Treatment of Mixture Pollutants with Combined Plasma Photocatalysis in Continuous Tubular Reactors with Atmospheric-Pressure Environment: Understanding Synergetic Effect Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analytical Methods
- The same gas volume (500 µL) was continuously sampled to monitor oxidation phenomena under the photocatalytic plasma reactor. The concentration of Toluene was determined using Gas Chromatography (GC) with a Clarus GC-500 chromatograph equipped with a flame ionization detector (FID) (Salt Lake City, UT, USA) and a 60 m × 0.25 mm polar DB-MS capillary column (film thickness, 0.25 μm). The FID detector was powered by an air and hydrogen mixture (H2). Helium (He) was used as the carrier gas at a flow rate of 1 mL.min−1. The analysis conditions included injection and detection temperatures of 250 °C for both, and the oven temperature was programmed to maintain 90 °C throughout each analysis (analysis time: 4 min). The analysis method for DMDS sulfur pollutant has been described in detail in our previous work [5].
- CO2 measurements were determined using a Fourier Transform Infrared Spectrophotometer (FTIR) from Environment SA (MIR 9000H). The mineralization step was continuously monitored during the oxidation (plasma/photocatalysis) process using a pump system to control the outlet gas stream. For CO analysis, samples were taken using a gas analyzer (NO/CO ZRE marketed by Fuji Electric France S.A.S.). The SO2 outlet concentration was measured using a MEDOR gas analyzer (THT MEDOR®-Houston, TX, USA).
- The amount of Ozone generated during the oxidation step with plasma was determined using sodium thiosulfate titration. A membrane pump (KNF lab N86k18) delivered part of the flow exits and then bubbled into a potassium iodide solution (KI, at 10−2 M). The chemical reaction between KI and Ozone (Equation (1)) resulted in the appearance of a yellow color, which was then neutralized through titration with a sodium thiosulfate solution (Na2S2O3, at 10−3 M) until a colorless solution was obtained (Equation (2)) [7]. The titration was carried out in an acid medium by adding concentrated hydrochloric acid (HCl) to the final solution.
3. Results and Discussion
- (Cinlet) and (Coutlet) represent the inlet and outlet concentration of pollutant (mg.m−3), respectively.
- The degradation rate of the pollutant with each process (%) = (1 − Coutlet/Cinlet) × 100.
- The value of the Synergetic Effect (SE) is calculated using the following expression:
- The Specific Energy SE (J/L) = [P(W)/Q(m3.s−1)]/1000, where P is the input power in the function of the applied voltage and Q is the flowrate.
- The sulfur dioxide selectivity (SSO2) = ([SO2]outlet − [SO2]inlet) × 104/(ns,cov × RE × [C]inlet).
- The carbon dioxide and monoxide selectivities (SCOx) = ([COx]outlet − [COx]inlet) × 104/(nc,cov × RE × [C]inlet), where [C] is the concentration of DMDS/Toluene and nc,VOC represents the number of carbons in the molecules (two for DMDS and seven for Toluene).
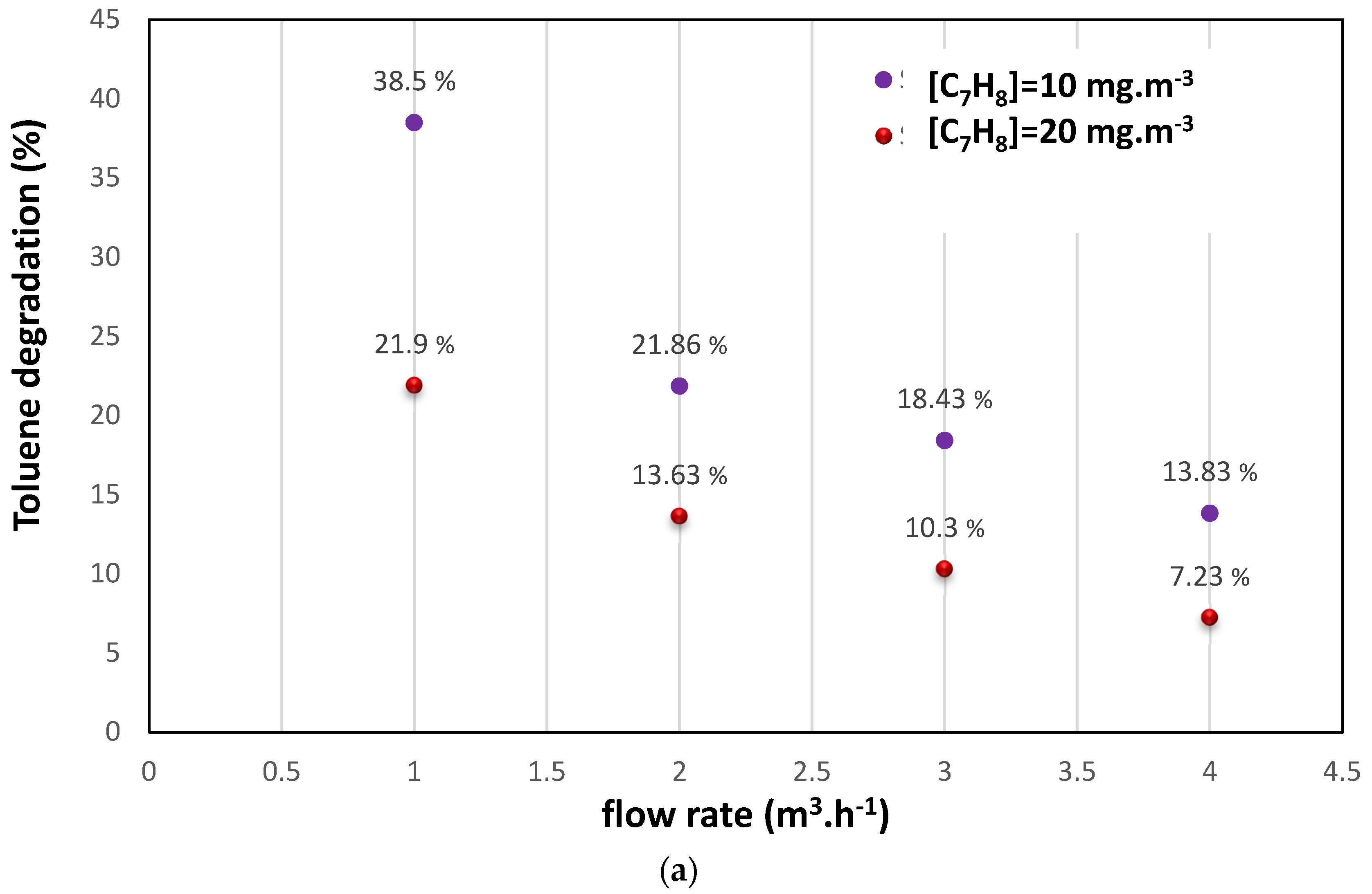
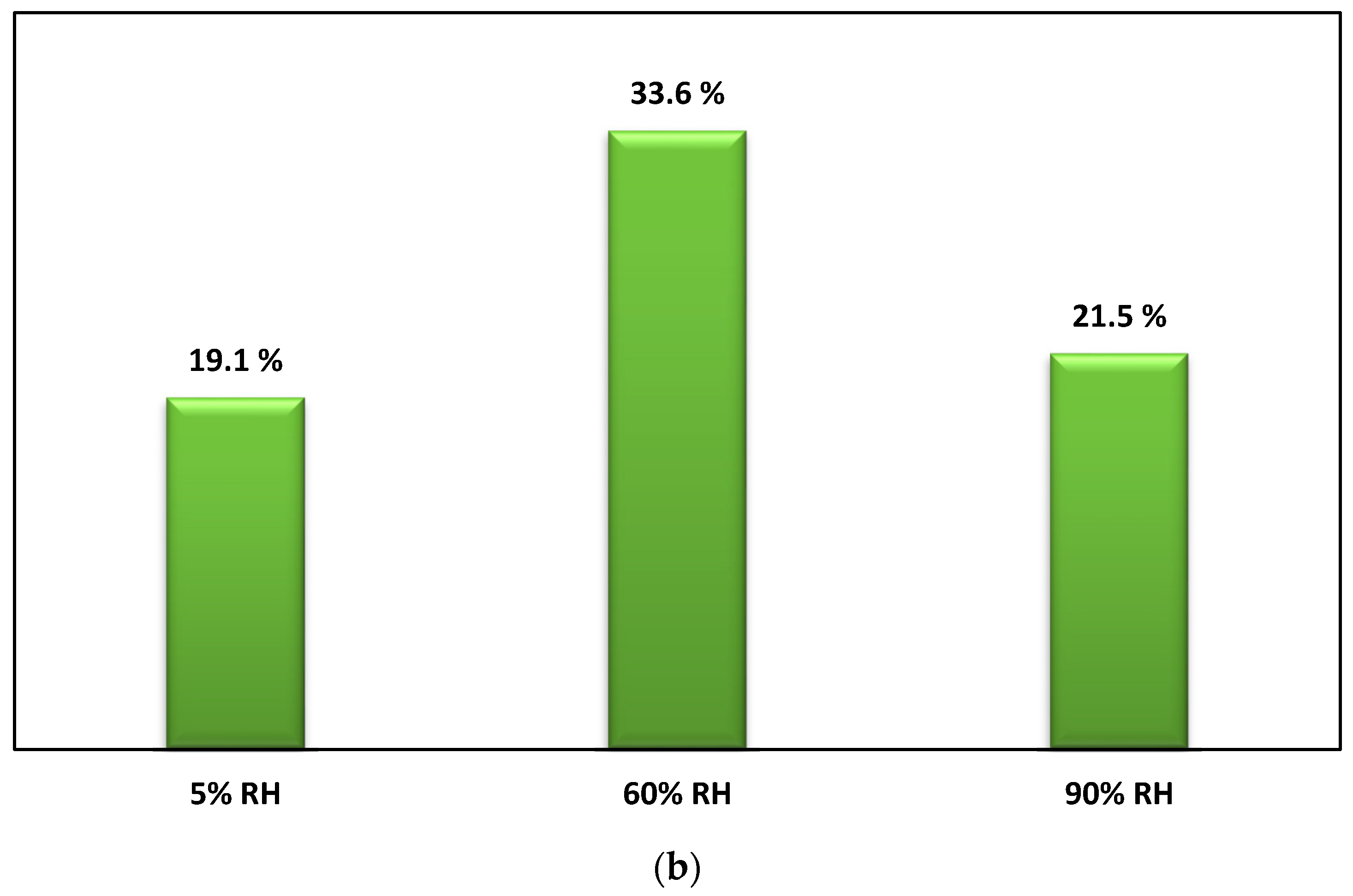
3.1. Photocatalysis Treatment: (i) Effects of Initial Pollutant Concentration and Air Flow on Degradation and (ii) Effect of Water Vapor
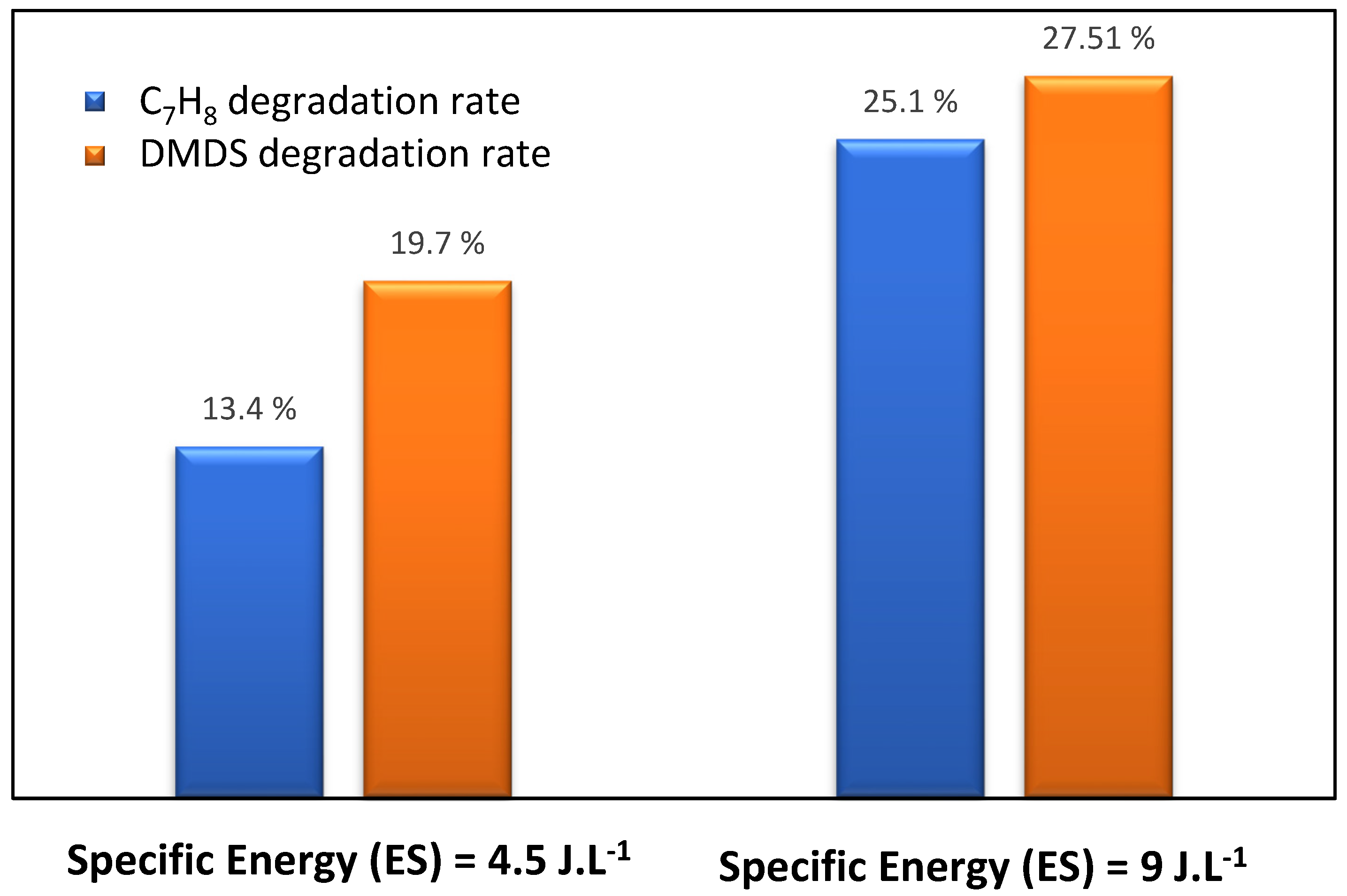
3.2. DBD Direct Plasma Treatment: Effect of Plasma Energy
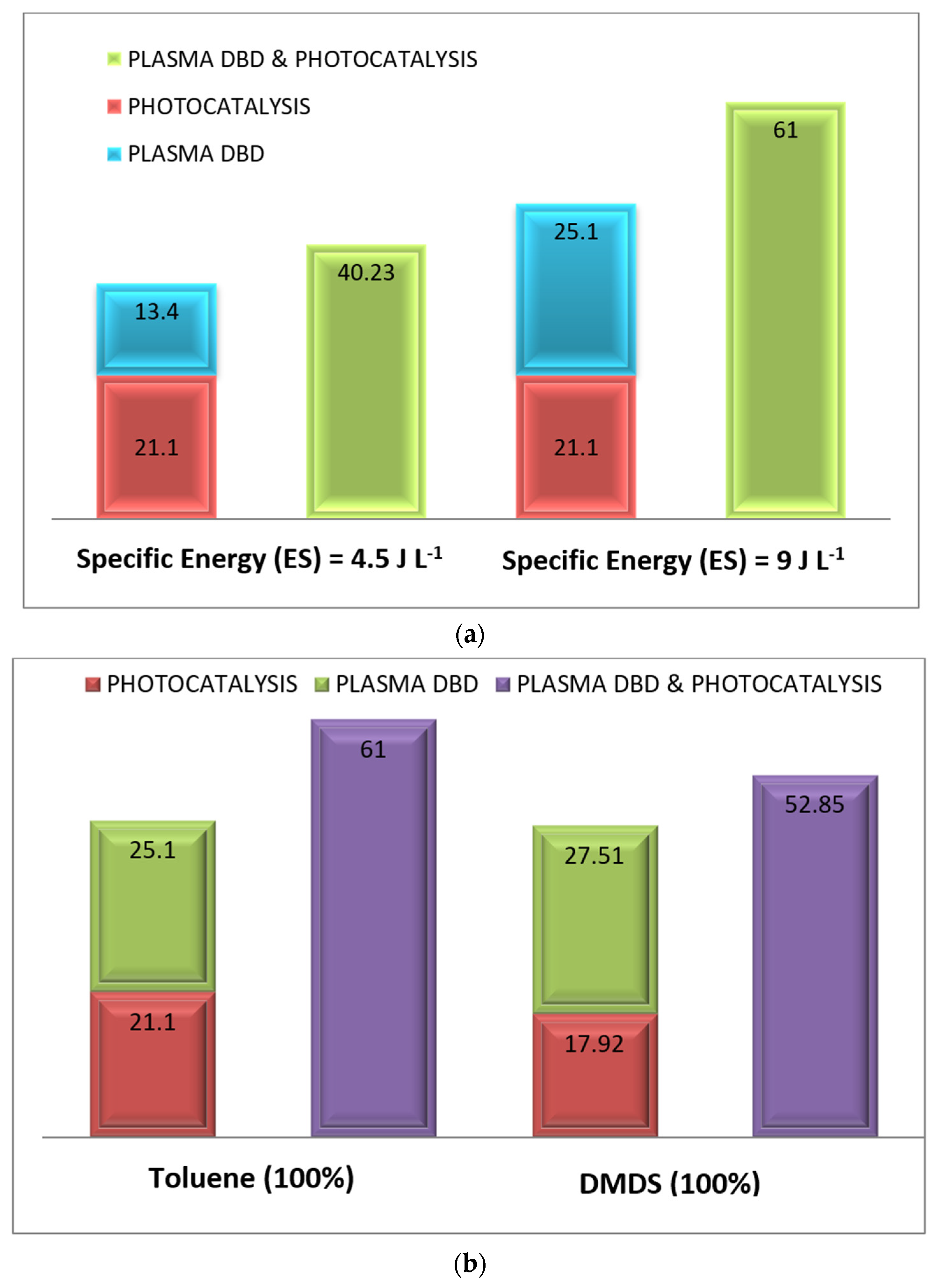
3.3. Treatment by Coupling Process (Photocatalysis/Plasma): Comparison of Process Performance
- (i).
- The action of plasma radicals (N°, O°, H°, OH°), renewing the catalytic site and improving the removal/mineralization step;
- (ii).
- The contribution of the reactive species generated by plasma to photocatalytic mechanisms;
- (iii).
- The Ozone and UV-light reactions generate highly reactive radicals that can activate TiO2 and enhance performance;
- (iv).
- The enhancement of mass transfer of pollutants by the ionic wind generated by plasma;
- (v).
- The in-situ regeneration of the catalytic surface in the presence of the micro discharge of plasma.
3.4. Treatment by Coupling Process (Photocatalysis/Plasma): Effect of Mixture (Toluene/DMDS) and Plasma Power
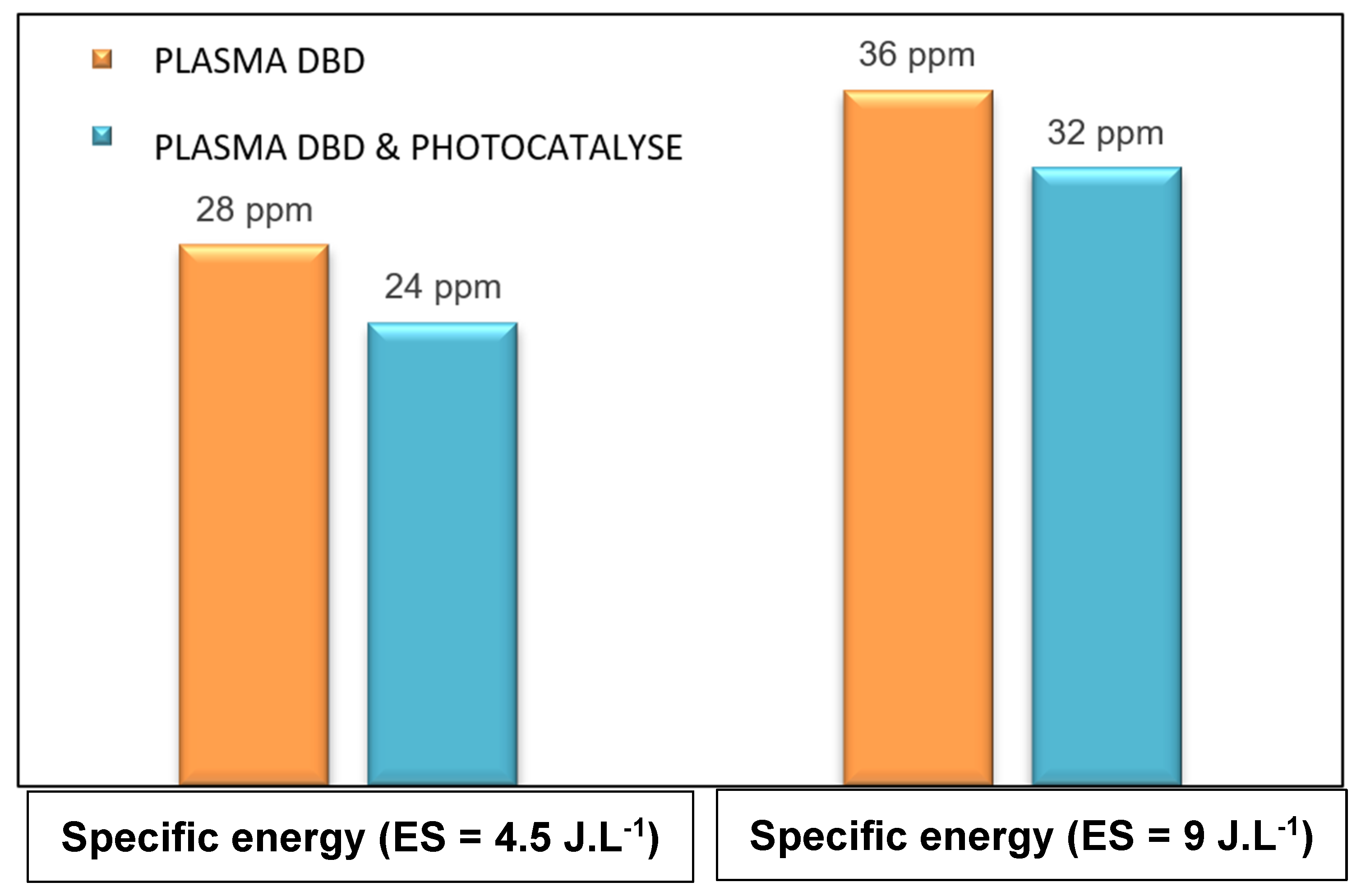
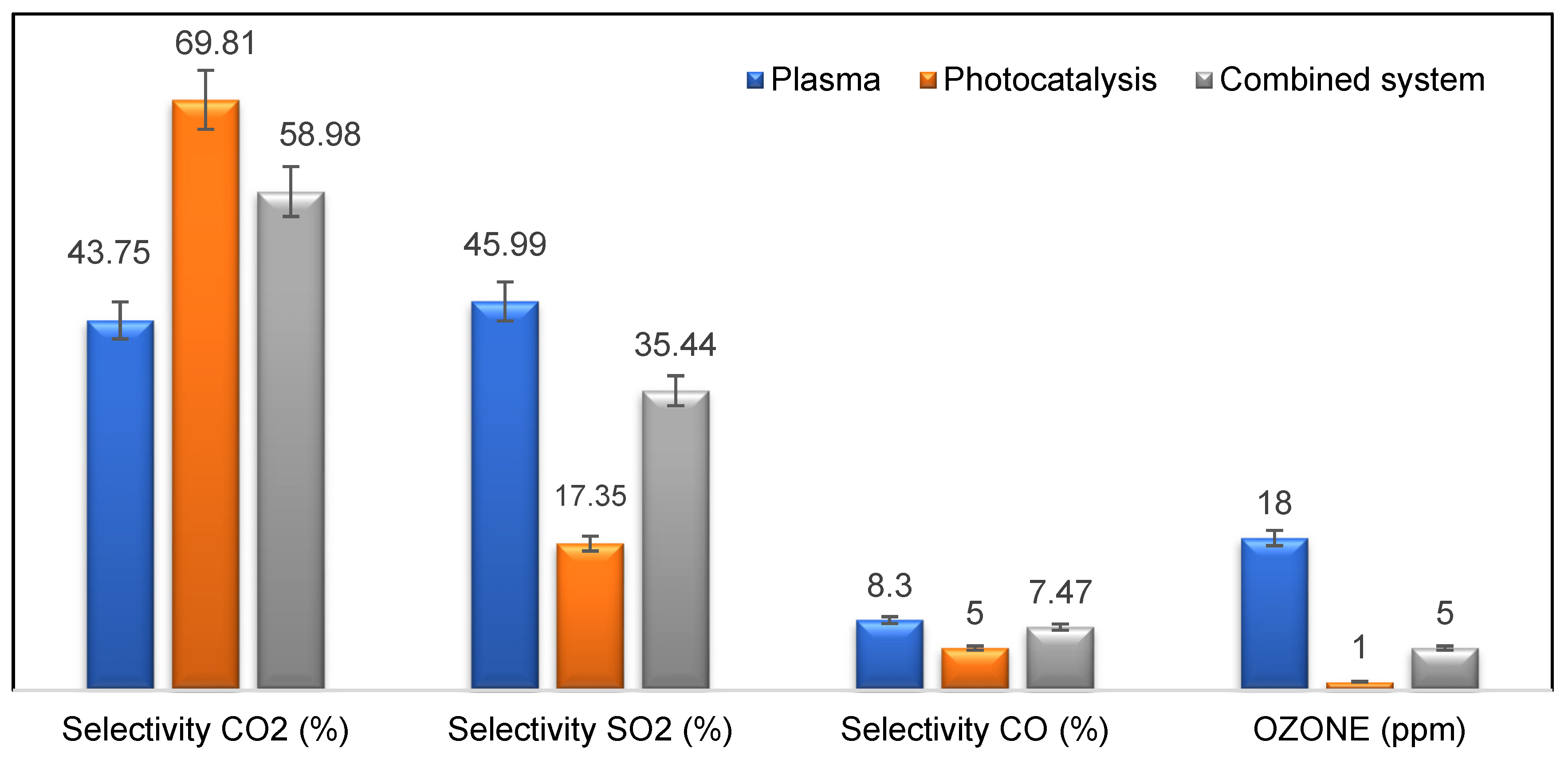
3.5. Study of By-Products Generation: Ozone Selectivity of CO/CO2/SO2
3.5.1. Monitoring of the Ozone Formed
3.5.2. CO2, SO2, CO Selectivity
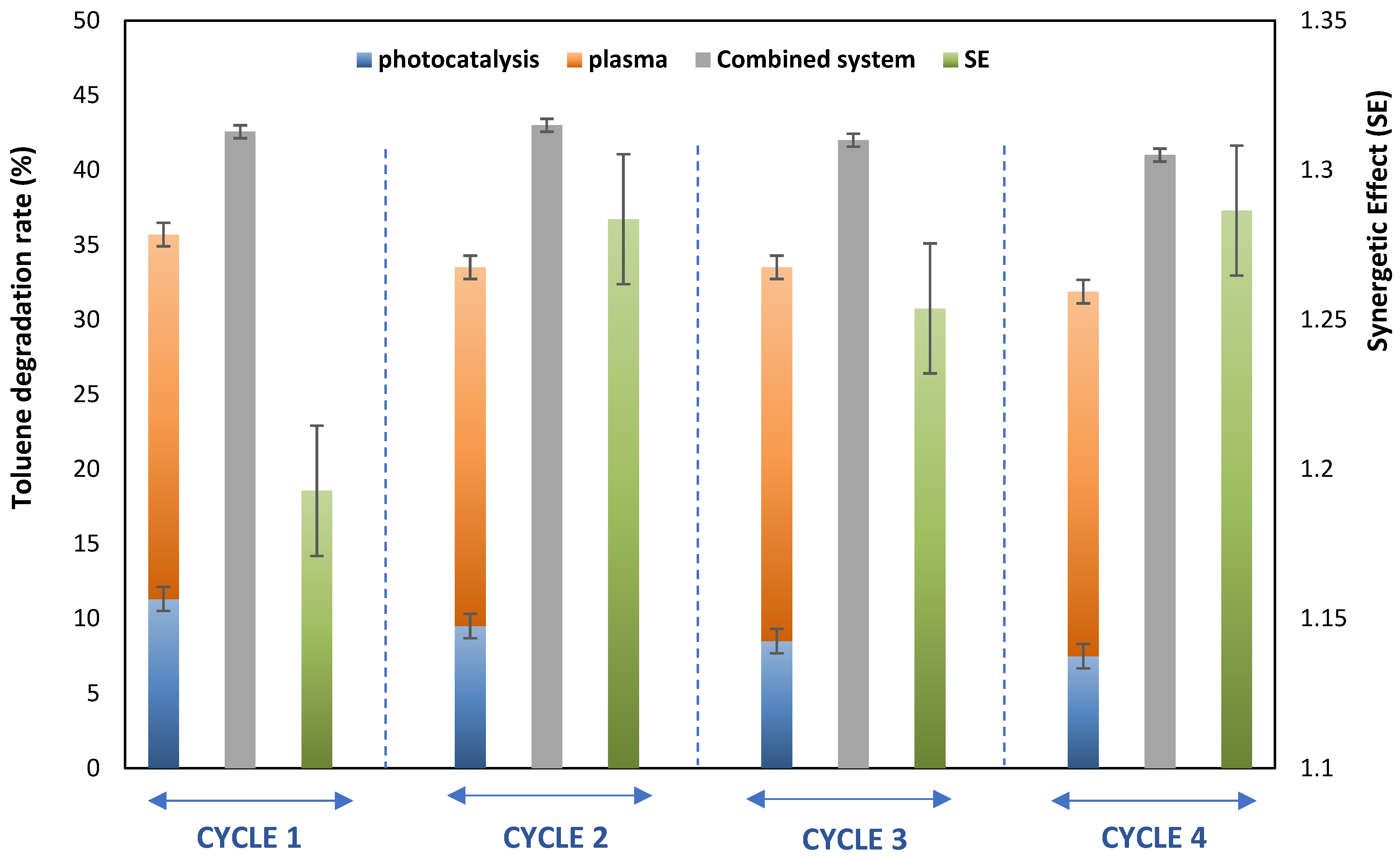
4. Reusability and In Situ Regeneration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, C.; Yu, C.W.; Cao, S.-J. Development of urban air environmental control policies and measures. Indoor Built Environ. 2022, 32, 299–304. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, M.; Shao, S.; Song, X.; Wang, H. Socioeconomic drivers and mitigating strategies of volatile organic compounds emissions in China’s industrial sector. Environ. Impact Assess. Rev. 2023, 101, 107102. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano, H.; Rodríguez-Couto, S. Quantification and Removal of Volatile Sulfur Compounds (VSCs) in Atmospheric Emissions in Large (Petro) Chemical Complexes in Different Countries of America and Europe. Sustainability 2022, 14, 11402. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Q.-Y.; Zheng, J.; Li, Y.-H.; Xiang, S.; Sun, X.-J.; He, X.-S. Volatile and semi-volatile organic compounds in landfill gas: Composition characteristics and health risks. Environ. Int. 2023, 174, 107886. [Google Scholar] [CrossRef]
- Saoud, W.A.; Assadi, A.A.; Kane, A.; Jung, A.-V.; Le Cann, P.; Gerard, A.; Bazantay, F.; Bouzaza, A.; Wolbert, D. Integrated process for the removal of indoor VOCs from food industry manufacturing: Elimination of Butane-2,3-dione and Heptan-2-one by cold plasma-photocatalysis combination. J. Photochem. Photobiol. A Chem. 2020, 386, 112071. [Google Scholar] [CrossRef]
- Acayanka, E.; Tarkwa, J.-B.; Nchimi, K.N.; Voufouo, S.A.; Tiya-Djowe, A.; Kamgang, G.Y.; Laminsi, S. Grafting of N-doped titania nanoparticles synthesized by the plasma-assisted method on textile surface for sunlight photocatalytic self-cleaning applications. Surf. Interfaces 2019, 17, 100361. [Google Scholar] [CrossRef]
- Guillard, C.; Baldassare, D.; Duchamp, C.; Ghazzal, M.; Daniele, S. Photocatalytic degradation and mineralization of a malodorous compound (dimethyldisulfide) using a continuous flow reactor. Catal. Today 2007, 122, 160–167. [Google Scholar] [CrossRef]
- Ramírez, M.; Fernández, M.; Granada, C.; Le Borgne, S.; Gómez, J.M.; Cantero, D. Biofiltration of reduced sulphur compounds and community analysis of sulphur-oxidizing bacteria. Bioresour. Technol. 2011, 102, 4047–4053. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, Z.; Nikiforov, A.; Chen, J.; Wang, J.; Zhao, L.; Zhang, X. The influence of relative humidity on double dielectric barrier discharge plasma for chlorobenzene removal. J. Clean. Prod. 2021, 288, 125502. [Google Scholar] [CrossRef]
- Ochiai, T.; Aoki, D.; Saito, H.; Akutsu, Y.; Nagata, M. Analysis of Adsorption and Decomposition of Odour and Tar Components in Tobacco Smoke on Non-Woven Fabric-Supported Photocatalysts. Catalysts 2020, 10, 304. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Q.; Xu, M.; Yan, C.; Song, X.; Liu, X.; Wang, H.; Zhou, W.; Huo, P. Dual-plasma enhanced 2D/2D/2D g-C3N4/Pd/MoO3-x S-scheme heterojunction for high-selectivity photocatalytic CO2 re-duction. Appl. Surf. Sci. 2023, 640, 158420. [Google Scholar] [CrossRef]
- Młotek, M.; Reda, E.; Jóźwik, P.; Krawczyk, K.; Bojar, Z. Plasma-catalytic decomposition of cyclohexane in gliding discharge reactor. Appl. Catal. A Gen. 2015, 505, 150–158. [Google Scholar] [CrossRef]
- Luo, S.; Lin, H.; Wang, Q.; Ren, X.; Hernández-Pinilla, D.; Nagao, T.; Xie, Y.; Yang, G.; Li, S.; Song, H.; et al. Triggering Water and Methanol Activation for Solar-Driven H2 Production: Interplay of Dual Active Sites over Plasmonic ZnCu Alloy. J. Am. Chem. Soc. 2021, 143, 12145–12153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ren, X.; Ma, F.; Jiang, X.; Wen, Y.; He, W.; Hao, L.; Zeng, C.; Liu, H.; Chen, R.; et al. MOF-derived Co(Ni)Ox species loading on two-dimensional cobalt phosphide: A Janus electrocatalyst toward efficient and stable overall water splitting. Appl. Mater. Today 2023, 34, 101912. [Google Scholar] [CrossRef]
- Ren, X.; Philo, D.; Li, Y.; Shi, L.; Chang, K.; Ye, J. Recent advances of low-dimensional phosphorus-based nanomaterials for solar-driven photocatalytic reactions. Coord. Chem. Rev. 2020, 424, 213516. [Google Scholar] [CrossRef]
- Assadi, A.A.; Loganathan, S.; Tri, P.N.; Gharib-Abou Ghaida, S.; Bouzaza, A.; Tuan, A.N.; Wolbert, D. Pilot scale deg-radation of mono and multi volatile organic compounds by surface discharge plasma/TiO2 reactor: Investigation of competition and synergism. J. Hazard. Mater. 2018, 357, 305–313. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Study of synergetic effect by surface discharge plasma/TiO2 combination for indoor air treatment: Sequential and continuous configurations at pilot scale. J. Photochem. Photobiol. A Chem. 2015, 310, 148–154. [Google Scholar] [CrossRef]
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Trablesi, K.; Makhlouf, H.; Rtimi, S.; Assadi, A.; Bessais, B. Simultaneous removal of bacteria and volatile organic compounds on Cu2O-NPs decorated TiO2 nanotubes: Competition effect and kinetic studies. J. Photochem. Photobiol. A Chem. 2020, 400, 112722. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wang, F.; Di, L.; Yang, S.; Zhu, S.; Yao, Y.; Ma, C.; Dai, B.; Yu, F. Enhanced Photocatalytic Degradation of Organic Dyes via Defect-Rich TiO2 Prepared by Dielectric Barrier Discharge Plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.A.; Bouzaza, A.; Lemasle, M.; Wolbert, D. Removal of trimethylamineand isovaleric acid from gas streams in a con-tinuous flow surface discharge plasma reactor. Chem. Eng. Res. Des. 2014, 93, 640–651. [Google Scholar] [CrossRef]
- Dobslaw, C.; Glocker, B. Plasma Technology and Its Relevance in Waste Air and Waste Gas Treatment. Sustainability 2020, 12, 8981. [Google Scholar] [CrossRef]
- Xu, Z.; Ren, Y.; Deng, X.; Xu, M.; Chai, W.; Qian, X.; Bian, Z. Recent Developments on Gas-Phase Volatile Organic Compounds Abatement Based on Photocatalysis. Adv. Energy Sustain. Res. 2022, 3, 2200105. [Google Scholar] [CrossRef]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Van Heyst, B. An overview of photocatalyst immobilization methods for air pollution remediation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Yu, X.; Dang, X.; Liu, X.; Cao, L. A novel double dielectric barrier discharge reactor for toluene abatement: Role of different discharge zones and reactive species. J. Clean. Prod. 2022, 368, 133073. [Google Scholar] [CrossRef]
- Hoshino, M.; Akimoto, H.; Okuda, M. Photochemical Oxidation of Benzene, Toluene, and Ethylbenzene Initiated by OH Radicals in the Gas Phase. Bull. Chem. Soc. Jpn. 1978, 51, 718–724. [Google Scholar] [CrossRef]
- Winayu, B.N.R.; Chen, S.-T.; Chang, W.-C.; Chu, H. rGO doped S0.05N0.1/TiO2 accelerated visible light driven photocatalytic degradation of dimethyl sulfide and dimethyl disulfide. Appl. Catal. A Gen. 2023, 655, 119113. [Google Scholar] [CrossRef]
- Junior, A.G.; Pereira, A.; Gomes, M.; Fraga, M.; Pessoa, R.; Leite, D.; Petraconi, G.; Nogueira, A.; Wender, H.; Miyakawa, W.; et al. Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts 2020, 10, 282. [Google Scholar] [CrossRef]
- Qi, L.-Q.; Yu, Z.; Chen, Q.-H.; Li, J.-X.; Xue, H.-B.; Liu, F. Toluene degradation using plasma-catalytic hybrid system over Mn-TiO2 and Fe-TiO2. Environ. Sci. Pollut. Res. 2023, 30, 23494–23509. [Google Scholar] [CrossRef] [PubMed]
- Maciuca, A.; Batiot-Dupeyrat, C.; Tatibouët, J.M. Synergetic effect by couplingphotocatalysis with plasma for low VOCs con-centration removal from air. Appl. Catal. B Environ. 2012, 43, 432–438. [Google Scholar] [CrossRef]
- Salvadores, F.; Juan Brandi, R.; Alfano, O.M.; de los Milagros Ballari, M. Modelling and experimental validation of reaction chamber simulating indoor air decontamination by photocatalytic paint. Appl. Catal. A Gen. 2023, 663, 119285. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zheng, J.; Du, M.; Wu, X.; Song, J.; Cheng, C.; Li, T.; Yang, W. Non-thermal plasma-assisted ammonia production: A review. Energy Convers. Manag. 2023, 293, 117482. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, J.-L.; Li, X.-S.; Liu, J.-B.; Zhu, X.; Zhu, A.-M. In Situ Regeneration of Au Nanocatalysts by Atmospheric-Pressure Air Plasma: Regeneration Characteristics of Square-Wave Pulsed Plasma. Top. Catal. 2017, 60, 914–924. [Google Scholar] [CrossRef]
- Malayeri, M.; Haghighat, F.; Lee, C.-S. Modeling of volatile organic compounds degradation by photocatalytic oxidation reactor in indoor air: A review. Build. Environ. 2019, 154, 309–323. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Y.; Chen, S.; Wang, H.; Liu, Z.; Wu, Z. Synergetic effect between non-thermal plasma and photocatalytic oxidation on the degradation of gas-phase toluene: Role of ozone. Chin. J. Catal. 2019, 40, 631–637. [Google Scholar] [CrossRef]
- Adhikari, B.C.; Lamichhane, P.; Lim, J.S.; Nguyen, L.N.; Choi, E.H. Generation of reactive species by naturally sucked air in the Ar plasma jet. Results Phys. 2021, 30, 104863. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, S.; Long, J.; Wu, T.; Fan, Z. The Synthesis of a Core-Shell Photocatalyst Material YF3:Ho3+@TiO2 and Investigation of Its Photocatalytic Properties. Materials 2017, 10, 302. [Google Scholar] [CrossRef]
- Parvari, R.; Ghorbani-Shahna, F.; Bahrami, A.; Azizian, S.; Assari, M.J.; Farhadian, M. α-Fe2O3/Ag/g-C3N4 Core-Discontinuous Shell Nanocomposite as an Indirect Z-Scheme Photocatalyst for Degradation of Ethylbenzene in the Air Under White LEDs Irradiation. Catal. Lett. 2020, 150, 3455–3469. [Google Scholar] [CrossRef]
- Liang, C.; Li, C.; Zhu, Y.; Du, X.; Yao, C.; Ma, Y.; Zhao, J. Recent advances of photocatalytic degradation for BTEX: Materials, operation, and mechanism. Chem. Eng. J. 2023, 455, 140461. [Google Scholar] [CrossRef]
- Liang, W.; Li, J.; Li, J.; Jin, Y. Abatement of toluene from gas streams via ferro-electric packed bed dielectric barrier discharge plasma. J. Hazard. Mater. 2009, 170, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, Y.; Yang, J.; Cao, J.; Su, L.; Wu, X.; Nengzi, L.-C.; Liu, S. Performance and mechanism analysis of degradation of toluene by DBD plasma-catalytic method with MnOx/Al2O3 catalyst. Fuel 2022, 319, 123721. [Google Scholar] [CrossRef]
- Huang, H.; Ye, D.; Leung, D.Y.; Feng, F.; Guan, X. Byproducts and pathways of toluene destruction via plasma-catalysis. J. Mol. Catal. A Chem. 2011, 336, 87–93. [Google Scholar] [CrossRef]




| Parameter | Value/Domain |
|---|---|
| Gas temperature | Ambient (293 K) |
| Gas pressure | Atmospheric pressure (1 atm) |
| Relative humidity | (5, 60, 90) ± 5% |
| Specific Energy (SE) | 4.5–9 J.L−1 |
| Target compound concentartion | 10–60 mg.m−3 |
| Residence time | 1.36 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khezami, L.; Assadi, A.A. Treatment of Mixture Pollutants with Combined Plasma Photocatalysis in Continuous Tubular Reactors with Atmospheric-Pressure Environment: Understanding Synergetic Effect Sources. Materials 2023, 16, 6857. https://doi.org/10.3390/ma16216857
Khezami L, Assadi AA. Treatment of Mixture Pollutants with Combined Plasma Photocatalysis in Continuous Tubular Reactors with Atmospheric-Pressure Environment: Understanding Synergetic Effect Sources. Materials. 2023; 16(21):6857. https://doi.org/10.3390/ma16216857
Chicago/Turabian StyleKhezami, Lotfi, and Aymen Amin Assadi. 2023. "Treatment of Mixture Pollutants with Combined Plasma Photocatalysis in Continuous Tubular Reactors with Atmospheric-Pressure Environment: Understanding Synergetic Effect Sources" Materials 16, no. 21: 6857. https://doi.org/10.3390/ma16216857







