Antimicrobial and Antiproliferative Coatings for Stents in Veterinary Medicine—State of the Art and Perspectives
Abstract
:1. Introduction
- (1)
- To focus on the knowledge of stenting in veterinary medicine for the most common diseases;
- (2)
- To present the knowledge gathered so far on the applications of modified stents in veterinary medicine;
- (3)
- To describe the possible applications of antimicrobial and antiproliferative coatings and their possible use in veterinary medicine;
- (4)
- To determine the direction of future research in the context of stenting procedures and their clinical use in veterinary medicine.
2. Stents in Veterinary Medicine
2.1. Indications for Stenting in Veterinary Medicine
2.2. Comparison of Stenting in Human and Veterinary Medicine
2.3. Types of Stents and Their Coatings Used in Veterinary Medicine
3. Stent Modifications and Its Possible Use in Veterinary Medicine
3.1. Stents Modifications Related to Tracheal Collapse—Granulation Tissue Prevention
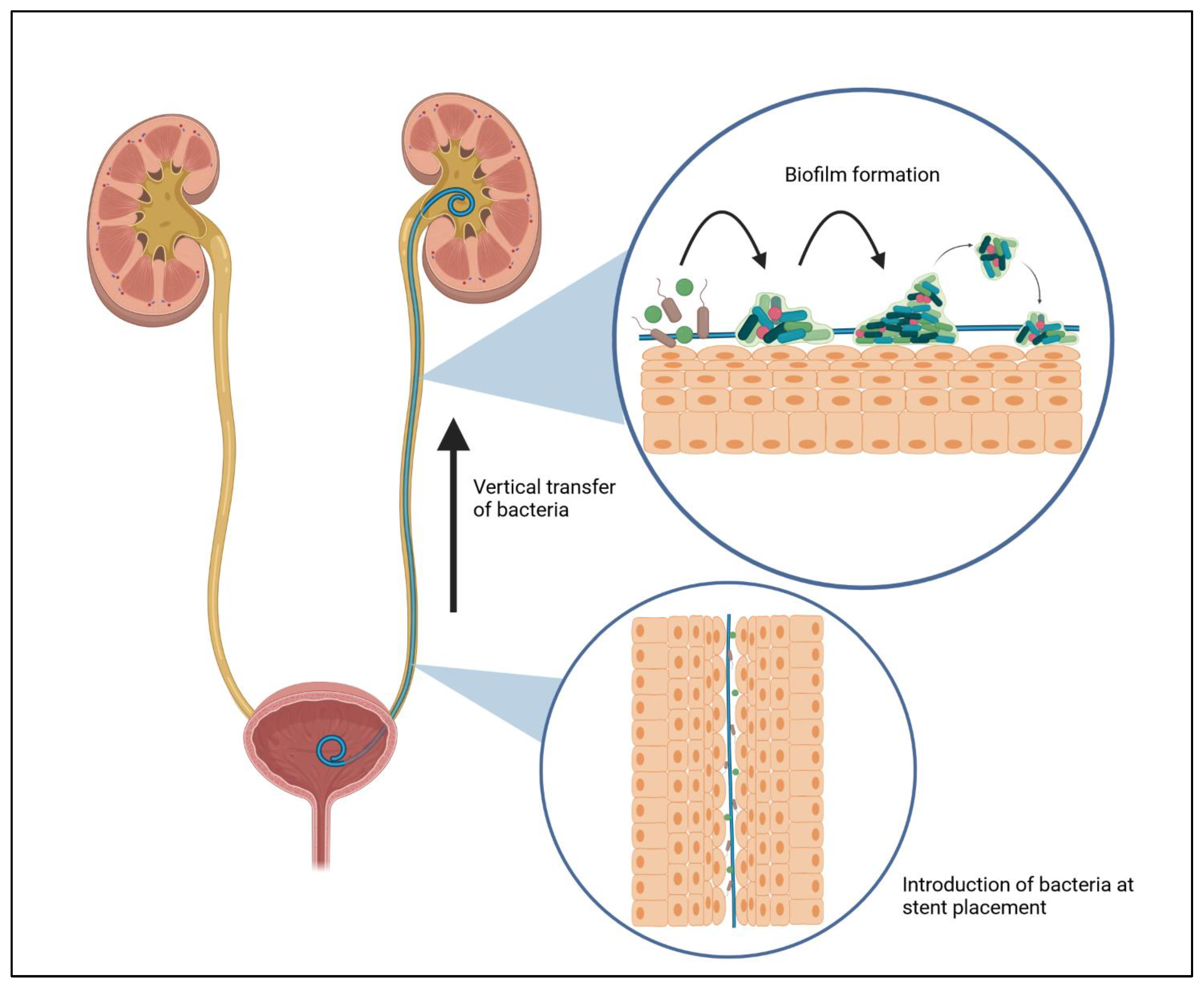
3.2. Biofilm Formation in Urinary Tract Infection Related to Stent Procedures
3.3. Strategies for Biofilm Prevention in Stent Designed for Ureter Obstructions
4. Novel Nanotech-Based Coatings for a Better Tomorrow
5. Limitations
6. Conclusions and Future Perspectives
- (1)
- This review brings together information on the most common conditions treated with stenting procedures in veterinary medicine. Despite promising results shortly after the procedure, serious post-surgical complications occur over time, forcing owners to undertake further surgical measures. Undoubtedly, this is a major economic expense for the owner as well as another interference with the animal’s body, which significantly affects its welfare. So far, no commercial solutions have been introduced to effectively counteract the excessive growth of granulation tissue in the case of tracheal collapse, or bacterial infections after placing a ureteral stent on the veterinary market.
- (2)
- Currently, the most commonly used material in the production of stents in veterinary medicine is nitinol with a cross-braided stent. However, solutions have emerged that modify the design as well as the method of stent implantation, which has translated into a much better postoperative outcome compared to commercially used stents [68,69]. However, they are not available for widespread use, but it seems that the use of such solutions may be justified because they do not need additional procedures related to stent modification, which will also reduce production costs. This is one option that may find viable application in veterinary medicine.
- (3)
- Compared to human medicine, veterinarians do not have available stents that fit into the DES category using coatings of polymers, drugs, nanoparticles, or antimicrobial peptides. Nevertheless, with the available literature focusing on the study of new coatings using in vitro testing, we can determine the future direction of stenting research in veterinary medicine. In addition, there are many reports using in vivo animal models. Through animal studies, we can confirm high efficacy against granulation tissue hyperresponsiveness through the use of cytostatics such as paclitaxel and cisplatin, as well as compounds that inhibit cell proliferation—sirolimus. In addition, antimicrobial compounds based on antimicrobial peptides, immunomodulatory drugs, and in particular, nanoparticles, effectively inhibited bacterial growth and biofilm formation.
- (4)
- In consideration of the above, prospects should focus on implementing new solutions related to surface modification of applied stents. Multicomponent coatings containing both antiproliferative and antimicrobial compounds seem to play a crucial role [79]. Their synergistic action allows for effective protection and reduction of postoperative complications. In addition, stent modifications that change their shape or braided nature also seem to be a good option. A different distribution of the stent surface forces about the surrounding tissue, allowed the tracheal epithelial cells to adapt better, reducing the negative effects after stent placement [68,69]. It should be noted that most of the studies were scientific, where the animal models were mice, rabbits, pigs, and even healthy dogs. However, there are no studies relating to veterinary patients with real-world clinical problems such as tracheal collapse or ureteral obstruction using modified stents. In addition to developing new solutions, there is a need for clinical trials using veterinary patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scafa Udriște, A.; Niculescu, A.G.; Grumezescu, A.M.; Bădilă, E. Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials 2021, 14, 2498. [Google Scholar] [CrossRef] [PubMed]
- Liatsikos, E.N.; Karnabatidis, D.; Kagadis, G.C.; Katsakiori, P.F.; Stolzenburg, J.U.; Nikiforidis, G.C.; Perimenis, P.; Siablis, D. Metal Stents in the Urinary Tract. EAU-EBU Update Ser. 2007, 5, 77–88. [Google Scholar] [CrossRef]
- Johnson, C.M.; Luke, A.S.; Jacobsen, C.; Novak, N.; Dion, G.R. State of the Science in Tracheal Stents: A Scoping Review. Laryngoscope 2022, 132, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Matulevičiūtė, I.; Sidaraitė, A.; Tatarūnas, V.; Veikutienė, A.; Dobilienė, O.; Žaliūnienė, D. Retinal and Choroidal Thinning—A Predictor of Coronary Artery Occlusion? Diagnostics 2022, 12, 2016. [Google Scholar] [CrossRef]
- Bianchi, D.; Vespasiani, G.; Bove, P. Acute Kidney Injury Due to Bilateral Ureteral Obstruction in Children. World J. Nephrol. 2014, 3, 182. [Google Scholar] [CrossRef]
- Majid, A.; Alape, D.; Kheir, F.; Folch, E.; Ochoa, S.; Folch, A.; Gangadharan, S.P. Short-Term Use of Uncovered Self-Expanding Metallic Airway Stents for Severe Expiratory Central Airway Collapse. Respiration 2016, 92, 389–396. [Google Scholar] [CrossRef]
- Remine, W.H.; Grindlay, J.H. Skin-lined omentum and plastic sponge tubes for experimental choledochoduodenostomy. AMA Arch. Surg. 1954, 69, 255–262. [Google Scholar] [CrossRef]
- Stoeckel, D.; Pelton, A.; Duerig, T. Self-Expanding Nitinol Stents: Material and Design Considerations. Eur. Radiol. 2004, 14, 292–301. [Google Scholar] [CrossRef]
- Graczyk, S.; Pasławski, R.; Grzeczka, A.; Litwińska, L.; Jagielski, D.; Pasławska, U. Stents in Veterinary Medicine. Materials 2023, 16, 1480. [Google Scholar] [CrossRef]
- Mittleman, E.; Weisse, C.; Mehler, S.J.; Lee, J.A. Fracture of an endoluminal nitinol stent used in the treatment of tracheal collapse in a dog. J. Am. Vet. Med. Assoc. 2004, 225, 1217–1221. [Google Scholar] [CrossRef]
- Johnson, L.R.; Pollard, R.E. Tracheal Collapse and Bronchomalacia in Dogs: 58 Cases (7/2001–1/2008). J. Vet. Intern. Med. 2010, 24, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Konan, P.G.; Manzan, K.; Dekou, A.; Djedje Mady, A.; Yao Dje, C. Nosocomial Urinary Stent Infection at the Urology Service of the Cocody University Hospital Center. Dakar Med. 1999, 44, 186–189. [Google Scholar] [PubMed]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. Bionanoscience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- John, T.; Rajpurkar, A.; Smith, G.; Fairfax, M.; Triest, J. Antibiotic Pretreatment of Hydrogel Ureteral Stent. J. Endourol. 2007, 21, 1211–1215. [Google Scholar] [CrossRef]
- Zhang, J.M.; Liu, J.; Wang, K.; Zhang, X.; Zhao, T.; Luo, H.M. Observations of Bacterial Biofilm on Ureteral Stent and Studies on the Distribution of Pathogenic Bacteria and Drug Resistance. Urol. Int. 2018, 101, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sindeeva, O.A.; Prikhozhdenko, E.S.; Schurov, I.; Sedykh, N.; Goriainov, S.; Karamyan, A.; Mordovina, E.A.; Inozemtseva, O.A.; Kudryavtseva, V.; Shchesnyak, L.E.; et al. Patterned Drug-Eluting Coatings for Tracheal Stents Based on Pla, Plga, and Pcl for the Granulation Formation Reduction: In Vivo Studies. Pharmaceutics 2021, 13, 1437. [Google Scholar] [CrossRef]
- Liu, J.; Yao, X.; Wang, Z.; Ye, J.; Luan, C.; He, Y.; Lin, H.; Fu, J. A Flexible Porous Chiral Auxetic Tracheal Stent with Ciliated Epithelium. Acta Biomater. 2021, 124, 153–165. [Google Scholar] [CrossRef]
- Defarges, A.; Berent, A.; Dunn, M. New alternatives for minimally invasive management of uroliths: Ureteroliths. Compend. Contin. Educ. Vet. 2013, 35, E4. [Google Scholar]
- Weisse, C.; Berent, A.; Violette, N.; McDougall, R.; Lamb, K. Short-, Intermediate-, and Long-Term Results for Endoluminal Stent Placement in Dogs with Tracheal Collapse. J. Am. Vet. Med. Assoc. 2019, 254, 380–392. [Google Scholar] [CrossRef]
- Gellasch, K.L.; Da Costa Gomez, T.; McAnulty, J.F.; Bjorling, D.E. Use of intraluminal nitinol stents in the treatment of tracheal collapse in a dog. J. Am. Vet. Med. Assoc. 2002, 221, 1719–1723. [Google Scholar] [CrossRef]
- Sura, P.A.; Krahwinkel, D.J. Self-Expanding Nitinol Stents for the Treatment of Tracheal Collapse in Dogs: 12 Cases (2001–2004). J. Am. Vet. Med. Assoc. 2008, 232, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Berent, A.C.; Weisse, C.; Beal, M.W.; Brown, D.C.; Todd, K.; Bagley, D. Use of Indwelling, Double-Pigtail Stents for Treatment of Malignant Ureteral Obstruction in Dogs: 12 Cases (2006–2009). J. Am. Vet. Med. Assoc. 2011, 238, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.K.; Knapp, D.W.; Ramos-Vara, J.A.; Bonney, P.L.; Adams, L.G. Outcome of Urethral Stent Placement for Management of Urethral Obstruction Secondary to Transitional Cell Carcinoma in Dogs: 19 Cases (2007–2010). J. Am. Vet. Med. Assoc. 2012, 241, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Durant, A.M.; Sura, P.; Rohrbach, B.; Bohling, M.W. Use of Nitinol Stents for End-Stage Tracheal Collapse in Dogs. Vet. Surg. 2012, 41, 807–817. [Google Scholar] [CrossRef]
- Blackburn, A.L.; Berent, A.C.; Weisse, C.W.; Brown, D.C. Evaluation of Outcome Following Urethral Stent Placement for the Treatment of Obstructive Carcinoma of the Urethra in Dogs: 42 Cases (2004–2008). J. Am. Vet. Med. Assoc. 2013, 242, 59–68. [Google Scholar] [CrossRef]
- Berent, A.C.; Weisse, C.W.; Todd, K.; Bagley, D.H. Technical and clinical outcomes of ureteral stenting in cats with benign ureteral obstruction: 69 cases (2006–2010). J. Am. Vet. Med. Assoc. 2014, 244, 559–576. [Google Scholar] [CrossRef]
- Kuntz, J.A.; Berent, A.C.; Weisse, C.W.; Bagley, D.H. Double Pigtail Ureteral Stenting and Renal Pelvic Lavage for Renal-Sparing Treatment of Obstructive Pyonephrosis in Dogs: 13 Cases (2008–2012). J. Am. Vet. Med. Assoc. 2015, 246, 216–225. [Google Scholar] [CrossRef]
- Tinga, S.; Thieman Mankin, K.M.; Peycke, L.E.; Cohen, N.D. Comparison of Outcome After Use of Extra-Luminal Rings and Intra-Luminal Stents for Treatment of Tracheal Collapse in Dogs. Vet. Surg. 2015, 44, 858–865. [Google Scholar] [CrossRef]
- Wormser, C.; Clarke, D.L.; Aronson, L.R. Outcomes of Ureteral Surgery and Ureteral Stenting in Cats: 117 Cases (2006–2014). J. Am. Vet. Med. Assoc. 2016, 248, 518–525. [Google Scholar] [CrossRef]
- Pavia, P.R.; Berent, A.C.; Weisse, C.W.; Neiman, D.; Lamb, K.; Bagley, D. Outcome of Ureteral Stent Placement for Treatment of Benign Ureteral Obstruction in Dogs: 44 Cases (2010–2013). J. Am. Vet. Med. Assoc. 2018, 252, 721–731. [Google Scholar] [CrossRef]
- Violette, N.P.; Weisse, C.; Berent, A.C.; Lamb, K.E. Correlations among Tracheal Dimensions, Tracheal Stent Dimensions, and Major Complications after Endoluminal Stenting of Tracheal Collapse Syndrome in Dogs. J. Vet. Intern. Med. 2019, 33, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A.; Ozai, Y.; Hamabe, L.; Yoshida, T.; Tanaka, R. Surgical Outcomes in Dogs with Tracheal Collapse Treated with a Novel Cross-and-Hook Braided Endoluminal Stent. J. Vet. Sci. 2022, 23, e46. [Google Scholar] [CrossRef] [PubMed]
- Della Maggiore, A. An Update on Tracheal and Airway Collapse in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Pereira, Y.M. Approach to the Coughing Dog. Practice 2013, 35, 503–517. [Google Scholar] [CrossRef]
- Ferasin, L.; Linney, C. Coughing in Dogs: What Is the Evidence for and against a Cardiac Cough? J. Small Anim. Pract. 2019, 60, 139–145. [Google Scholar] [CrossRef]
- Corrêa Reis, J.G.; Takiya, C.M.; Lima Carvalho, A.; Souza Mota, R.; De Ary-Pires, B.; Pires-Neto, M.A.; De Ary-Pires, R. Myofibroblast Persistence and Collagen Type I Accumulation in the Human Stenotic Trachea. Head Neck 2012, 34, 1283–1293. [Google Scholar] [CrossRef]
- Lesnikowski, S.; Weisse, C.; Berent, A.; Le Roux, A.; Tozier, E. Bacterial Infection before and after Stent Placement in Dogs with Tracheal Collapse Syndrome. J. Vet. Intern. Med. 2020, 34, 725–733. [Google Scholar] [CrossRef]
- Congiusta, M.; Weisse, C.; Berent, A.C.; Tozier, E. Comparison of Short-, Intermediate-, and Long-Term Results between Dogs with Tracheal Collapse That Underwent Multimodal Medical Management Alone and Those That Underwent Tracheal Endoluminal Stent Placement. J. Am. Vet. Med. Assoc. 2021, 258, 279–289. [Google Scholar] [CrossRef]
- Beal, M.W. Tracheal Stent Placement for the Emergency Management of Tracheal Collapse in Dogs. Top Companion Anim. Med. 2013, 28, 106–111. [Google Scholar] [CrossRef]
- White, R.A.S.; Williams, J.M. Tracheal Collapse in the Dog—Is There Really a Role for Surgery? A Survey of 100 Cases. J. Small Anim. Pract. 1994, 35, 191–196. [Google Scholar] [CrossRef]
- Pardali, D.; Adamama-Moraitou, K.K.; Rallis, T.S.; Raptopoulos, D.; Gioulekas, D. Tidal Breathing Flow-Volume Loop Analysis for the Diagnosis and Staging of Tracheal Collapse in Dogs. J. Vet. Intern. Med. 2010, 24, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.M.; Sponseller, B.A.; Riedesel, E.A.; Couëtil, L.L.; Kersh, K. The Use of Intraluminal Stents for Tracheal Collapse in Two Horses: Case Management and Long-Term Treatment. Equine Vet. Educ. 2008, 20, 80–90. [Google Scholar] [CrossRef]
- Kopecny, L.; Palm, C.A.; Drobatz, K.J.; Balsa, I.M.; Culp, W.T.N. Risk Factors for Positive Urine Cultures in Cats with Subcutaneous Ureteral Bypass and Ureteral Stents (2010–2016). J. Vet. Intern. Med. 2019, 33, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Berent, A. Interventional Management of Canine and Feline Benign Ureteral Obstructions. In Veterinary Image-Guided Interventions, 1st ed.; Weisse, C., Berent, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; Volume 29, pp. 309–335. [Google Scholar] [CrossRef]
- Radhakrishnan, A. Urethral Stenting for Obstructive Uropathy Utilizing Digital Radiography for Guidance: Feasibility and Clinical Outcome in 26 Dogs. J. Vet. Intern. Med. 2017, 31, 427–433. [Google Scholar] [CrossRef]
- Beal, M.W. Interventional Management of Urethral Obstructions. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 863–874. [Google Scholar] [CrossRef]
- Lulich, J.P. Evaluation of Temporary Urethral Stents in the Management of Malignant and Nonmalignant Urethral Diseases in Dogs. Vet. Sci. 2022, 9, 63. [Google Scholar] [CrossRef]
- Hoehne, S.N.; Milovancev, M.; Hyde, A.J.; deMorais, H.A.; Scollan, K.F.; Nemanic, S. Placement of a Caudal Vena Cava Stent for Treatment of Budd-Chiari–like Syndrome in a 4-Month-Old Ragdoll Cat. J. Am. Vet. Med. Assoc. 2014, 245, 414–418. [Google Scholar] [CrossRef]
- Bertolini, G.; Caldin, M. Percutaneous Cava Stenting in a Dog with Symptomatic Azygos Continuation of the Caudal Vena Cava. Case Rep. Vet. Med. 2020, 2020, 7523247. [Google Scholar] [CrossRef]
- Borgeat, K.; Gomart, S.; Kilkenny, E.; Chanoit, G.; Hezzell, M.J.; Payne, J.R. Transvalvular Pulmonic Stent Angioplasty: Procedural Outcomes and Complications in 15 Dogs with Pulmonic Stenosis. J. Vet. Cardiol. 2021, 38, 1–11. [Google Scholar] [CrossRef]
- Morgan, K.R.S.; Stauthammer, C.D.; Gruenstein, D. Transmembrane Stent Placement for Cor Triatriatum Dexter in Six Dogs. J. Vet. Cardiol. 2022, 41, 79–87. [Google Scholar] [CrossRef]
- Schreiber, N.; Toaldo, M.B.; Wolfer, N.; Dennler, M.; Corona, D.; Henze, I.; Kovacevic, A.; Glaus, T. Long-Term Palliation of Right-Sided Congestive Heart Failure after Stenting a Recurrent Cor Triatriatum Dexter in a 10½-Year-Old Pug. J. Vet. Cardiol. 2022, 41, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.N.; MacPhail, C.M.; Perry, J.A.; Jensen, T.D. Use of a Nitinol Stent to Palliate a Colorectal Neoplastic Obstruction in a Dog. J. Am. Vet. Med. Assoc. 2011, 239, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Monnet, E. Biliary Stenting. In Gastrointestinal Surgical Techniques in Small Animals, 1st ed.; Monnet, E., Smeak, D.D., Eds.; John Willey & Sons: Hoboken, NJ, USA, 2020; Volume 39, pp. 293–296. [Google Scholar] [CrossRef]
- Krohn, J.; Ennen, S.; Hospes, R.; Nieth, J.; Wehrend, A. Use of a Cervical Stent for Long-Term Treatment of Pyometra in the Mare: A Report of Three Cases. Reprod. Domest. Anim. 2019, 54, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Mahley, R.W.; Weisgraber, K.H.; Innerarity, T. Canine Lipoproteins and Atherosclerosis. Circ. Res. 1974, 35, 722–733. [Google Scholar] [CrossRef]
- Ginzinger, D.G.; Wilson, J.E.; Redenbach, D.; Lewis, M.E.; Clee, S.M.; Excoffon, K.J.; Rogers, Q.R.; Hayden, M.R.; McManus, A.B.M. Diet-induced atherosclerosis in the domestic cat. Lab. Investig. 1997, 77, 409–419. [Google Scholar]
- Yang, L.; Whiteside, S.; Cadieux, P.A.; Denstedt, J.D. Ureteral Stent Technology: Drug-Eluting Stents and Stent Coatings. Asian. J. Urol. 2015, 2, 194–201. [Google Scholar] [CrossRef]
- Lee, D.H.; de la Torre Hernandez, J.M. The Newest Generation of Drug-Eluting Stents and Beyond. Eur. Cardiol. Rev. 2018, 13, 54. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Su, H.; Gu, Y.; Ma, Q.; Zhang, Y. Recent Developments in Antibacterial or Antibiofilm Compound Coating for Biliary Stents. Colloids Surf. B Biointerfaces 2022, 219, 112837. [Google Scholar] [CrossRef]
- Serrano-Casorran, C.; Lopez-Minguez, S.; Rodriguez-Zapater, S.; Bonastre, C.; Guirola, J.A.; De Gregorio, M.A. A New Airway Spiral Stent Designed to Maintain Airway Architecture with an Atraumatic Removal after Full Epithelization—Research of Feasibility and Viability in Canine Patients with Tracheomalacia. Pediatr. Pulmonol. 2020, 55, 1757–1764. [Google Scholar] [CrossRef]
- Zaid, M.S.; Berent, A.C.; Weisse, C.; Caceres, A. Feline Ureteral Strictures: 10 Cases (2007–2009). J. Vet. Intern. Med. 2011, 25, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kulendra, E.; Kulendra, N.; Halfacree, Z. Management of Bilateral Ureteral Trauma Using Ureteral Stents and Subsequent Subcutaneous Ureteral Bypass Devices in a Cat. J. Feline Med. Surg. 2014, 16, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Wormser, C.; Clarke, D.L.; Aronson, L.R. End-to-End Ureteral Anastomosis and Double-Pigtail Ureteral Stent Placement for Treatment of Iatrogenic Ureteral Trauma in Two Dogs. J. Am. Vet. Med. Assoc. 2015, 247, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A.; Schneider, M.; Bauer, N. Management of Advanced Tracheal Collapse in Dogs Using Intraluminal Self-Expanding Biliary Wallstents. J. Vet. Intern. Med. 2004, 18, 31–42. [Google Scholar] [CrossRef]
- Tanaka, M.; Uemura, A. Self-Expanding Tracheal Stent Placement in a Cat with Primary Tracheal Collapse. Vet. Med. Sci. 2022, 8, 1347–1351. [Google Scholar] [CrossRef]
- Radlinsky, M.G.; Fossum, T.W.; Walker, M.A.; Aufdemorte, T.B.; Thompson, J.A. Evaluation of the Palmaz Stent in the Trachea and Mainstem Bronchi of Normal Dogs. Vet. Surg. 1997, 26, 99–107. [Google Scholar] [CrossRef]
- Lopez-Minguez, S.; Rodriguez-Zapater, S.; Bonastre, C.; Rodriguez, J.; De Gregorio, M.A.; Guirola, J.A.; Serrano-Casorran, C. A New Removable Helical Metallic Stent for the Treatment of Tracheomalacia in Children: Study in Pathological Animal Model. J. Clin. Med. 2022, 11, 6757. [Google Scholar] [CrossRef]
- Liu, K.S.; Liu, Y.H.; Peng, Y.J.; Liu, S.J. Experimental Absorbable Stent Permits Airway Remodeling. J. Thorac. Cardiovasc. Surg. 2011, 141, 463–468. [Google Scholar] [CrossRef]
- Shi, J.; Lv, Y.; Yu, L.; Zhang, B.; Zhang, X.; Fan, C.; Geng, Z. Interest of a New Biodegradable Stent Coated with Paclitaxel on Anastomotic Wound Healing after Biliary Reconstruction. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1415–1423. [Google Scholar] [CrossRef]
- Grolich, T.; Crha, M.; Novotný, L.; Kala, Z.; Hep, A.; Nečas, A.; Hlavsa, J.; Mitáš, L.; Misík, J. Self-Expandable Biodegradable Biliary Stents in Porcine Model. J. Surg. Res. 2015, 193, 606–612. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.F.; Yin, Y.X.; Shi, Z.Z.; Li, T.; Feng, X.Y.; Zhang, J.W.; Song, C.X.; Cui, X.S.; Xu, K.L.; et al. Long-Term in Vivo Study of Biodegradable Zn-Cu Stent: A 2-Year Implantation Evaluation in Porcine Coronary Artery. Acta Biomater. 2019, 97, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Gwon, D.; Lee, S.S.; Kim, E.Y. Cefotaxime-Eluting Covered Self-Expandable Stents in a Canine Biliary Model: Scanning Electron Microscopic Study of Biofilm Formation. Acta Radiol. 2012, 53, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Wu, L.; Wang, J.; Yang, Z.; Tu, Q.; Zhang, X.; Huang, N. Surface-Degradable Drug-Eluting Stent with Anticoagulation, Antiproliferation, and Endothelialization Functions. Biomolecules 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S. Tracheal Stent: Biocompatibility. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2018. [Google Scholar]
- Pakseresht, S.; Alogaili, A.W.M.; Akbulut, H.; Placha, D.; Pazdziora, E.; Klushina, D.; Konvičková, Z.; Kratošová, G.; Holešová, S.; Martynková, G.S. Silver/Chitosan Antimicrobial Nanocomposites Coating for Medical Devices: Comparison of Nanofiller Effect Prepared via Chemical Reduction and Biosynthesis. J. Nanosci. Nanotechnol. 2018, 19, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Vladkova, T.G.; Staneva, A.D.; Gospodinova, D.N. Surface Engineered Biomaterials and Ureteral Stents Inhibiting Biofilm Formation and Encrustation. Surf. Coat. Technol. 2020, 404, 126424. [Google Scholar] [CrossRef]
- Li, Z.; Tian, C.; Jiao, D.; Li, J.; Li, Y.; Zhou, X.; Zhao, H.; Zhao, Y.; Han, X. Synergistic Effects of Silver Nanoparticles and Cisplatin in Combating Inflammation and Hyperplasia of Airway Stents. Bioact. Mater. 2022, 9, 266–280. [Google Scholar] [CrossRef]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Chen, X.; Assadsangabi, B.; Hsiang, Y.; Takahata, K.; Chen, X.; Assadsangabi, B.; Takahata, K.; Hsiang, Y. Enabling Angioplasty-Ready “Smart” Stents to Detect In-Stent Restenosis and Occlusion. Adv. Sci. 2018, 5, 1700560. [Google Scholar] [CrossRef]
- Wilson, A.C.; Neuenschwander, P.F.; Chou, S.-F. Engineering Approaches to Prevent Blood Clotting from Medical Implants. Arch. Biomed. Eng. Biotechnol. 2019, 1, 000510. [Google Scholar] [CrossRef]
- Horwitz, S.B. Taxol (Paclitaxel): Mechanisms of Action. Ann. Oncol. 1994, 5 (Suppl. S6), S3–S6. [Google Scholar]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N. Sirolimus: Its Discovery, Biological Properties, and Mechanism of Action. Transplant. Proc. 2003, 35, S7–S14. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.E.; Kuntz, R.E.; Schwartz, L.B. Zotarolimus (ABT-578) Eluting Stents. Adv. Drug Deliv. Rev. 2006, 58, 437–446. [Google Scholar] [CrossRef] [PubMed]
- LeFrock, J.L.; Prince, R.A.; Left, R.D. Mechanism of Action, Antimicrobial Activity, Pharmacology, Adverse Effects, and Clinical Efficacy of Cefotaxime. J. Hum. Pharmac. Drug Therap. 1982, 2, 174–184. [Google Scholar] [CrossRef]
- Liu, C.; Qi, J.; Shan, B.; Ma, Y. Tachyplesin Causes Membrane Instability That Kills Multidrug-Resistant Bacteria by Inhibiting the 3-Ketoacyl Carrier Protein Reductase FabG. Front. Microbiol. 2018, 9, 356510. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V.; Vila, M.M.D.C.; Teixeira, J.A.; Balcão, V.M. Alternatives to Overcoming Bacterial Resistances: State-of-the-Art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef]
- Heath, R.J.; Rubin, J.R.; Holland, D.R.; Zhang, E.; Snow, M.E.; Rock, C.O. Mechanism of Triclosan Inhibition of Bacterial Fatty Acid Synthesis. J. Biol. Chem. 1999, 274, 11110–11114. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Olivi, M.; Alfè, M.; Gargiulo, V.; Valle, F.; Mura, F.; Di Giosia, M.; Rapino, S.; Palleschi, C.; Uccelletti, D.; Fiorito, S. Antimicrobial Properties of Graphene-like Nanoparticles: Coating Effect on Staphylococcus aureus. J. Nanopart. Res. 2016, 18, 358. [Google Scholar] [CrossRef]
- Bansal, V.; Sharma, P.K.; Sharma, N.; Pal, O.P.; Malviya, R. Applications of Chitosan and Chitosan Derivatives in Drug Delivery. Adv. Biol. Res. 2011, 5, 28–37. [Google Scholar]
- Pawar, R.; Pathan, A.; Nagaraj, S.; Kapare, H.; Giram, P.; Wavhale, R. Polycaprolactone and Its Derivatives for Drug Delivery. Polym. Adv. Technol. 2023, 34, 3296–3316. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly (Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Chavan, Y.R.; Tambe, S.M.; Jain, D.D.; Khairnar, S.V.; Amin, P.D. Redefining the Importance of Polylactide-Co-Glycolide Acid (PLGA) in Drug Delivery. Ann. Pharm. Fr. 2022, 80, 603–616. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Li, M.; Yin, Z.; Butt, H.A. The Synthesis, Mechanisms, and Additives for Bio-Compatible Polyvinyl Alcohol Hydrogels: A Review on Current Advances, Trends, and Future Outlook. J. Vinyl. Addit. Technol. 2022. [Google Scholar] [CrossRef]
- Haas, J.; Lehr, C.M. Developments in the Area of Bioadhesive Drug Delivery Systems. Expert Opin. Biol. Ther. 2002, 2, 287–298. [Google Scholar] [CrossRef]
- Debiane, L.; Reitzel, R.; Rosenblatt, J.; Gagea, M.; Chavez, M.A.; Adachi, R.; Grosu, H.B.; Sheshadri, A.; Hill, L.R.; Raad, I.; et al. A Design-Based Stereologic Method to Quantify the Tissue Changes Associated with a Novel Drug-Eluting Tracheobronchial Stent. Respiration 2019, 98, 60–69. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Zhang, J.; Wang, T.; Qiu, X.J.; Wang, Y.L. Preparation and Characterization of Paclitaxel-Loaded Poly Lactic Acid-Co-Glycolic Acid Coating Tracheal Stent. Chin. Med. J. 2014, 127, 2236–2240. [Google Scholar] [CrossRef]
- Arellano-Orden, E.; Serrano, C.; Montes-Worboys, A.; Sánchez-López, V.; Laborda, A.; Lostalé, F.; Lahuerta, C.; Rodríguez-Panadero, F.; de Gregorio, M.Á. Stent-Induced Tracheal Stenosis Can Be Predicted by IL-8 Expression in Rabbits. Eur. J. Clin. Investig. 2017, 47, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Wang, J.; Pei, Y.H.; Qiu, X.J.; Wang, Y.L. Paclitaxel Drug-Eluting Tracheal Stent Could Reduce Granulation Tissue Formation in a Canine Model. Chin. Med. J. 2016, 129, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, Y.; Wang, X.; Liu, Y.; Ren, K.; Liu, X.; Zhang, H.; Li, Z.; Han, X.; Uyama, H.; et al. Microinjection Molded Biopolymeric Airway Stent with Antibacterial and Anti-Hyperplastic Properties. Macromol. Biosci. 2023, 2023, 2300113. [Google Scholar] [CrossRef]
- Chao, Y.K.; Liu, K.S.; Wang, Y.C.; Huang, Y.L.; Liu, S.J. Biodegradable Cisplatin-Eluting Tracheal Stent for Malignant Airway Obstruction: In Vivo and In Vitro Studies. Chest 2013, 144, 193–199. [Google Scholar] [CrossRef]
- Freitag, L.; Gördes, M.; Zarogoulidis, P.; Darwiche, K.; Franzen, D.; Funke, F.; Hohenforst-Schmidt, W.; Dutau, H. Towards Individualized Tracheobronchial Stents: Technical, Practical and Legal Considerations. Respiration 2017, 94, 442–456. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Murdoch, D.; Goa, K.L. The Sirolimus-Eluting Stent: A Review of Its Use in the Treatment of Coronary Artery Disease. Am. J. Cardiovasc. Drugs 2003, 3, 211–230. [Google Scholar] [CrossRef]
- Sigler, M.; Klötzer, J.; Quentin, T.; Paul, T.; Möller, O. Stent Implantation into the Tracheo-Bronchial System in Rabbits: Histopathologic Sequelae in Bare Metal vs. Drug-Eluting Stents. Moll. Cell. Pediatr. 2015, 2, 10. [Google Scholar] [CrossRef]
- Duvvuri, M.; Motz, K.; Murphy, M.; Feeley, M.; Ding, D.; Lee, A.; Elisseeff, J.H.; Hillel, A.T. Engineering an Immunomodulatory Drug-Eluting Stent to Treat Laryngotracheal Stenosis. Biomater. Sci. 2019, 7, 1863–1874. [Google Scholar] [CrossRef]
- Mei, Y.; Yu, K.; Lo, J.C.Y.; Takeuchi, L.E.; Hadjesfandiari, N.; Yazdani-Ahmadabadi, H.; Brooks, D.E.; Lange, D.; Kizhakkedathu, J.N. Polymer-Nanoparticle Interaction as a Design Principle in the Development of a Durable Ultrathin Universal Binary Antibiofilm Coating with Long-Term Activity. ACS Nano 2018, 12, 11881–11891. [Google Scholar] [CrossRef]
- Obermeier, A.; Würstle, S.; Tübel, J.; Stolte, P.; Feihl, S.; Lipovcic, N.; Lanzinger, S.; Mühlhofer, H.; Weber, A.; Schmid, R.M.; et al. Novel Antimicrobial Coatings Based on Polylactide for Plastic Biliary Stents to Prevent Post-Endoscopic Retrograde Cholangiography Cholangitis. J. Antimicrob. Chemother. 2019, 74, 1911–1920. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, D.; Zhang, W.; Ren, K.; Qiu, L.; Tian, C.; Li, Y.; Li, J.; Zhou, X.; Zhao, Y.; et al. Antibacterial and Antihyperplasia Polylactic Acid/Silver Nanoparticles Nanofiber Membrane-Coated Airway Stent for Tracheal Stenosis. Colloids Surf. B Biointerfaces 2021, 206, 111949. [Google Scholar] [CrossRef] [PubMed]
- Maity, N.; Mansour, N.; Chakraborty, P.; Bychenko, D.; Gazit, E.; Cohn, D.; Maity, N.; Mansour, N.; Cohn, D.; Chakraborty, P.; et al. A Personalized Multifunctional 3D Printed Shape Memory-Displaying, Drug Releasing Tracheal Stent. Adv. Funct. Mater. 2021, 31, 2108436. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, Z.; Yang, L.; Liu, Y.; Liu, J.; Cai, S.; Shen, Y.; Guo, S. A Self-Expandable C-Shaped 3D Printing Tracheal Stent for Combinatorial Controlled Paclitaxel Release and Tracheal Support. Mater. Today Chem. 2022, 24, 100760. [Google Scholar] [CrossRef]
- Paick, S.H.; Park, H.K.; Oh, S.J.; Kim, H.H. Characteristics of Bacterial Colonization and Urinary Tract Infection after Indwelling of Double-J Ureteral Stent. Urology 2003, 62, 214–217. [Google Scholar] [CrossRef]
- Lock, J.Y.; Wyatt, E.; Upadhyayula, S.; Whall, A.; Nuñez, V.; Vullev, V.I.; Liu, H. Degradation and Antibacterial Properties of Magnesium Alloys in Artificial Urine for Potential Resorbable Ureteral Stent Applications. J. Biomed. Mater. Res. A 2014, 102, 781–792. [Google Scholar] [CrossRef]
- Gabi, M.; Hefermehl, L.; Lukic, D.; Zahn, R.; Vörös, J.; Eberli, D. Electrical Microcurrent to Prevent Conditioning Film and Bacterial Adhesion to Urological Stents. Urol. Res. 2011, 39, 81–88. [Google Scholar] [CrossRef]
- Farsi, H.M.A.; Mosli, H.A.; Al-Zemaity, M.F.; Bahnassy, A.A.; Alvarez, M. Bacteriuria and Colonization of Double-Pigtail Ureteral Stents: Long-Term Experience with 237 Patients. J. Endourol. 2009, 9, 469–472. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wuertz, S. Bacteria and Archaea on Earth and Their Abundance in Biofilms. Nat. Rev. Mirobiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 530515. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Vladkova, T.G. Surface Engineering of Polymeric Biomaterials, 1st ed.; Smithers Rapra Technology: Shrewsbury, UK, 2012. [Google Scholar]
- Minardi, D.; Ghiselli, R.; Cirioni, O.; Giacometti, A.; Kamysz, W.; Orlando, F.; Silvestri, C.; Parri, G.; Kamysz, E.; Scalise, G.; et al. The Antimicrobial Peptide Tachyplesin III Coated Alone and in Combination with Intraperitoneal Piperacillin-Tazobactam Prevents Ureteral Stent Pseudomonas Infection in a Rat Subcutaneous Pouch Model. Peptides 2007, 28, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Kamysz, W.; Silvestri, C.; Riva, A.; Della Vittoria, A.; Abbruzzetti, A.; Łukasiak, J.; Scalise, G. In Vitro Activities of Tachyplesin III against Pseudomonas Aeruginosa. Peptides 2007, 28, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Gao, R.; Liu, C.; Shan, B.; Gao, F.; He, J.; Yuan, M.; Xie, H.; Jin, S.; Ma, Y. Potential Role of the Antimicrobial Peptide Tachyplesin III against Multidrug-Resistant P. Aeruginosa and A. Baumannii Coinfection in an Animal Model. Infect. Drug Resist. 2019, 12, 2865–2874. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, J.; Pan, G.; Chen, B. Mussel-Inspired Clickable Antibacterial Peptide Coating on Ureteral Stents for Encrustation Prevention. ACS Appl. Mater. Interfaces 2022, 14, 36473–36486. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, B.; Bai, J.; He, W.; Chen, X.; Geng, D.; Pan, G. Bio-Inspired Antibacterial Coatings on Urinary Stents for Encrustation Prevention. J. Mater. Chem. B 2022, 10, 2584–2596. [Google Scholar] [CrossRef]
- Cauda, F.; Cauda, V.; Fiori, C.; Onida, B.; Garrone, E. Heparin Coating on Ureteral Double J Stents Prevents Encrustations: An in Vivo Case Study. J. Endourol. 2008, 22, 465–472. [Google Scholar] [CrossRef]
- Chew, B.H.; Cadieux, P.A.; Reid, G.; Denstedt, J.D. Second Prize: In-Vitro Activity of Triclosan-Eluting Ureteral Stents against Common Bacterial Uropathogens. J. Endourol. 2006, 20, 949–958. [Google Scholar] [CrossRef]
- Cadieux, P.A.; Chew, B.H.; Knudsen, B.E.; DeJong, K.; Rowe, E.; Reid, G.; Denstedt, J.D. Triclosan Loaded Ureteral Stents Decrease Proteus Mirabilis 296 Infection in a Rabbit Urinary Tract Infection Model. J. Urol. 2006, 175, 2331–2335. [Google Scholar] [CrossRef]
- Cadieux, P.A.; Chew, B.H.; Nott, L.; Seney, S.; Elwood, C.N.; Wignall, G.R.; Goneau, L.W.; Denstedt, J.D. Use of Triclosan-Eluting Ureteral Stents in Patients with Long-Term Stents. J. Endourol. 2009, 23, 1187–1194. [Google Scholar] [CrossRef]
- Mendez-Probst, C.E.; Goneau, L.W.; MacDonald, K.W.; Nott, L.; Seney, S.; Elwood, C.N.; Lange, D.; Chew, B.H.; Denstedt, J.D.; Cadieux, P.A. The Use of Triclosan Eluting Stents Effectively Reduces Ureteral Stent Symptoms: A Prospective Randomized Trial. BJU Int. 2012, 110, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Kallidonis, P.; Kitrou, P.; Karnabatidis, D.; Kyriazis, I.; Kalogeropoulou, C.; Tsamandas, A.; Apostolopoulos, D.J.; Vrettos, T.; Liourdi, D.; Spiliopoulos, S.; et al. Evaluation of Zotarolimus-Eluting Metal Stent in Animal Ureters. J. Endourol. 2011, 25, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Uurto, I.; Kotsar, A.; Isotalo, T.; Mikkonen, J.; Martikainen, P.M.; Kellomäki, M.; Törmälä, P.; Tammela, T.L.J.; Talja, M.; Salenius, J.P. Tissue Biocompatibility of New Biodegradable Drug-Eluting Stent Materials. J. Mater. Sci. Mater. Med. 2007, 18, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Kotsar, A.; Isotalo, T.; Uurto, I.; Mikkonen, J.; Martikainen, P.; Talja, M.; Kellomäki, M.; Salenius, J.P.; Tammela, T.L.J. Urethral in Situ Biocompatibility of New Drug-Eluting Biodegradable Stents: An Experimental Study in the Rabbit. BJU Int. 2009, 103, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Kotsar, A.; Nieminen, R.; Isotalo, T.; Mikkonen, J.; Uurto, I.; Kellomäki, M.; Talja, M.; Moilanen, E.; Tammela, T.L.J. Biocompatibility of New Drug-Eluting Biodegradable Urethral Stent Materials. Urology 2010, 75, 229–234. [Google Scholar] [CrossRef]
- Kotsar, A.; Nieminen, R.; Isotalo, T.; Mikkonen, J.; Uurto, I.; Kellomäki, M.; Talja, M.; Moilanen, E.; Tammela, T.L.J. Preclinical Evaluation of New Indomethacin-Eluting Biodegradable Urethral Stent. J. Endourol. 2012, 26, 387–392. [Google Scholar] [CrossRef]
- Shin, J.H.; Song, H.Y.; Choi, C.G.; Yuk, S.H.; Kim, J.S.; Kim, Y.M.; Yoon, C.J.; Kim, T.H.; Suh, J.Y.; He, X. Tissue Hyperplasia: Influence of a Paclitaxel-Eluting Covered Stent—Preliminary Study in a Canine Urethral Model. Radiology 2005, 234, 438–444. [Google Scholar] [CrossRef]
- Wang, Z.X.; Hong, B.F.; Zhang, X.; Fu, W.J.; Cui, F.Z.; Hu, K. New Biodegradable Drug-Eluting Stents for Urethral Strictures in a Rabbit Model. J. Bioact. Compat. Polym. 2010, 26, 89–98. [Google Scholar] [CrossRef]
- Liatsikos, E.N.; Karnabatidis, D.; Kagadis, G.C.; Rokkas, K.; Constantinides, C.; Christeas, N.; Flaris, N.; Voudoukis, T.; Scopa, C.D.; Perimenis, P.; et al. Application of Paclitaxel-Eluting Metal Mesh Stents within the Pig Ureter: An Experimental Study. Eur. Urol. 2007, 51, 217–223. [Google Scholar] [CrossRef]
- Kram, W.; Rebl, H.; Wyrwa, R.; Laube, T.; Zimpfer, A.; Maruschke, M.; Frank, M.; Vollmar, B.; Kundt, G.; Schnabelrauch, M.; et al. Paclitaxel-Coated Stents to Prevent Hyperplastic Proliferation of Ureteral Tissue: From In Vitro to In Vivo. Urolithiasis 2020, 48, 47–56. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Advanced Applications of Nanotechnology in Veterinary Medicine. Environ. Sci. Pollut. Res. 2020, 27, 19073–19086. [Google Scholar] [CrossRef]
- Prasad, R.D.; Charmode, N.P.; Shrivastav, O.P.; Prasad, S.R.; Moghe, A.; Samant, A.; Sarvalkar, P.D.; Prasad, N.R.D. A Review on Concept of Nanotechnology in Veterinary Medicine. ES Food Agrofor. 2021, 4, 28–60. [Google Scholar] [CrossRef]
- Ziąbka, M.; Kiszka, J.; Trenczek-Zając, A.; Radecka, M.; Cholewa-Kowalska, K.; Bissenik, I.; Kyzioł, A.; Dziadek, M.; Niemiec, W.; Królicka, A. Antibacterial Composite Hybrid Coatings of Veterinary Medical Implants. Mater. Sci. Eng. C 2020, 112, 110968. [Google Scholar] [CrossRef]
- Ziąbka, M.; Matysiak, K.; Cholewa-Kowalska, K.; Kyzioł, A.; Królicka, A.; Sapierzyński, R.; Januchta-Kurmin, M.; Bissenik, I. In Vitro and In Vivo Studies of Antibacterial Coatings on Titanium Alloy Implants for Veterinary Application. Int. J. Mol. Sci. 2023, 24, 8114. [Google Scholar] [CrossRef]
- Das, C.G.A.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Govindaraju, K.; Joselin, J.M.; Baalamurugan, J. Antibacterial Activity of Silver Nanoparticles (Biosynthesis): A Short Review on Recent Advances. Biocatal. Agric. Biotechnol. 2020, 27, 101593. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Health Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Wang, L.; Periyasami, G.; Aldalbahi, A.; Fogliano, V. The Antimicrobial Activity of Silver Nanoparticles Biocomposite Films Depends on the Silver Ions Release Behaviour. Food Chem. 2021, 359, 129859. [Google Scholar] [CrossRef]
- Hoque, J.; Yadav, V.; Prakash, R.G.; Sanyal, K.; Haldar, J. Dual-Function Polymer-Silver Nanocomposites for Rapid Killing of Microbes and Inhibiting Biofilms. ACS Biomater. Sci. Eng. 2019, 5, 81–91. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Keatch, R.; Corner, G.; Nabi, G.; Murdoch, S.; Davidson, F.; Zhao, Q. In-Vitro Antibacterial and Anti-Encrustation Performance of Silver-Polytetrafluoroethylene Nanocomposite Coated Urinary1 Catheters. J. Hosp. Infect. 2019, 103, 55–63. [Google Scholar] [CrossRef]
- Arkowski, J.; Obremska, M.; Kędzierski, K.; Sławuta, A.; Wawrzyńska, M. Applications for Graphene and Its Derivatives in Medical Devices: Current Knowledge and Future Applications. Adv. Clin. Exp. Med. 2020, 29, 1497–1504. [Google Scholar] [CrossRef]
- Ge, S.; Xi, Y.; Du, R.; Ren, Y.; Xu, Z.; Tan, Y.; Wang, Y.; Yin, T.; Wang, G. Inhibition of In-Stent Restenosis after Graphene Oxide Double-Layer Drug Coating with Good Biocompatibility. Regen. Biomater. 2019, 6, 299–309. [Google Scholar] [CrossRef]
- Some, S.; Ho, S.M.; Dua, P.; Hwang, E.; Shin, Y.H.; Yoo, H.; Kang, J.S.; Lee, D.K.; Lee, H. Dual Functions of Highly Potent Graphene Derivative-Poly-l-Lysine Composites to Inhibit Bacteria and Support Human Cells. ACS Nano 2012, 6, 7151–7161. [Google Scholar] [CrossRef]


| Properties | Substances | Mechanism of Action | Author |
|---|---|---|---|
| Antiproliferative | Paclitaxel | Disrupting the balance between polymerization and depolymerization of microtubules, thus disrupting cytoskeletal function leading to cell death | [83] |
| Cisplatin | Crosslinking with the purine bases, which interferes with DNA repair, causing damage | [84] | |
| Sirolimus | Complex sirolimus-FKBP12 acts as an inhibitor of kinase mTOR which finally results in the blocking of the cellular pathway at the junction of G1 and S-phase phase | [85] | |
| Zotarolius | Inhibition of mTOR kinase by the complex of zotarolimus-FKBP12 leading to inhibition of cell proliferation | [86] | |
| Antibacterial | Cefotaxyme | Inhibition of bacterial wall synthesis | [87] |
| Tachyplesin III | Inhibition of FabG enzyme activity and blocks NADPH bindings of bacteria | [88] | |
| Antibacterial peptides | Permeabilizing cell membrane and disrupting intracellular function | [89,90] | |
| Triclosan | Inhibition of bacterial fatty acid synthesis | [91] | |
| AgNPs | Penetration of cell membranes by nanoparticles leads to the production of reactive oxygen species leading to the disruption of bacterial cell signaling pathways | [92] | |
| Graphene | Free edges of GR sheets containingcarboxyl groups provide an attachment site for bacteria by disrupting the integrity of their membranes | [93] | |
| Graphene oxidate | Disruption of phospholipid integrity leads to bacteria membrane damage, the generation of reactive oxygen species causing DNA fragmentation and cell death | [93,94] | |
| Polymers | Chitosan | Drug carrier, controlled and targeted release of drug in different formulations | [95] |
| Polycaprolactone (PCL) | Drug carrier, controlled and targeted release, enhanced properties of used drugs | [96] | |
| Poly-l-lactic acid (PLA) | Drug carrier for a variety of substances in different formulations, controlled and targeted release | [97] | |
| Poly(D, L-lactide-co-glycolide) (PLGA) | Controllable drug release profile, ability to alter surface with targeting agents for diagnosis and treatment | [98] | |
| Polyvinyl alcohol (PVA) | Drug sustained release carrier, targeted releasing at specific sites of the body, controlled rate of drug release | [99] | |
| Polytetrafluoroethylene (PTFE) | Drug carrier | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graczyk, S.; Pasławski, R.; Grzeczka, A.; Pasławska, U.; Świeczko-Żurek, B.; Malisz, K.; Popat, K.; Sionkowska, A.; Golińska, P.; Rai, M. Antimicrobial and Antiproliferative Coatings for Stents in Veterinary Medicine—State of the Art and Perspectives. Materials 2023, 16, 6834. https://doi.org/10.3390/ma16216834
Graczyk S, Pasławski R, Grzeczka A, Pasławska U, Świeczko-Żurek B, Malisz K, Popat K, Sionkowska A, Golińska P, Rai M. Antimicrobial and Antiproliferative Coatings for Stents in Veterinary Medicine—State of the Art and Perspectives. Materials. 2023; 16(21):6834. https://doi.org/10.3390/ma16216834
Chicago/Turabian StyleGraczyk, Szymon, Robert Pasławski, Arkadiusz Grzeczka, Urszula Pasławska, Beata Świeczko-Żurek, Klaudia Malisz, Ketul Popat, Alina Sionkowska, Patrycja Golińska, and Mahendra Rai. 2023. "Antimicrobial and Antiproliferative Coatings for Stents in Veterinary Medicine—State of the Art and Perspectives" Materials 16, no. 21: 6834. https://doi.org/10.3390/ma16216834






