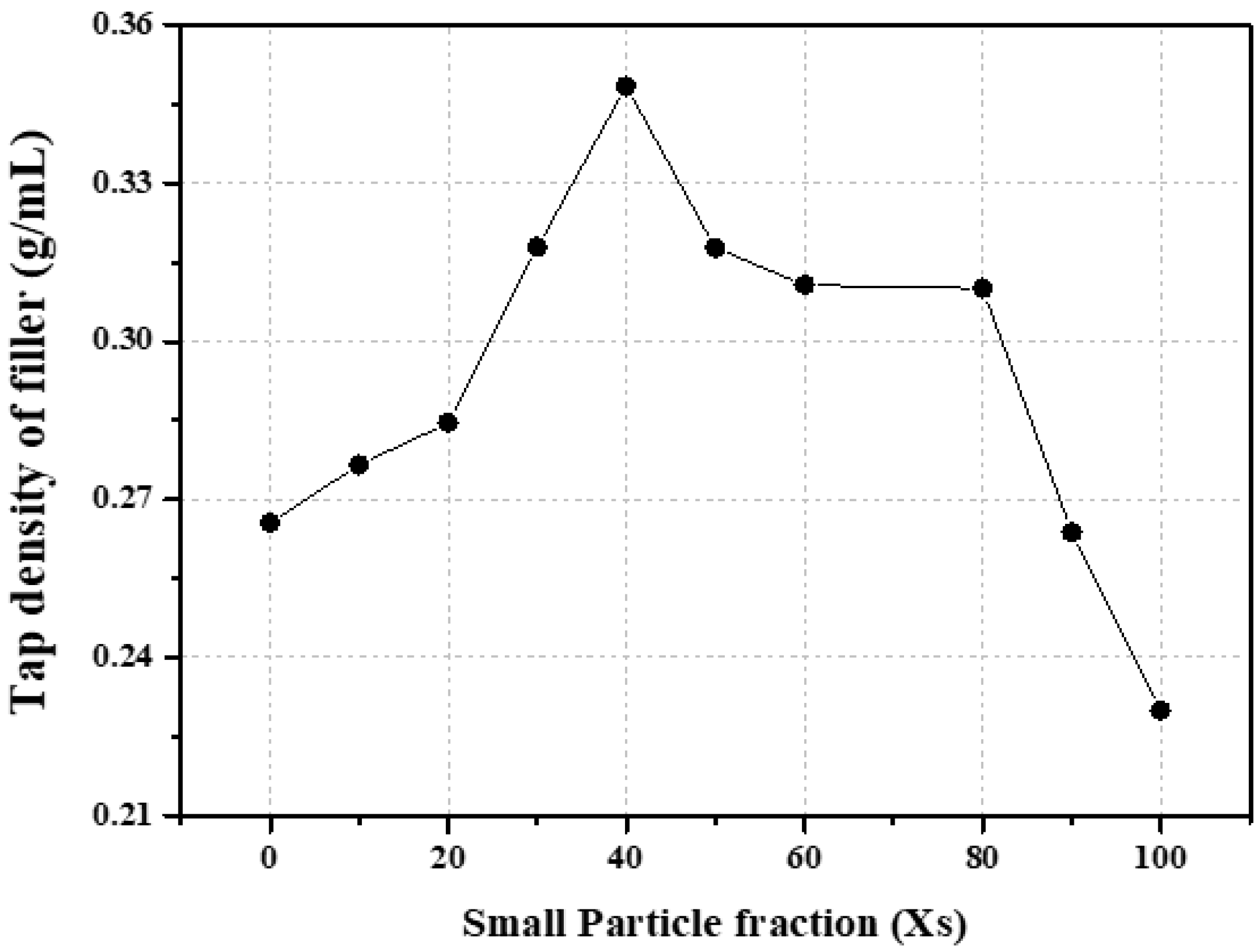
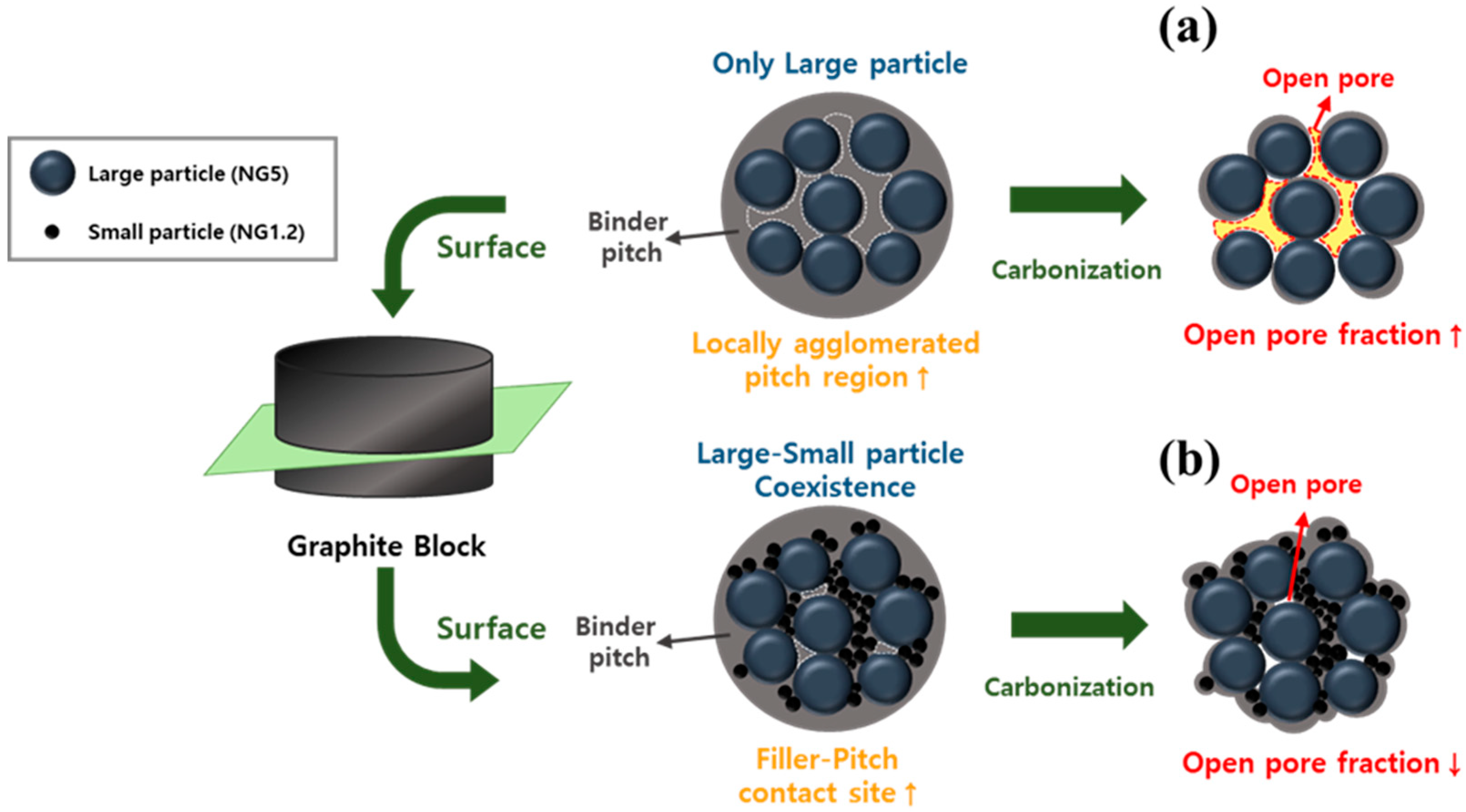
3.1. Effect of Graphite Filler Size Distribution on Block Porosity
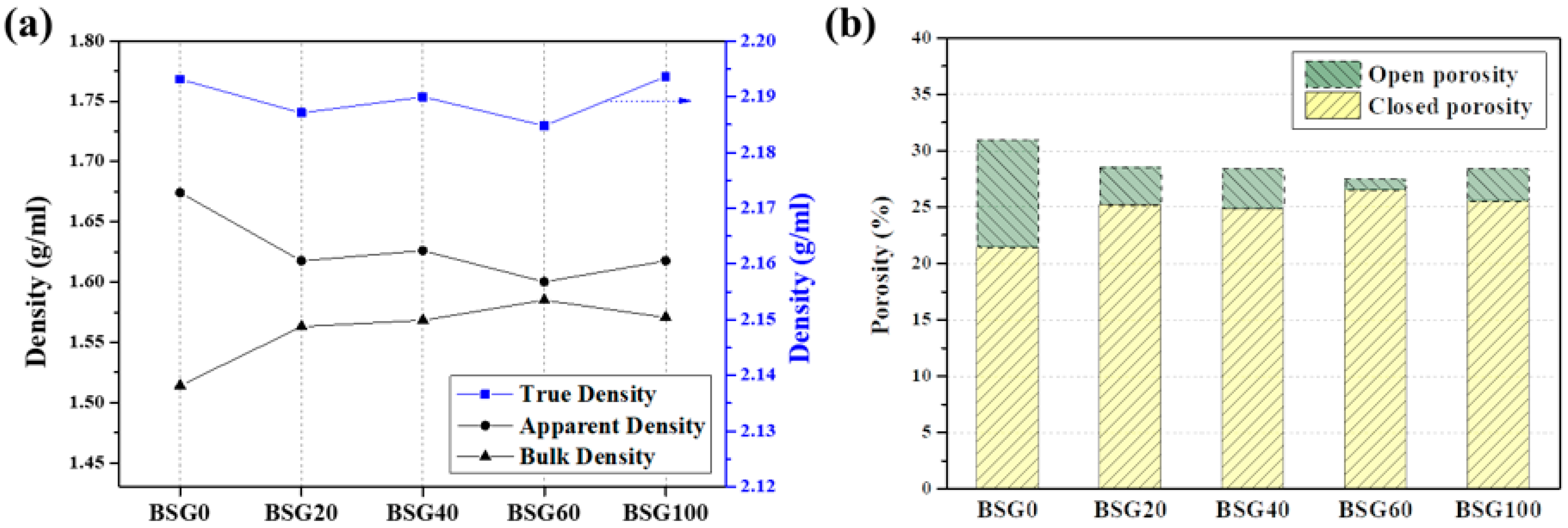
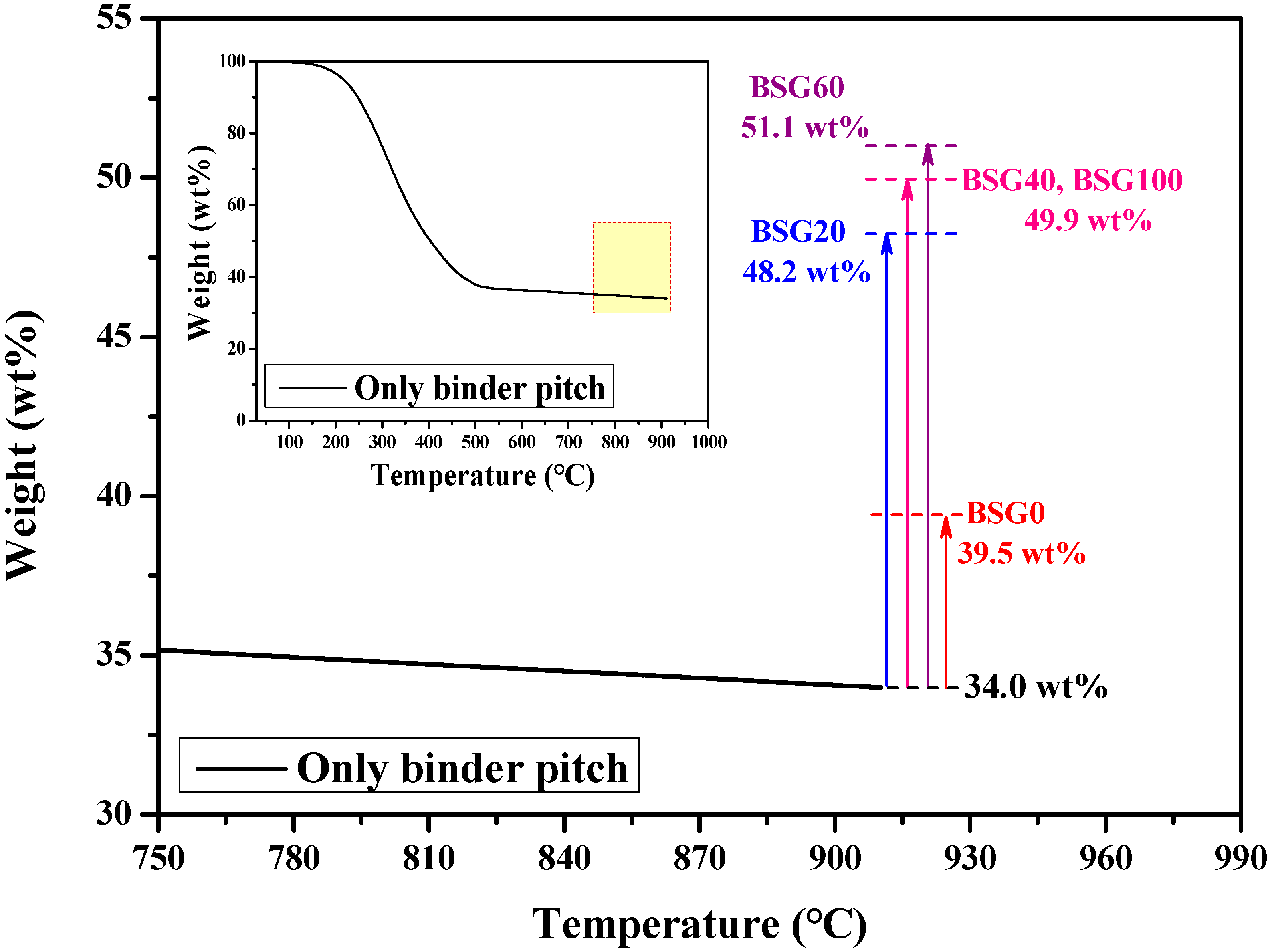
3.2. Characteristics of Assembled Graphite According to the Porosity of the Graphite Block
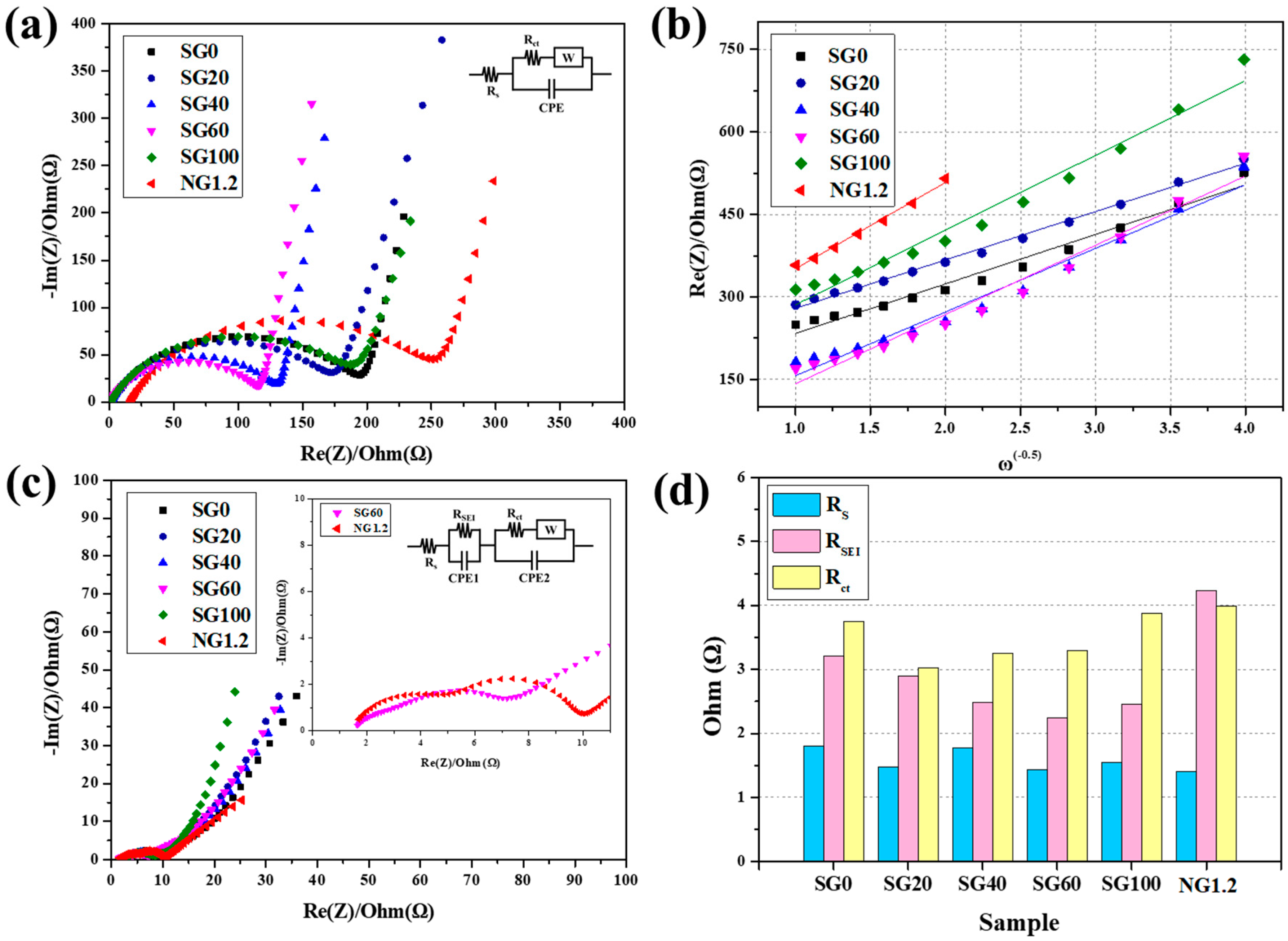
3.3. Electrochemical Characteristics of Assembled Graphite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, D.; Chen, L.; Tian, J.; Su, Y.; Li, N.; Chen, G.; Hu, Y.; Dou, Y.; Chen, S.; Wu, F. Research Progress of Lithium Plating on Graphite Anode in Lithium-Ion Batteries. Chin. J. Chem. 2021, 39, 165–173. [Google Scholar] [CrossRef]
- Zhan, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Woo, S.-W.; Lee, Y.-J.; Kim, J.-Y.; Lee, S.-M. Achieving High-Performance Spherical Natural Graphite Anode through a Modified Carbon Coating for Lithium-Ion Batteries. Energies 2021, 14, 1946. [Google Scholar] [CrossRef]
- Fischer, S.; Doose, S.; Müller, J.; Höfels, C.; Kwade, A. Impact of Spheroidization of Natural Graphite on Fast-Charging Capability of Anodes for LIB. Batteries 2023, 9, 305. [Google Scholar] [CrossRef]
- Im, U.-S.; Hwang, J.U.; Yun, J.H.; Ahn, W.; Kim, K.S.; Im, J.S. The effect of mild activation on the electrochemical performance of pitch-coated graphite for the lithium-ion battery anode material. Mater. Lett. 2020, 278, 128421. [Google Scholar] [CrossRef]
- Chang, H.; Wu, Y.-R.; Han, X.; Yi, Y.-F. Recent developments in advanced anode materials for lithium-ion batteries. Energy Mater. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, B.; Qin, X.-Y.; Wang, Z.; Lv, W.; He, Y.-B.; Yang, Q.-H.; Kang, F. Revisiting the Roles of Natural Graphite in Ongoing Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2106704. [Google Scholar] [CrossRef]
- Heng, S.; Shan, X.; Wang, W.; Wang, Y.; Zhu, G.; Qu, Q.; Zheng, H. Controllable solid electrolyte interphase precursor for stabilizing natural graphite anode in lithium ion batteries. Carbon 2020, 159, 390–400. [Google Scholar] [CrossRef]
- Biber, B.; Sander, S.; Martin, J.; Wohlfahrt-Mehrens, M.; Mancini, M. Improved production process with new spheroidization machine with high efficiency and low energy consumption for rounding natural graphite for Li-ion battery applications. Carbon 2023, 201, 847–855. [Google Scholar] [CrossRef]
- Moradi, B.; Botte, G.G. Recycling of graphite anodes for the next generation of lithium ion batteries. J. Appl. Electrochem. 2016, 46, 123–148. [Google Scholar] [CrossRef]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, Y.J. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 25, 671–689. [Google Scholar] [CrossRef]
- Wu, X.; Song, B.; Chein, P.-H.; Everett, S.M.; Zhao, K.; Liu, J.; Du, Z. Structural Evolution and Transition Dynamics in Lithium Ion Battery under Fast Charging: An Operando Neutron Diffraction Investigation. Adv. Sci. 2021, 8, 2102318. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Huang, T.; Li, L.; Xiong, W.; Huang, S.; Ren, X.; Ouyang, X.; Hu, J.; Zhang, Q.; et al. Cost-effective natural graphite reengineering technology for lithium ion batteries. Chin. Chem. Lett. 2023, in press. [CrossRef]
- Fu, Y.; Jin, Y.; Ma, J.; Liu, J.; Wang, Z.; Wang, B.; Gong, X. Lithium-ion transfer strengthened by graphite tailings and coking coal for high-rate performance anode. Chem. Eng. J. 2022, 442, 136184. [Google Scholar] [CrossRef]
- Abrego-Martinez, J.C.; Wang, Y.; Vanpeene, V.; Roué, L. From waste graphite fines to revalorized anode material for Li-ion batteries. Carbon 2023, 209, 118004. [Google Scholar] [CrossRef]
- Escher, I.; Mancini, M.; Martin, J.; Janßen, K.A.; Axmann, P.; Adelhelm, P. Towards low-cost sodium-ion batteries: Electrode behavior of graphite electrodes obtained from spheroidization waste fractions and their structure-property relations. J. Phys. Energy 2023, 5, 014011. [Google Scholar] [CrossRef]
- Yang, X.; Zhan, C.; Ren, X.; Wang, C.; Wei, L.; Yu, Q.; Xu, D.; Nan, D.; Lv, R.; Shen, W.; et al. Nitrogen-doped hollow graphite granule as anode materials for high-performance lithium-ion batteries. J. Solid State Chem. 2021, 303, 122500. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Wang, D.-K.; Zhang, L.-H.; Liu, H.-Y.; Liu, Z.-B.; Xing, T.; Ma, Z.-K.; Chen, X.-H.; Song, H.-H. A wet granulation method to prepare graphite particles with a high tap density for high volumetric energy density lithium-ion storage. New Carbon Mater. 2022, 37, 402–410. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, Y.; Xu, M.; Huang, S.; Yuan, G. One-Step Binding and Wrapping Fragmented Natural Microcrystalline Graphite via Phenolic Resin into Secondary Particles for High-Performance Lithium-Ion Battery Anode. JOM 2023, in press. [CrossRef]
- Mu, Y.; Han, M.; Li, J.; Liang, J.; Yu, J. Growing vertical graphene sheets on natural graphite for fast charging lithium-ion batteries. Carbon 2021, 173, 477–484. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Wang, B.; Wang, K.; Xie, J. Spherical nanostructured Si/C composite prepared by spray drying technique for lithium ion batteries anode. Mater. Sci. Eng. B 2006, 131, 186–190. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Zhang, F.; Cai, L.; Zhang, L.; Wen, Z. Carbon-coated isotropic natural graphite spheres as anode material for lithium-ion batteries. Ceram. Int. 2017, 43, 9458–9464. [Google Scholar] [CrossRef]
- Methods of Measuring Density and Specific Gravity of Solid, 12th ed.; Japanese Standards Association (JSA): Japan, 2022.
- Kim, J.H. Control of the properties of a binder pitch to enhance the density and strength of graphite blocks. Carbon Lett. 2023, 33, 1757–1766. [Google Scholar] [CrossRef]
- Liu, Z.-R.; Ye, W.-M.; Zhang, Z.; Wang, Q.; Chen, Y.-G.; Cui, Y.-J. Particle size ratio and distribution effects on packing behaviour of crushed GMZ bentonite pellets. Powder Technol. 2019, 351, 92–101. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, K.; Zhang, Y.; Jiang, J. Packing Fraction, Tortuosity, and Permeability of Granular-Porous Media With Densely Packed Spheroidal Particles: Monodisperse and Polydisperse Systems. Water Resour. Res. 2022, 58, e2021WR031433. [Google Scholar] [CrossRef]
- Petrova, B.; Budinova, T.; Ekinci, E.; Petrov, N.; Yardim, F. Influence of pitch composition and surface properties of petroleum coke on their interaction during the preparation of carbon/carbon composites. Carbon 2007, 45, 704–709. [Google Scholar] [CrossRef]
- Lahaye, J.; Aubert, J.-P. Interaction between a coke and a tar. 1. Influence of the surface chemical functions of coke. Fuel 1977, 56, 185–187. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hwang, H.-I.; Im, J.-S. Optimization of the Filler-and-Binder Mixing Ratio for Enhanced Mechanical Strength of Carbon–Carbon Composites. Materials 2023, 16, 4084. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Kim, J.; Jeghan, S.M.N.; Lee, G. Superior fast-charging capability of graphite anode via facile surface treatment for lithium-ion batteries. Microporous Mesoporous Mater. 2020, 305, 110325. [Google Scholar] [CrossRef]
- Yu, K.; Li, J.; Qi, H.; Liang, C. High-capacity activated carbon anode material for lithium-ion batteries prepared from rice husk by a facile method. Diam. Relat. Mater. 2018, 86, 139–145. [Google Scholar] [CrossRef]
- Mundszinger, M.; Farsi, S.; Rapp, M.; Golla-Schindler, U.; Kaiser, U.; Wachtler, M. Morphology and texture of spheroidized natural and synthetic graphites. Carbon 2017, 111, 764–773. [Google Scholar] [CrossRef]
- Sutton, A.L.; Howard, V.C. The role of porosity in the accommodation of thermal expansion in graphite. J. Nucl. Mater. 1962, 7, 58–71. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Wu, J.; Shao, R.; Jiang, R.; Tie, Z.; Jin, Z. A Review on Recent Advances for Boosting Initial Coulombic Efficiency of Silicon Anodic Lithium Ion batteries. Small 2022, 18, 2102894. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Hu, X.; Fan, F.; Cai, S.; Zheng, C.; Stucky, G.D. Review on comprehending and enhancing the initial Coulombic efficiency of anode materials in lithium-ion/sodium-ion batteries. Nano Energy 2020, 77, 105143. [Google Scholar] [CrossRef]
- Tian, J.; Yang, H.; Fu, C.; Sun, M.; Wang, L.; Liu, T. In-situ synthesis of microspherical Sb@C composite anode with high tap density for lithium/sodium-ion batteries. Compos. Commun. 2020, 17, 177–181. [Google Scholar] [CrossRef]
- Kim, K.H.; Cho, J.H.; Hwang, J.U.; Im, J.S.; Lee, Y.-S. A key strategy to form a LiF-based SEI layer for a lithium-ion battery anode with enhanced cycling stability by introducing a semi-ionic C-F bond. J. Ind. Eng. Chem. 2021, 25, 48–54. [Google Scholar] [CrossRef]
- Li, X.; Geng, D.; Zhang, Y.; Meng, X.; Li, R.; Sun, X. Superior cycle stability of nitrogen-doped graphene nanosheets as anodes for lithium ion batteries. Electrochem. Commun. 2011, 13, 822–825. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, J.H.; Lee, Y.-S.; Im, J.S. Effect of coke orientation on the electrochemical properties of lithium-ion battery anode. J. Appl. Electrochem. 2021, 51, 1407–1418. [Google Scholar] [CrossRef]
- Jeong, C.-U.; Umirov, N.; Jung, D.-H.; Seo, D.-H.; Lee, B.-M.; Choi, B.-S.; Kim, S.-S.; Choi, J.-H. Li-incorporated porous carbon monoliths derived from carboxymethyl cellulose as anode material for high power lithium-ion batteries. J. Power Sources 2021, 506, 230050. [Google Scholar] [CrossRef]
- Gao, P.; Tang, H.; Xing, A.; Bao, Z. Porous silicon from the magnesiothermic reaction as a high-performance anode material for lithium ion battery applications. Electrochim. Acta 2017, 228, 545–552. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Zhang, K.; Feng, X.; Wang, M. Synthesis and high-performance of carbonaceous polypyrrole nanotubes coated with SnS2 nanosheets anode materials for lithium ion batteries. Chem. Eng. J. 2017, 330, 470–479. [Google Scholar] [CrossRef]
- Kurc, B.; Piglowska, M. An influence of temperature on the lithium ions behavior for starch-based carbon compared to graphene anode for LIBs by the electrochemical impedance spectroscopy (EIS). J. Power Sources 2021, 485, 229323. [Google Scholar] [CrossRef]
- Peng, J.; Li, W.; Wu, Z.; Li, H.; Zeng, P.; Chen, G.; Chang, B.; Zhang, X.; Wang, X. Si/C composite embedded nano-Si in 3D porous carbon matrix and enwound by conductive CNTs as anode of lithium-ion batteries. Sustain. Mater. Technol. 2022, 32, e00410. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Xie, Y.; He, L.; Chen, J.; Wu, K.; Xu, R.; Gao, Y. On the stress characteristics of graphite anode in commercial pouch lithium-ion battery. J. Power Sources 2013, 232, 29–33. [Google Scholar] [CrossRef]
- Han, Y.-J.; Kim, J.; Yeo, J.-S.; An, J.C.; Hong, I.-P.; Nakabayashi, K.; Miyawaki, J.; Jung, J.-D.; Yoon, S.-H. Coating of graphite anode with coal tar pitch as an effective precursor for enhancing the rate performance in Li-ion batteries: Effects of composition and softening points of coal tar pitch. Carbon 2015, 94, 432–438. [Google Scholar] [CrossRef]




| Sample Name | dB (1) (g/mL) | dA (2) (g/mL) | dT (3) (g/mL) | Closed Porosity (%) | Open Porosity (%) | Total Porosity (%) | Yield (wt%) |
|---|---|---|---|---|---|---|---|
| BSG0 | 1.51 | 1.67 | 2.1931 | 21.41 | 9.56 | 30.96 | 87.89 |
| BSG20 | 1.56 | 1.62 | 2.1871 | 25.16 | 3.37 | 28.54 | 89.63 |
| BSG40 | 1.57 | 1.63 | 2.1900 | 24.84 | 3.55 | 28.39 | 89.99 |
| BSG60 | 1.58 | 1.60 | 2.1848 | 26.51 | 0.94 | 27.45 | 90.22 |
| BSG100 | 1.57 | 1.62 | 2.1936 | 25.50 | 2.90 | 28.40 | 89.98 |
| Sample Name | dTap (1) (g/mL) | SBET (2) (m2/g) |
|---|---|---|
| NG1.2 | 0.2299 | 10.96 |
| NG5 | 0.2656 | 12.13 |
| SG0 | 0.3028 | 8.68 |
| SG20 | 0.3053 | 8.59 |
| SG40 | 0.2895 | 9.10 |
| SG60 | 0.3142 | 8.51 |
| SG100 | 0.2716 | 9.75 |
| 1st Charge Capacity (mAh/g) | 1st Discharge Capacity (mAh/g) | I.C.E (%) | |
|---|---|---|---|
| SG0 | 400.6 | 346.2 | 86.4 |
| SG20 | 368.8 | 317.2 | 86.0 |
| SG40 | 376.8 | 340.1 | 90.3 |
| SG60 | 340.8 | 314.5 | 92.3 |
| SG100 | 372.3 | 339.2 | 91.1 |
| NG1.2 | 395.7 | 346.8 | 87.6 |
| 1st Discharge Capacity (mAh/g) | 100th Discharge Capacity (mAh/g) | Capacity Retention after 100 Cycles (%) | |
|---|---|---|---|
| SG0 | 346.2 | 346.1 | 100.0 |
| SG20 | 317.2 | 329.6 | 103.9 |
| SG40 | 340.1 | 344.4 | 101.3 |
| SG60 | 314.5 | 332.3 | 105.7 |
| SG100 | 339.2 | 340.9 | 100.5 |
| NG1.2 | 346.8 | 317.2 | 91.5 |
| Sample | Before the Cycle | After 10 Cycles | |||
|---|---|---|---|---|---|
| RS (Ω) | Rct (Ω) | RS (Ω) | RSEI (Ω) | Rct (Ω) | |
| SG0 | 1.42 | 200.6 | 1.80 | 3.21 | 3.75 |
| SG20 | 1.32 | 179.4 | 1.47 | 2.89 | 3.02 |
| SG40 | 1.31 | 133.5 | 1.78 | 2.48 | 3.25 |
| SG60 | 1.13 | 119.3 | 1.43 | 2.24 | 3.30 |
| SG100 | 1.08 | 199.0 | 1.55 | 2.46 | 3.88 |
| NG1.2 | 15.9 | 249.3 | 1.40 | 4.23 | 3.99 |
| Sample | SG0 | SG20 | SG40 | SG60 | SG100 | NG |
|---|---|---|---|---|---|---|
| DiLi+ (cm2 s−1) | 2.8216 × 10−12 | 1.8314 × 10−12 | 2.0192 × 10−12 | 1.7675 × 10−12 | 1.1183 × 10−12 | 9.2007 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-J.; Lee, Y.-S.; Kim, J.-H.; Im, J.-S. Optimization of Pore Characteristics of Graphite-Based Anode for Li-Ion Batteries by Control of the Particle Size Distribution. Materials 2023, 16, 6896. https://doi.org/10.3390/ma16216896
Choi Y-J, Lee Y-S, Kim J-H, Im J-S. Optimization of Pore Characteristics of Graphite-Based Anode for Li-Ion Batteries by Control of the Particle Size Distribution. Materials. 2023; 16(21):6896. https://doi.org/10.3390/ma16216896
Chicago/Turabian StyleChoi, Yun-Jeong, Young-Seak Lee, Ji-Hong Kim, and Ji-Sun Im. 2023. "Optimization of Pore Characteristics of Graphite-Based Anode for Li-Ion Batteries by Control of the Particle Size Distribution" Materials 16, no. 21: 6896. https://doi.org/10.3390/ma16216896





