3.1. Superpave Binder Specification
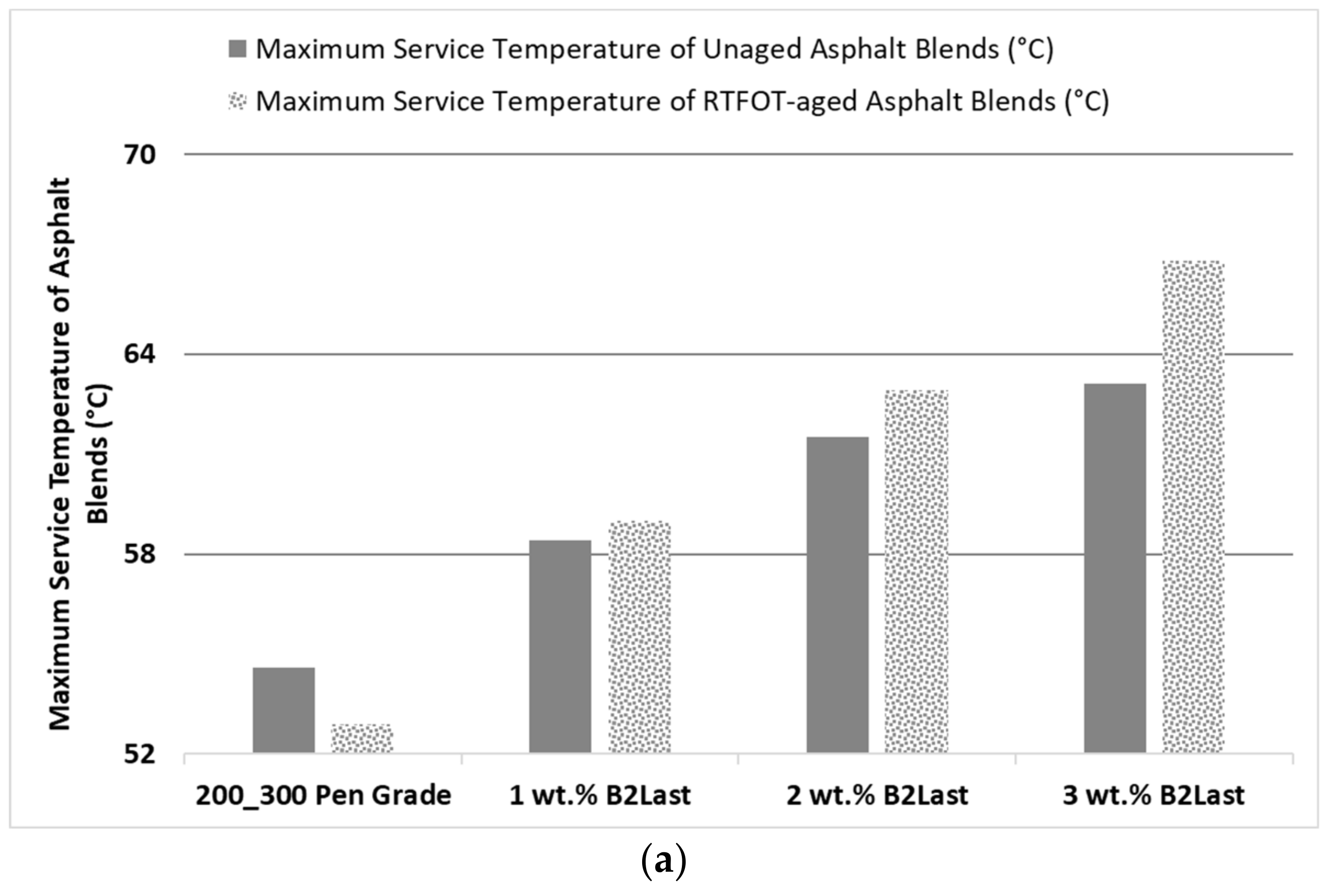
The effect of increasing concentration of B2Last on maximum service temperature of unaged (defined as |G*|/sinδ ≥ 1 kPa, [
35]) as well as RTFOT aged (defined as |G*|/sinδ ≥ 2.2 kPa [
35]) asphalt blends is shown in
Figure 1a. It is clear that linearly increasing concentration of B2Last resulted in linearly increasing maximum service temperature of unaged modified asphalts. Particularly interesting was to observe the further linear increase in maximum service temperature after RTFOT ageing. The reaction between the isocyanate group of mono- and oligomeric methylene diphenyl diisocyanate of B2Last and polar components of asphalt was optimized to achieve the highest possible conversion prior to the preparation of B2Last modified asphalt. This was carried out by monitoring the rheological properties and FTIR characteristics during blending. It seems, however, that the aggressive effect of air and high temperatures during RTFOT ageing resulted in additional B2Last conversion and/or reorganization of already created internal structure.
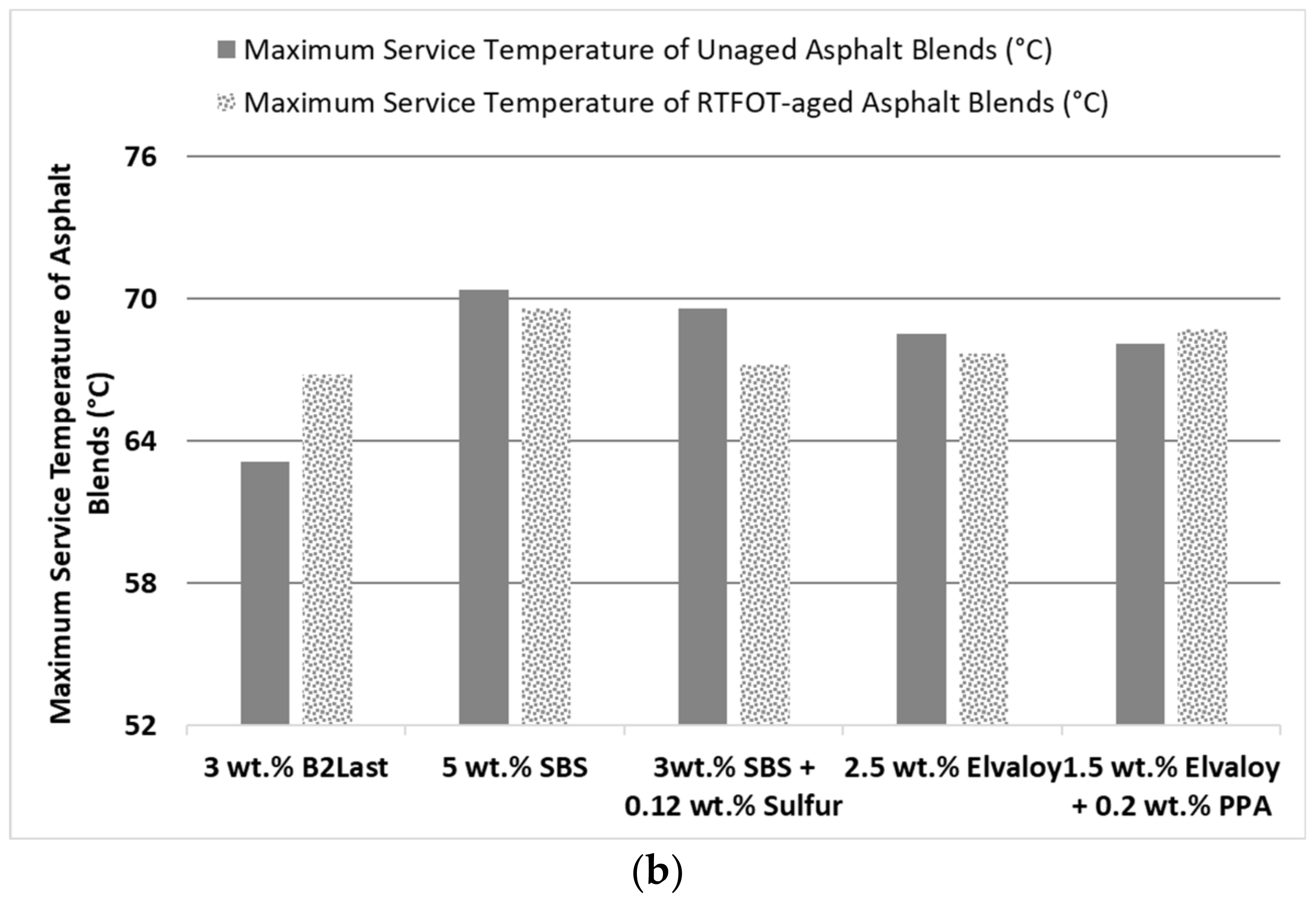
The highest resistance to rutting, defined by high temperature parameter |G*|/sinδ, was obtained at 3 wt.% of B2Last and was selected for comparison with modification technology using thermoplastic elastomer, styrene−butadiene−styrene, with and without sulfur as a crosslinking agent, and Elvaloy 4170 with and without polyphosphoric acid. For comparison and evaluation purposes of modification technologies, it was decided to prepare modified asphalts with high temperature performance grades of PG 68 ± 2 °C as determined after RTFOT ageing. This can be seen in
Figure 1b. The concentration of modifiers was carefully considered, so that each modifier had sufficient concentration in asphalt to demonstrate the main features of particular modification technology.
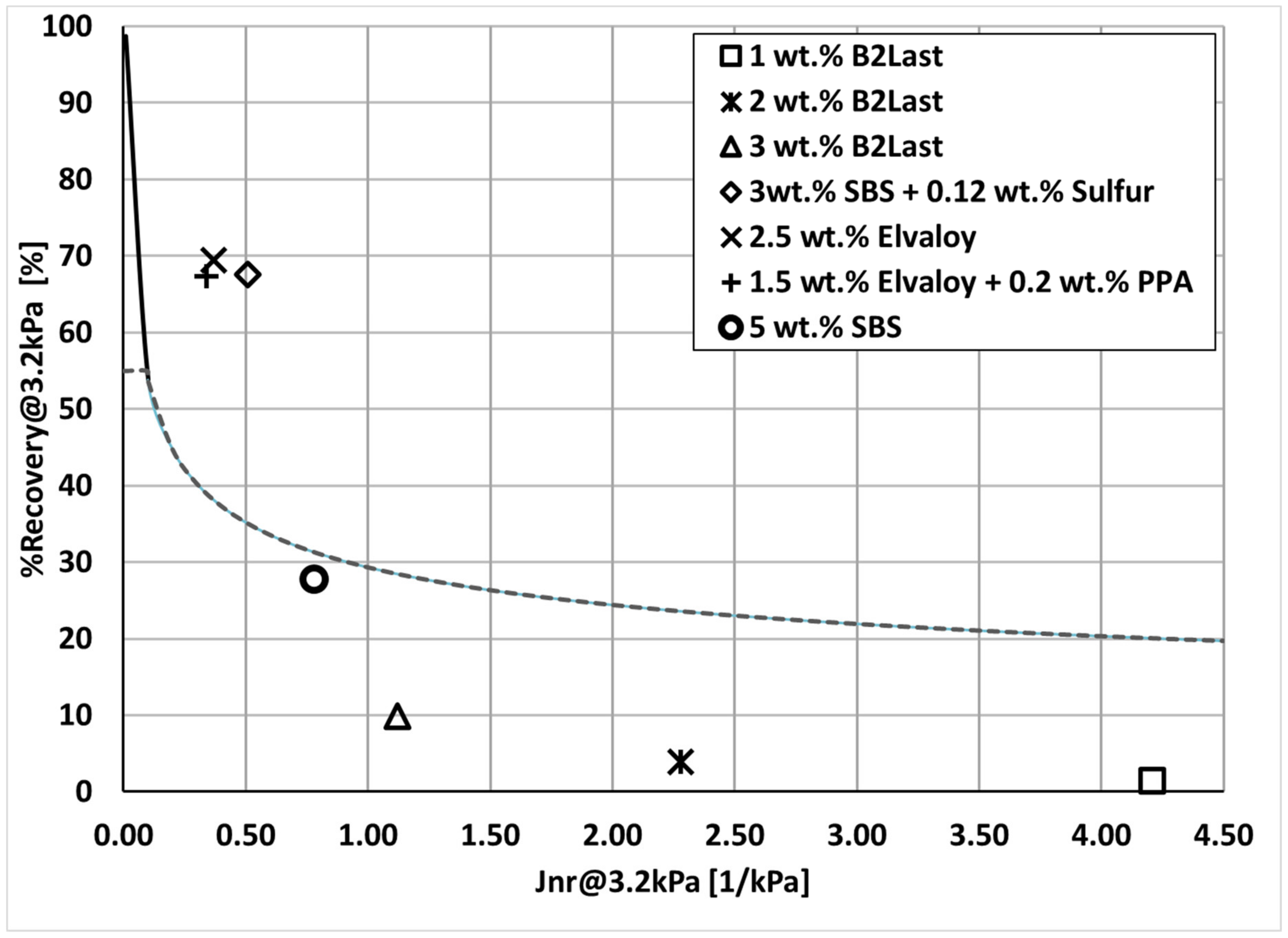
It was observed that the performance of asphalt with the same PG at high service temperature was not similar. On the contrary, the clear distinction of performance in MSCR Jnr
3.2kPa and %Recovery
3.2kPa measured at 58 °C was seen for each modification technology as shown in
Figure 2.
For reference, the values of Jnr3.2kPa and %Recovery3.2kPa measured at 58 °C for 200/300 Pen grade straight run asphalt, used as a base for modification, were 7.4 kPa−1 and 1%, respectively. These values were expected as this straight run asphalt with a relatively high value of penetration (low value of viscosity) was characterized only as PG 52–34. Although the introduction of 1, 2, and 3 wt.% of B2Last improved values of Jnr3.2kPa substantially, the values of %Recovery3.2kPa remained very low. Asphalt blend containing 5 wt.% of SBS had values of Jnr3.2kPa equal to 0.78 kPa−1 and surprisingly low value of %Recovery3.2kPa equal to 27.7%. Modified asphalts with the lowest values of Jnr3.2kPa (below 0.5 kPa−1) and the highest values %Recovery3.2kPa (above 65%) were prepared by technologies containing crosslinked SBS and Elvaloy with and without PPA.
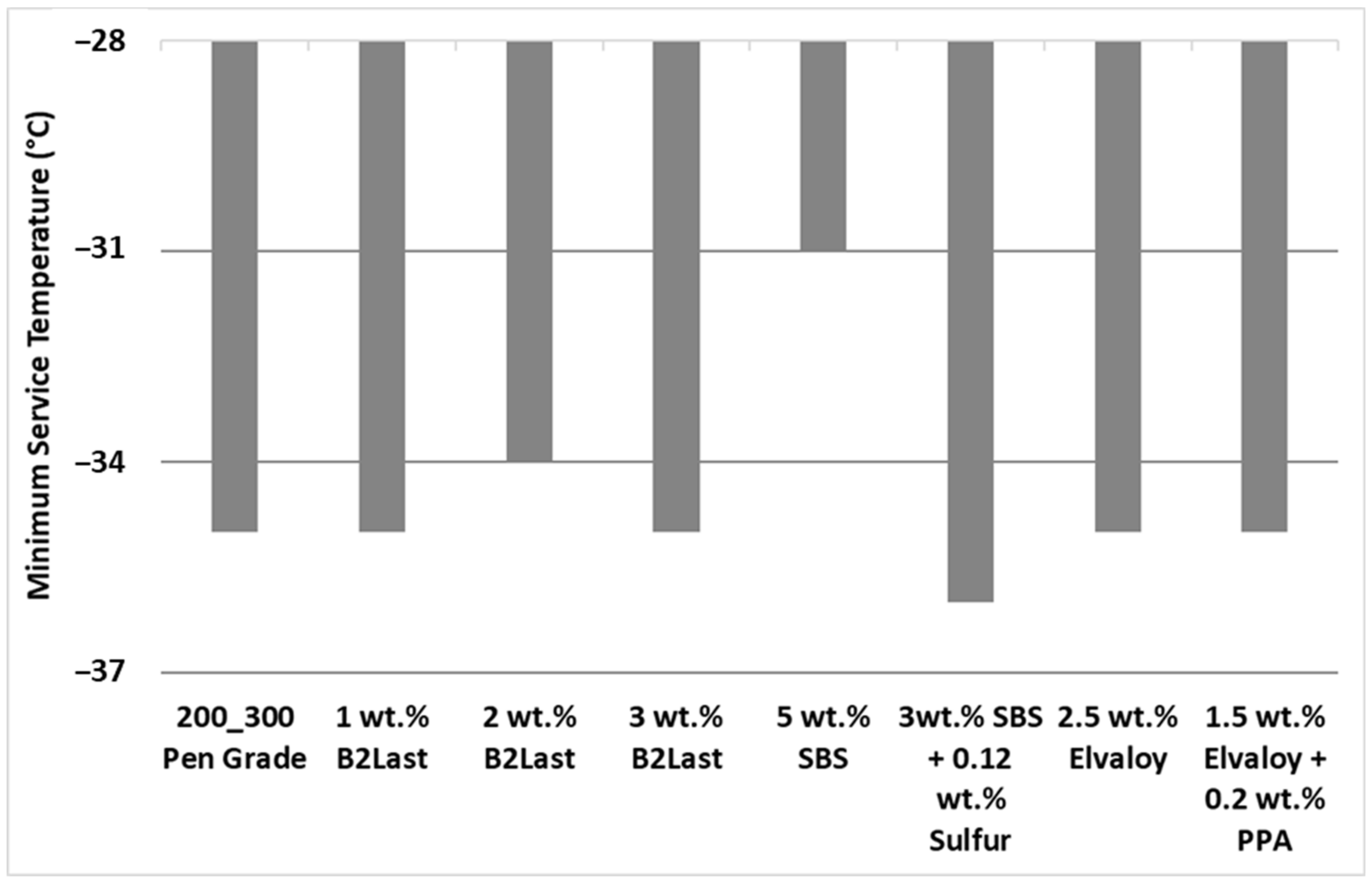
The minimum service temperature determined by a bending beam rheometer (BBR) [
35] on PAV aged samples of modified asphalts can be seen in
Figure 3. The effect of B2Last on minimum service temperature is somewhat controversial. Materials with 1 wt.% and 3 wt.% of B2Last did not have any effect on minimum service temperature. Sample of modified asphalt containing 2 wt. % of B2Last worsened the minimum service temperature by 1 °C.
The effects of the second reactive modifier, Elvaloy, either alone or in combination with PPA, did not have any effect on minimum service temperature. While 5 wt.% of SBS worsened the minimum service temperature by 4 °C, the introduction of sulfur as a crosslinking agent in asphalt modified by 3 wt.% of SBS improved the minimum service temperature by 1 °C.
Ho et al. [
37,
38] found that the BBR test has difficulties in recognizing the true impact of modifiers on low temperature properties. The low service temperatures of modified asphalts determined by BBR were on average 3 to 6 °C higher than those determined by DTT. This was explained by increasing values of failure stress, energy, and strain and lower values of secant modulus in polymer modified asphalts. Due to nonconclusive BBR results and due to the observation that reactive B2Last modifier is improving the stiffness of asphalt binder, the DTT was also used to assess the low temperature behavior. As a rule of thumb, the value of critical cracking temperature (determined as a crossover between the master curve of BBR stiffness and DTT thermal strength) worsened by 0.4 °C per addition of 1 wt.% of B2Last modifier (the results are not shown in this contribution).
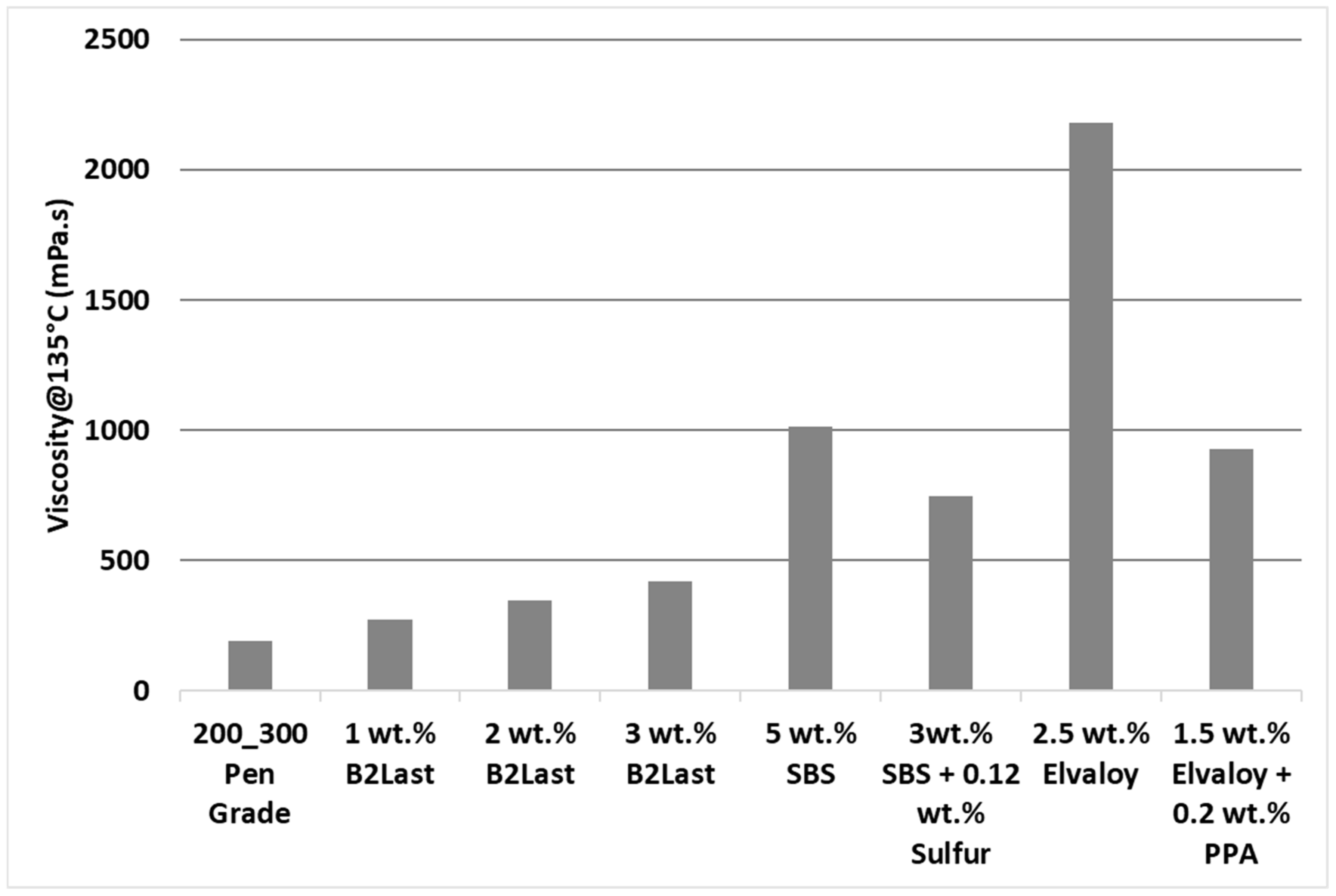
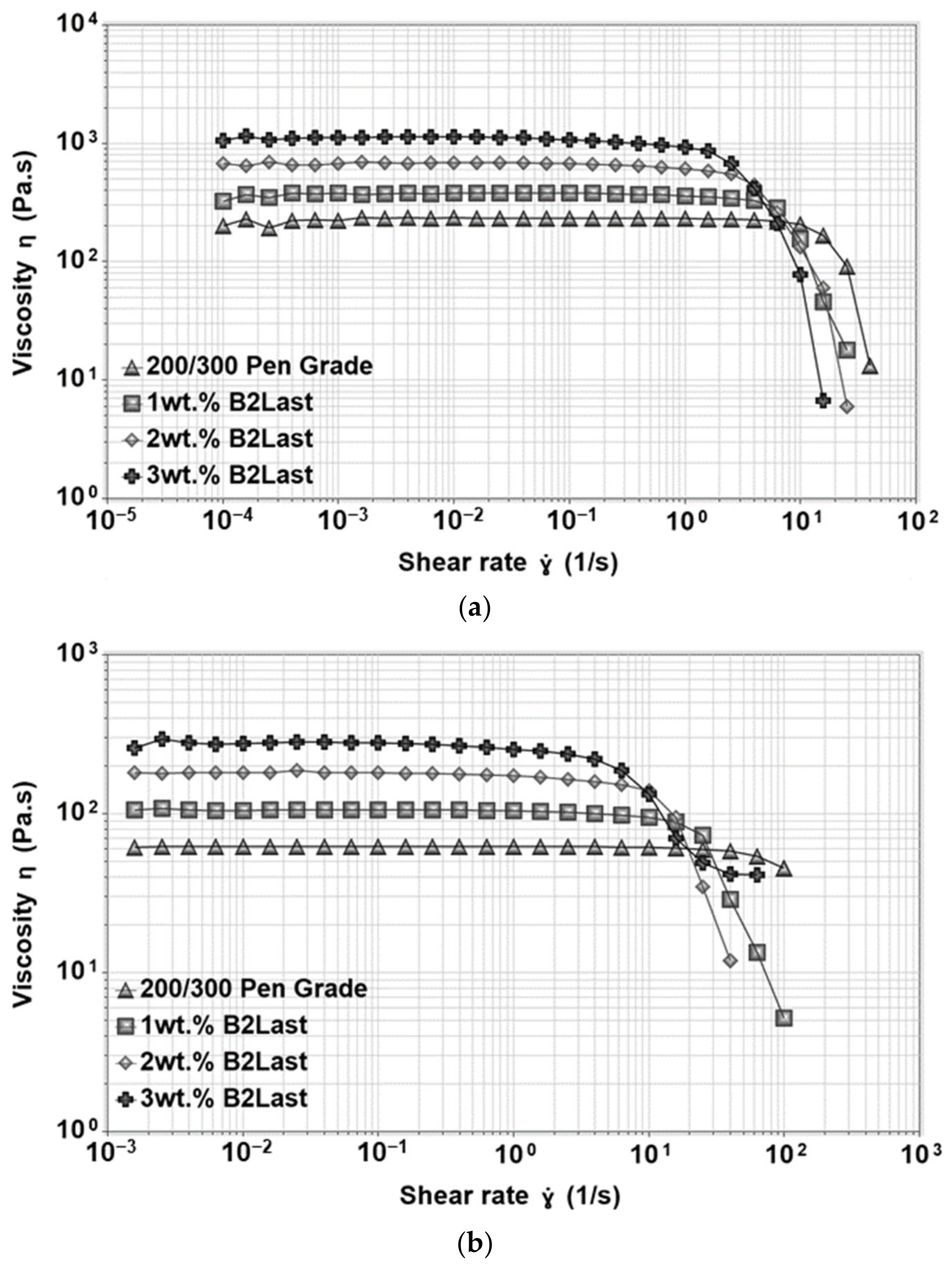
Figure 4 shows the viscosity of asphalts modified by different technologies measured at 135 °C [
35]. As can be seen, linearly increasing concentration of B2Last leads to linearly increasing values of viscosity. While the value of viscosity at 135 °C for 3 wt.% B2Last was below 500 mPa·s, the viscosity of asphalt blend containing 3 wt.% SBS crosslinked with 0.12 wt.% sulfur and blend containing 5 wt.% SBS without any crosslinking agent were 77% and 140% higher, respectively. The highest values of viscosity were recorded for modification technology using the reactive system Elvaloy. Although the viscosity value of the blend containing 1.5 wt.% of Elvaloy and 0.2 wt.% of PPA had similar viscosity to SBS modification technology, a sample containing 2.5 wt.% of Elvaloy without PPA had a viscosity of 2181 mPa·s. This result was expected due to the development of complex asphaltene–Elvaloy structures [
6,
39,
40,
41].
Lower values of viscosity at 135 °C are preferred from handling and transportation perspective. The significant increase in the maximum service temperature after RTFOT ageing, however, points to potentially higher values of viscosity of B2Last modified asphalts during the mixing and construction phase.
3.2. Mechanical Spectroscopy
Mechanical spectroscopy, together with creep and relaxation experiments, belong to the most common experimental techniques in rheology for a description of viscoelastic materials. While constant shear stress is applied in the creep experiment, changes in stress at constant deformation are monitored in the relaxation experiment. In mechanical spectroscopy, also known as dynamic material analysis, stress or strain varies sinusoidally with a given frequency.
In order to assess the behavior of viscoelastic material, the relaxation modulus over a wide range of time, ideally from time zero to infinity, should be achieved. Although this is experimentally not possible through the application of the time–temperature superposition principle, it is possible to extend the time interval for viscoelastic observation. When the studied material fulfills the condition of rheological simplicity, the experimental data can be shifted to master curves of dynamic material functions, described by discrete or continuous relaxation spectrum, {λ
i, g
i}, according to the following relations [
42]:
where i = 1,2, …, N;
is the frequency in
are the spectral strengths;
are the relaxation times.
The selection of reference temperature has to be carefully considered. The change in reference temperature alters relaxation times, thus resulting in the requirement for horizontal shifting factors, generally described by the Williams–Landel–Ferry (WLF) equation [
42]:
where T is the temperature;
The master curves of dynamic material functions were constructed using the commercial software IRIS RheoHub [
43].
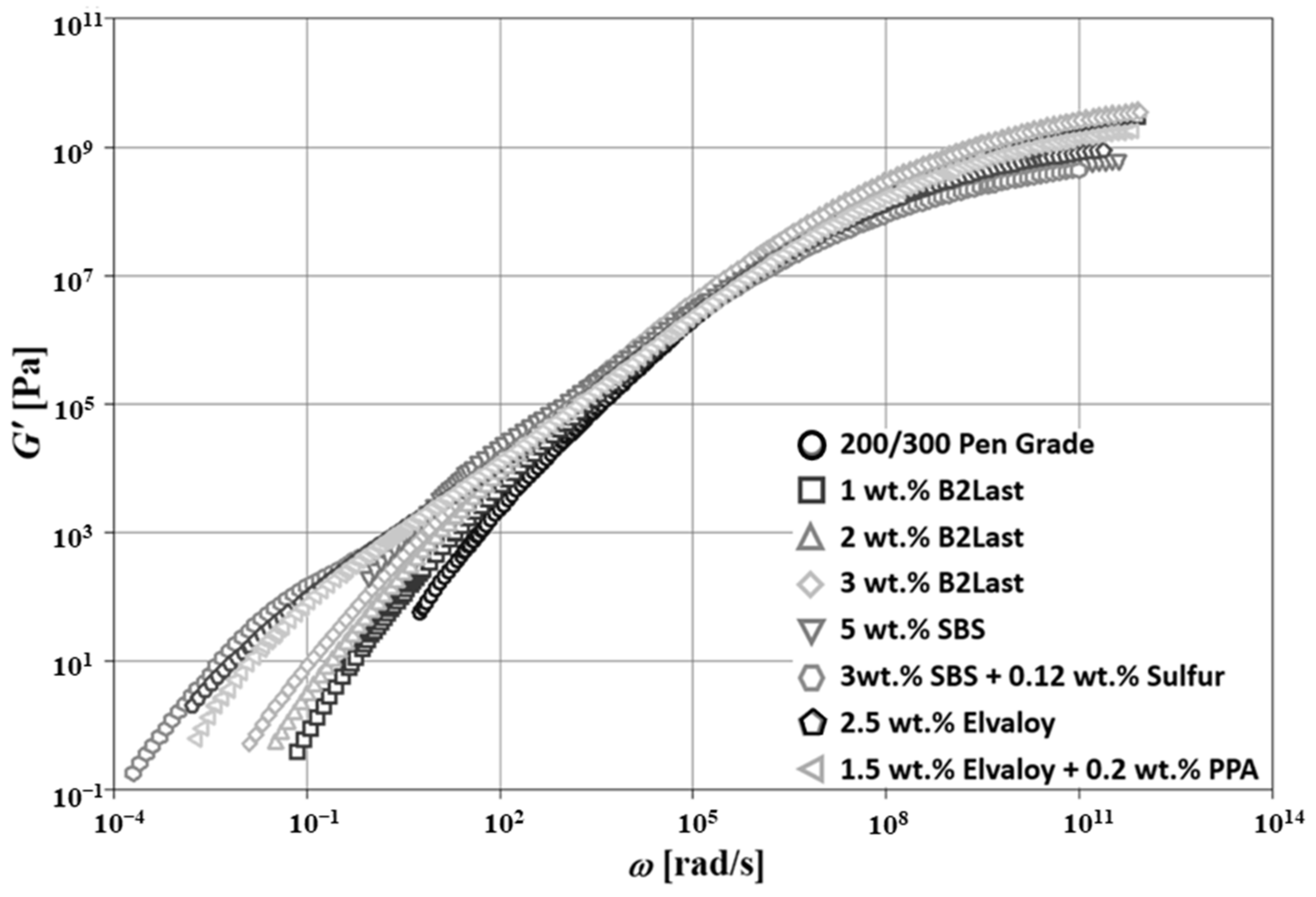
Figure 5 shows the master curves of
for asphalt blends prepared by different modification technologies at Tr = 50 °C. Three different regions generally dominate in straight run asphalts. In the first region, a solid-like behavior is observed at low temperatures (high frequencies). The magnitude of
dominates in this region and its value approaches 10
9 Pa. Magnitude of
reaches the maximum before its values start dropping. The second important region, characterized by the crossover of
and
is generally observed at intermediate frequencies. Newtonian zone observed at high temperatures (low frequencies) is the third region. Contrary to solid-like behavior, in the Newtonian zone, the magnitude of
is generally higher than the magnitude of
. The master curve of
for straight run asphalt, shown in
Figure 6, also confirms this behavior.
The microstructure of straight run asphalt can be indirectly observed from the master curves of dynamic material functions. Based on only two distinctive regions on the master curve of
, it could be deduced that the internal structure of this asphalt is relatively weak and can be associated only with intermolecular interactions [
28,
43]. The two distinctive regions on the master curve of
suggest the low mobility of asphalt components at low temperatures, however, the reduction in viscosity at higher temperatures, together with the weak internal structure of this sol-like material, result in a sharp linear increase in
[
44]. It is clear that this particular effect is the main reason for the formation of one of the most common pavement distresses, which is rutting.
Figure 5 and
Figure 6 show the effects of a variety of modifiers on the master curves of
and
, respectively. The modification of straight run asphalt with B2Last reactive modifier led to a higher magnitude of
. The increase in
was proportional to the concentration of this modifier. Quite interesting was the observation of the shape of
master curves for asphalts modified by B2Last. Although the modification effect of B2Last increased stiffness, potentially due to the chemical reaction of the -NCO functional group with polar components of asphalt, the internal structure did not seem to be significantly affected. The same behavior was also observed on the master curves of
. Although the modification effect of 1, 2, and 3 wt.% of B2Last seemed to be relatively small, as reflected by dynamic material functions, the further development of properties of these modified asphalts was observed after RTFOT ageing.
The modification effects of the second reactive system, Elvaloy with and without the presence of PPA, showed substantial changes in the master curves of
and
. The asphalt blend modified with 2.5 wt.% of Elvaloy 4170 showed the onset of the shoulder on the master curve of
, which is characteristic for the formation of the network-like internal structure [
6,
25]. The mechanism of modification of Elvaloy appears to be similar to B2Last reactive modifier, although both modifiers carry different reactive chemical groups. While the reactive group for B2Last is -NCO, the chemical reaction of Elvaloy is based on the opening of the epoxy functional group and subsequent reaction with polar components of asphalt.
The main difference between these two reactive systems, besides different functional groups, is the chemical character of modifiers. While B2Last is a low molecular weight mono- and oligomer which reacts and/or converges to macromolecules when blended with asphalt, Elvaloy is already in the form of polymer which must be dispersed in asphalt matrix for the reaction to occur. The higher molecular weight of the modifier, together with the reaction with asphalt components, lead to the formation of asphaltene–Elvaloy complexes [
6,
25]. The formation of these complexes depends on the chemical composition of asphalt and the concentration of Elvaloy, i.e., the number of functional groups present on the chain. As a result, even the small addition of Elvaloy, generally above 2.5 wt.%, could lead to gelation, and thus formation of an infusible and insoluble material [
22]. The dynamic material functions for asphalt modified by Elvaloy could suggest not only the formation of asphaltene–Elvaloy complexes, but also the significant transition of colloidal structure from sol-like to partial gel-like.
Interestingly, despite a lower content of the polymer, the asphalt modified by 1.5 wt.% of Elvaloy and 0.2 wt.% of PPA showed similar behavior to asphalt modified by 2.5 wt.% of Elvaloy. The slight deviation between these two modified asphalts was observed at
, with a higher tendency to flow for asphalt modified with 1.5 wt.% of Elvaloy and 0.2 wt.% of PPA. This effect could be attributed to several potential mechanisms: (1) the catalytic effect of PPA on the Elvaloy reaction with asphalt’s polar components [
45]; (2) the precipitation of asphaltenes [
46] and formation of new asphaltenes with PPA through the conversion of the portion of naphthene to polar aromatics, and polar aromatics to asphaltenes [
47]. This mechanism could not only result in the creation of new active centers able to react with oxirane, but also in the changes in the colloidal system. According to [
25], the combination of both mechanisms could be considered. In general, however, the development of properties does not depend only on the chemistry of reactive modification systems, but also on the source and manufacturing technology of asphalt binder.
The evolution of the fourth region, demonstrated by a shoulder of
as well as shorter region of local maximum followed by local minimum observed on the master curve of
(
Figure 5 and
Figure 6, respectively) was also observed in modification technology using 5 wt.% of thermoplastic elastomer SBS. Although very similar features between Elvaloy and SBS copolymer could be observed on the master curves of dynamic material functions, their origin is completely different. The presence of oxirane in Elvaloy promotes a reaction with polar asphalt components, which enhances the stiffness of modified asphalt [
48,
49,
50]. The formation of asphaltene–Elvaloy complexes depends on the amount of functional groups present in asphalt as well as Elvaloy.
The non-vulcanized SBS copolymer is generally dispersed in asphalt in the form of ultra-fine particles. The butadiene part of the polymer is able to absorb light oily asphalt’s components which leads to volumetric expansion. The swelling ability of the polybutadiene phase is a function of several different factors, e.g., molecular weight of polymer, chemistry and amount of light oily fraction in asphalt, morphology of polymer, temperature, etc. Consequently, dispersed polymer particles swell four to ten times. At a critical concentration of SBS, the polymer-rich phase becomes continuous, which explains the change in deformation–relaxation processes at intermediate to high temperatures, and thus a decrease in temperature susceptibility of modified asphalt [
10].
Without any stabilization mechanisms, such as vulcanization, the SBS particles act independently as an active, swollen, elastomeric filler and the transfer of its properties to asphalt binder is limited [
8]. Furthermore, over longer storage at high temperatures, the SBS particles coalesce and the polymer phase may separate from asphalt.
The introduction of a small amount of sulfur is able to eliminate the issues with the separation of SBS polymer. As the crosslinking with sulfur leads to the formation of a three-dimensional polymer network, the transfer of the elastomeric properties to modified asphalt is also more effective [
8]. This effect can be seen on the well pronounced shoulder of
as well as the large region of local maximum followed by local minimum observed on the master curve of
which is generally associated with the behavior of diluted polymer systems. These phenomena can be seen in
Figure 5 and
Figure 6, respectively.
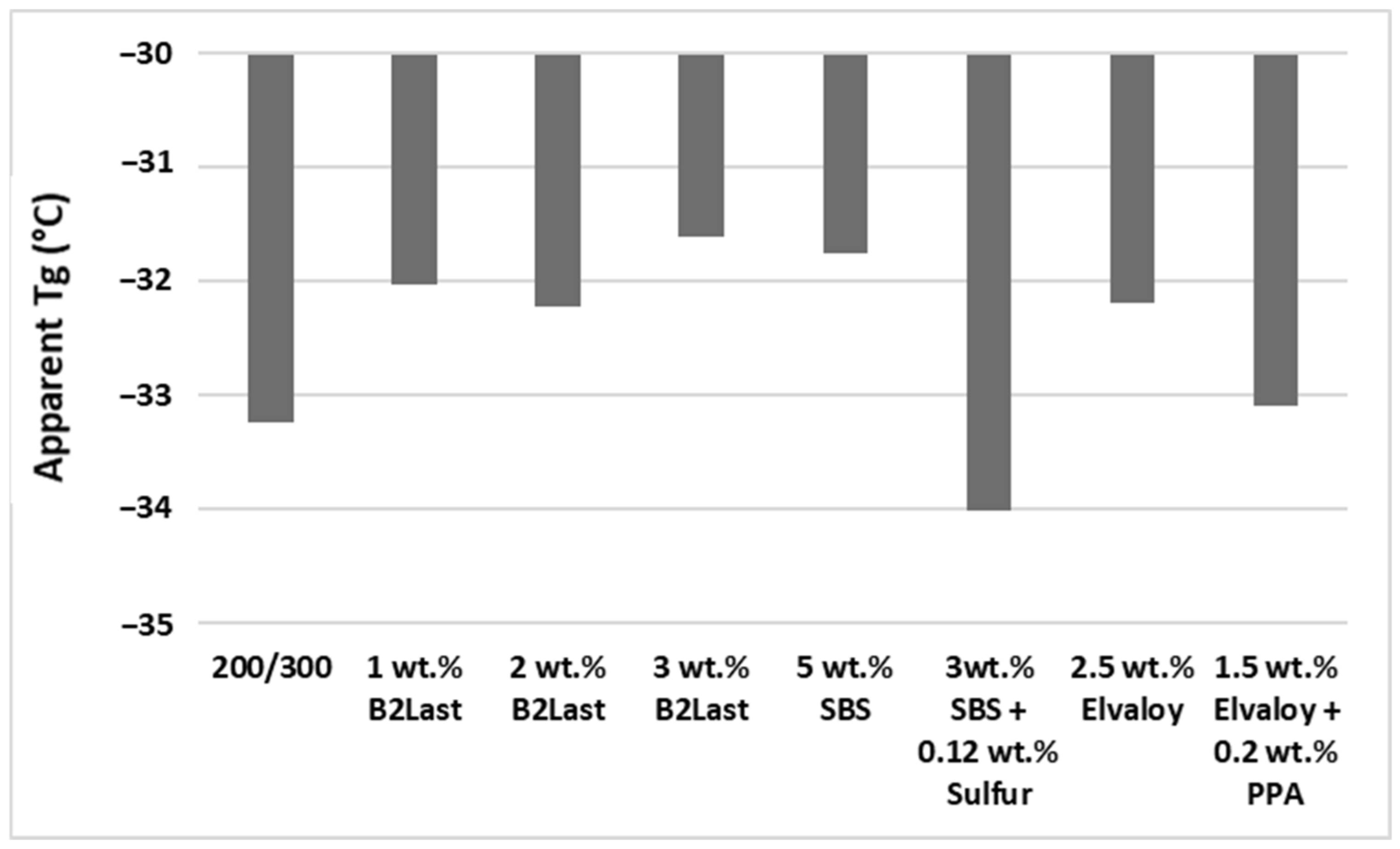
Figure 7 shows the values of apparent T
g for asphalt blends prepared by different modification technologies. In order to obtain values of apparent T
g, new master curves of dynamic material functions superposed to a reference temperature of −20 °C were prepared. The transformation of frequency to temperature domain was based on the WLF equation. As discussed in [
8,
25], the value of T
g depends on the selected value of testing frequency
used for the transformation of frequency to temperature domain. Although the values of apparent T
g, defined as a maximum on the master curves of
, have only informative character, they can be used to evaluate and compare the effects of particular modification systems at low temperatures.
It can be seen from
Figure 7 that asphalt modified by Elvaloy and asphalts modified by B2Last had higher values of apparent T
g than reference straight run asphalt 200/300 Pen grade. The higher (worse) values of apparent T
g of both reactive modifiers are very peculiar. Since B2Last prior to the modification is in the form of low molecular weight liquid, it should decrease the value of apparent T
g. On the other hand, although it was expected that Elvaloy would slightly decrease values of T
g, given the very low swelling ability in combination with the very low concentration of this modifier, the T
g was not significantly improved. As was already presented, even though the chemistry of both reactive modifiers is different, the mechanism of modification could be considered similar, i.e., the reaction of modifiers’ active groups with polar components of asphalt. Particularly, the formation of covalent bonds and increase in molecular weight could be the main factors for higher values of T
g [
28]. In the case of a low concentration of PPA in combination with Elvaloy, it could be hypothesized that disintegration and to a small extent formation of new asphaltenes could reduce the molecular weight of formed asphaltene–Elvaloy complexes. Thus, slightly improved values of T
g could be observed.
The values of apparent T
g of asphalt modified by 5 wt.% SBS, together with a sample of asphalt modified by 3 wt.% B2Last, were the highest (worst) of all tested modified asphalts. The ultra-fine dispersed and swollen particles of SBS can form only a weak network of a physical character. Despite the T
g of the butadiene phase below −80 °C, the T
g of such modified asphalt is mostly controlled by the reduction in its free volume [
18,
21]. As was observed from
Figure 5 and
Figure 6, the saturation of C=C double bonds present in butadiene by sulfur leads to the formation of a polymer network. Although it seems that the formation of a three-dimensional SBS network is solely responsible for the decrease in the T
g, other important effects must be also considered [
25,
28]. The formation of a three-dimensional SBS network is generally achieved by the process of dynamic vulcanization at high temperatures. As the vulcanization is not controlled, the formation of polysulfide bonds can be expected. Generally, the vulcanization increases T
g, however, given the low concentration of sulfur in this process, the created network could be considered to have only weak character. Thus, even when vulcanization reduces the molecular motion, the transfer of properties of the crosslinked polybutadiene network has a more significant effect.
3.3. Steady Shear Viscosity
The steady shear viscosity measurements were selected as a complementary method to dynamic material analysis. While in the latter the structural response is given by disturbance of the studied material to a negligible extent via a small amplitude oscillation in the linear viscoelastic region, the viscosity measurements could be used to investigate the response of internal structure when subjected to flow and to determine the value of critical shear rate at which the studied material stops behaving as a Newtonian liquid.
The viscosity functions of straight run asphalt measured at 50 °C and 60 °C can be seen in
Figure 8a,b, respectively. As can be seen, the viscosity function of straight run asphalt at 50 °C displayed long interval at which the viscosity was independent of the applied shear rate. This region, characterized by one value of viscosity, is generally called zero shear viscosity or Newtonian region. When the testing temperature increased to 60 °C, the magnitude of viscosity of straight run asphalt was lower, and the zero shear viscosity region was observed almost through the whole interval of applied shear rate. This behavior of straight run asphalt was expected not only due to the very weak internal structure, but also due to the significant influence of temperature on the viscosity function [
22].
The viscosity functions at 50 °C and 60 °C were also investigated for asphalt modified by 1, 2, and 3 wt.% of B2Last. Results can be seen in
Figure 8. Similarly to previous results, the magnitude of the viscosity function was linearly increasing with the increasing content of the B2Last modifier. Since the behavior of B2Last modified asphalts is very similar to straight run asphalt, the potential changes in its behavior were identified by fitting the viscosity curves to the Carreau–Yasuda model [
51]. As expected, besides the reduction in the value of the critical shear rate at which the shear thinning occurred, no changes that could be related to the change in internal structure were found for asphalt modified by 1 wt.% B2Last. Two shear thinning regions were observed for asphalt modified with 2 wt.% of B2Last. The first, mild shear thinning occurred at a shear rate of 0.25 s
−1 and the second sharp shear thinning region was recorded at 2.5 s
−1. Very weak viscosity overshoot (shear thickening region) was observed at a shear rate of 0.0016 s
−1 for asphalt modified by 3 wt.% B2Last. The correlation between the viscosity overshoot and the development of a network (physical or chemical) in modified asphalt has been found in our previous research. The presence of viscosity overshoot thus confirms the formation of a network based on the reaction of B2Last with asphalt components, however, given to a small magnitude of the overshoot, the internal network within asphalt is very weak. It should be noted, however, that the optimum conversion of B2Last was observed after the RTFOT ageing, which suggests that the internal structure is more pronounced.
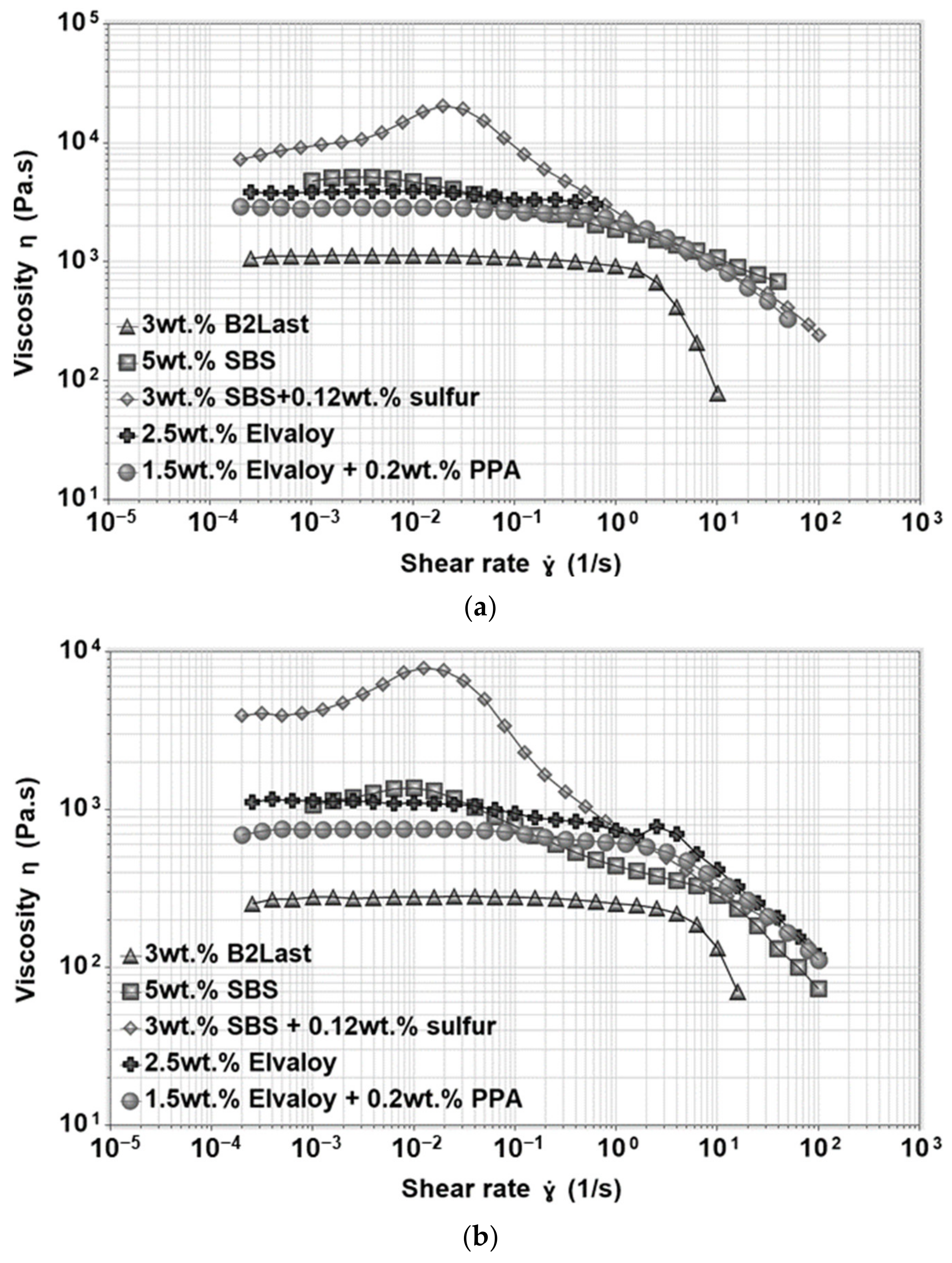
The magnitude of viscosity in asphalt modified by Elvaloy with and without the presence of PPA was three times higher than asphalt modified by the B2Last modifier. The shape of the viscosity function for both reactive systems had very similar features—a short zero shear viscosity region followed by two shear thinning regions. Functional groups of Elvaloy are responsible for the formation of covalent bonds with polar asphalt components. The resulting Elvaloy–asphaltene complexes can vary in size, mobility, and different levels of internal strength producing two shear thinning regions [
25]. A minor shear thickening was observed in asphalt modified by Elvaloy and PPA. Interestingly, replacing polymer with a small amount of PPA delayed the critical shear rate at which the shear thinning occurred even though materials had the same PG grade.
The shear viscosity of asphalt modified by thermoplastic elastomer, SBS, with and without the presence of sulfur is also shown in
Figure 9. It is clear that SBS modified asphalts had generally higher values of viscosity at 50 and 60 °C. In the case of asphalt modified by 5 wt.% of SBS, the short zero shear viscosity region was followed by a large viscosity overshoot. The shear thickening could be explained not only by the orientation of the independently acting swollen particles of polybutadiene in the direction of flow, but also by forming a weak non-permanent polymer network through physical interactions. The latter could be responsible for the reorganization of the internal network through an increase in a number of physical crosslinks. The shear thinning observed at high shear rates could be explained as the breaking of interactions within the internal structure [
22]. The formation of a three-dimensional polymer network by crosslinking of SBS with a small amount of sulfur resulted in the highest value of zero shear viscosity followed by a large viscosity overshoot. As it was mentioned above, the formation of viscosity overshoot can be associated with the presence of a network in modified asphalts [
8,
25].
3.4. Start-Up of Steady Shear Experiments
It is generally accepted that the majority of pavement distresses occur out of the linear viscoelastic zone. Thus, the strength of internal networks of asphalts modified by different modification technologies was investigated by the start-up of steady shear experiments. In these types of transient experiments, the shear rate is immediately applied to the tested material and held constant during the test. Rheologically, the behavior of paving asphalts could be presented as a diluted system of entanglements. Further modification of already complex internal structure with polymers or additives could result in the appearance of stress overshoot [
20,
52]. The stress overshoot does not only provide information about the strength of the internal network subjected to sudden large deformation, but also about the structural development of modified asphalts [
20].
The results from the steady shear viscosity measurements demonstrated that modification of asphalt binder by different modification systems could significantly affect its resistance to flow as well as change the shape of flow curves. The presence of shear thickening or two different shear thinning regions suggests the transition of these materials to non-Newtonian liquids. The complex responses of the formed internal structures cannot be described by either classical viscosity models or mesoscopic non-Newtonian viscosity models.
The stress growth function,
, of asphalts modified by different concentrations of B2Last is shown in
Figure 10. In order to avoid issues with overloading of the rheometer, the start-up of the steady shear experiment was carried out at 60 °C at three different shear rates of 0.5 s
−1, 2.0 s
−1, and 5.0 s
−1. In the start-up of the steady shear experiment, the response of asphalt modified by 1 wt.% of B2Last was within the linear viscoelastic domain. Such behaviors can be expressed as
, where
is equivalent to zero shear viscosity at the tested shear rate. For asphalt modified by 2 wt.% B2Last, very mild stress overshoot was observed only at the highest shear rate. At a maximum used concentration of B2Last, 3 wt.%, the stress overshoot was noticeable at 2.0 s
−1 and 5.0 s
−1.
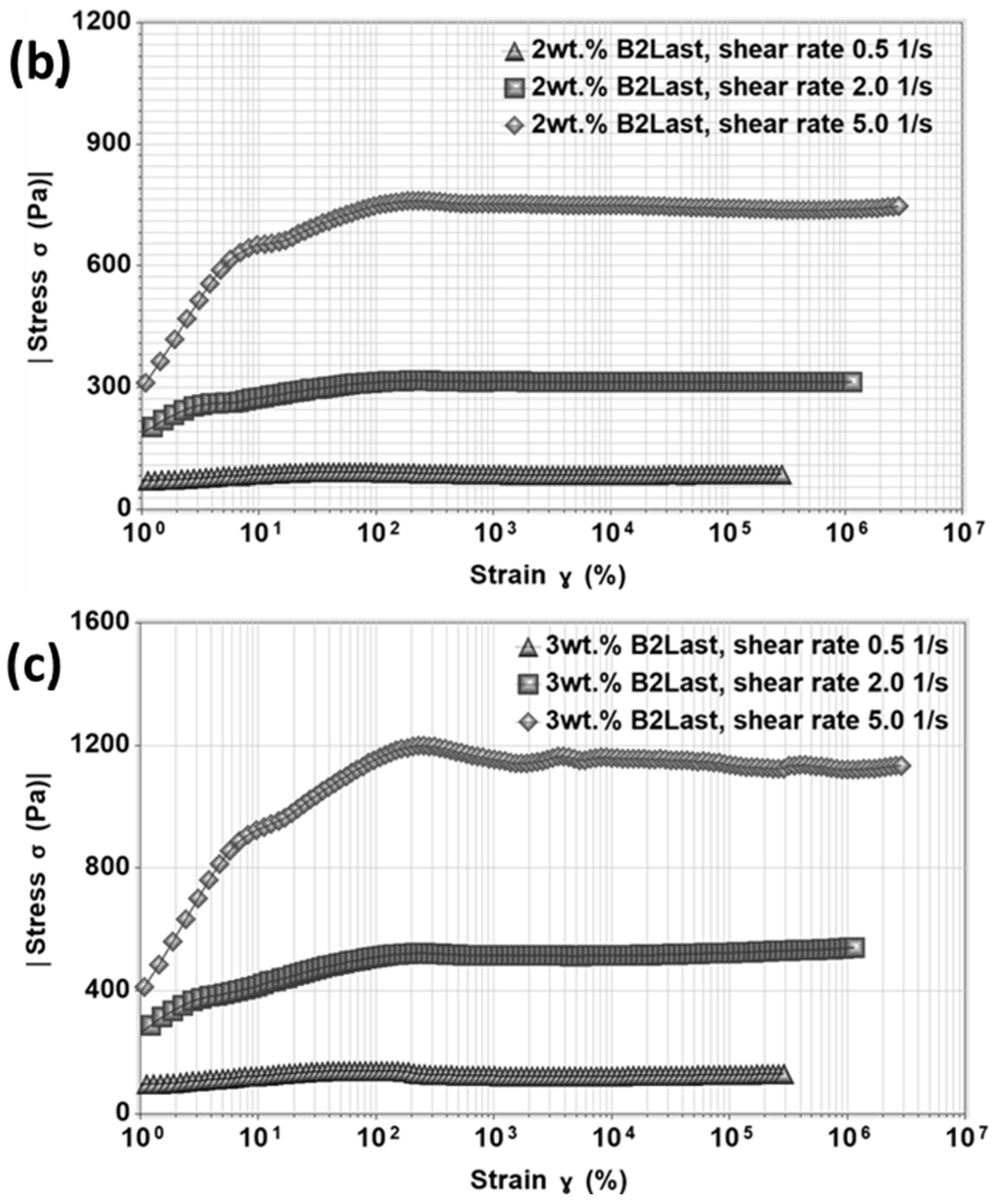
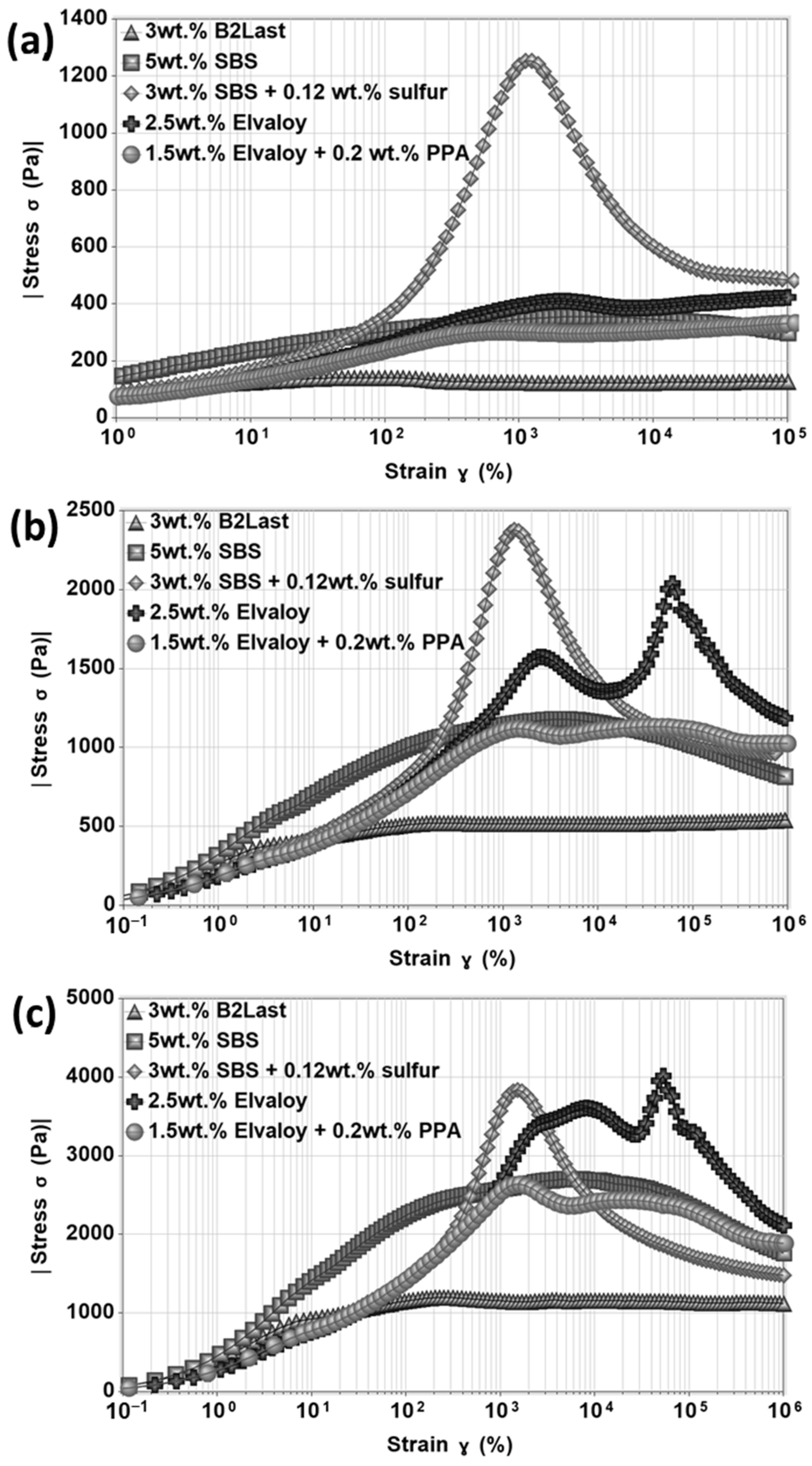
Stress overshoot as a function of strain for asphalts modified by different modification technologies at 60 °C at three different shear rates of 0.5 s
−1, 2.0 s
−1, and 5.0 s
−1 is shown in
Figure 11. Similarly to differences observed in steady shear viscosity, stress growth function has shown responses strongly influenced by the type of modification. The magnitudes of
of B2Last modified asphalts were the lowest compared to other modification technologies.
The internal network created by thermoplastic elastomer, SBS, without the presence of sulfur had a parabolic shape with stretched values of stress overshoot. This behavior could be explained by different phenomena. The swelling ability of SBS in asphalt increases the volume of the polymer phase which could lead to a reduction in asphalt’s liquid fraction. This favors aggregation and agglomeration of SBS particles [
8]. Thus, the parabolic response in the start-up of the steady shear experiment can be the result of different speeds of orientation of particles of active filler in the direction of flow, breaking of large polymer droplets to smaller droplets and their subsequent deformation. The ability of polystyrene domains to split, jump, and recombine under large deformation should also be considered [
25]. The formation of a three-dimensional SBS network upon vulcanization resulted in the highest magnitude of stress overshoot at all tested shear rates.
Asphalts modified by Elvaloy with and without PPA demonstrated at the start-up of steady shear experiment a peculiar stress overshoot. At a shear rate of 0.5 s−1, only mild stress overshoot with similar magnitude was observed for both modified asphalts. Two maxima of were detected when the shear rate was increased. The single stress overshoot can be generally associated with the diluted system of entanglements with high molecular weight or with a non-branched polymer system. Asphalt blend containing 2.5 wt.% of Elvaloy, however, could be in a metastable condition. The additional increase in the concentration of modifier, additional curing, or sudden change in curing conditions during preparation of modified asphalt could likely result in gelation of modified asphalt. The formed non-reversible internal network could be represented by asphaltene–Elvaloy complexes which could vary in size. Although the multiple overshoots could be explained by the orientation of chemically crosslinked asphaltenes and polymer complexes followed by their destruction, the origin of could be linked to the formation of either network or system of interacting asphaltene–Elvaloy micelles.
Due to the significantly reduced amount of Elvaloy in Elvaloy and PPA modified asphalt, the presence of multiple
was not expected. As can be seen in
Figure 11, however, this modified asphalt demonstrated two
overshoots of the same magnitude at a shear rate of 2 s
−1 and two
overshoots with a larger peak of
at 5 s
−1. To some extent, the PPA may disintegrate already present asphaltenes and form new asphaltenes. This could help with the formation of more active asphaltene centers for reaction with oxirane present in Elvaloy and potentially more efficient curing reaction. This phenomenon could suggest a possible combination of both mechanisms of PPA in asphalt as presented by Orange et al. [
46] and Baumgardner et al. [
47]. The appearance of two
overshoots in this case, however, should not be only attributed to the strength of formed chemically crosslinked asphaltene–Elvaloy complexes, but also to the increase in asphaltene micelles by PPA.
The stress growth function measured in the start-up of the steady shear experiments suggested that while asphalt modified by thermoplastic elastomer had the strongest network at all tested shear rates, the differences in the strength of the network between reactive (Elvaloy) and elastomeric modifiers seemed to be diminished at higher shear rates. Furthermore, the position of the maximum of stress overshoot in the case of reactive modifier with or without the presence of PPA was shifted to higher deformation. The appearance of the second stress overshoot also suggests an improved resistance of Elvaloy modified asphalts to deformation.