Effects of Chemicals Exposure on the Durability of Geopolymer Concrete Incorporated with Silica Fumes and Nano-Sized Silica at Varying Curing Temperatures
Abstract
:1. Introduction
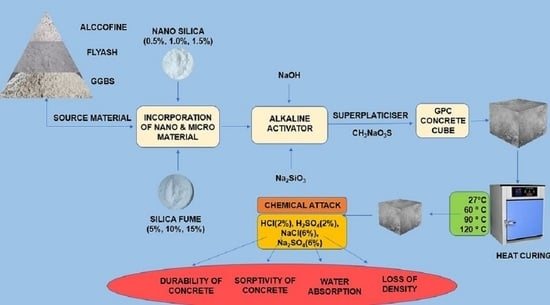
2. Experimental Program
2.1. Materials Used in GPC
2.2. Design Proportions for Mixture of GPC
2.3. Methodology
2.3.1. Mass Loss
2.3.2. Water Absorption
2.3.3. Sorptivity
2.3.4. Compressive Strength
3. Results and Discussion
3.1. Visual Appearance of Exposed GPC Specimens
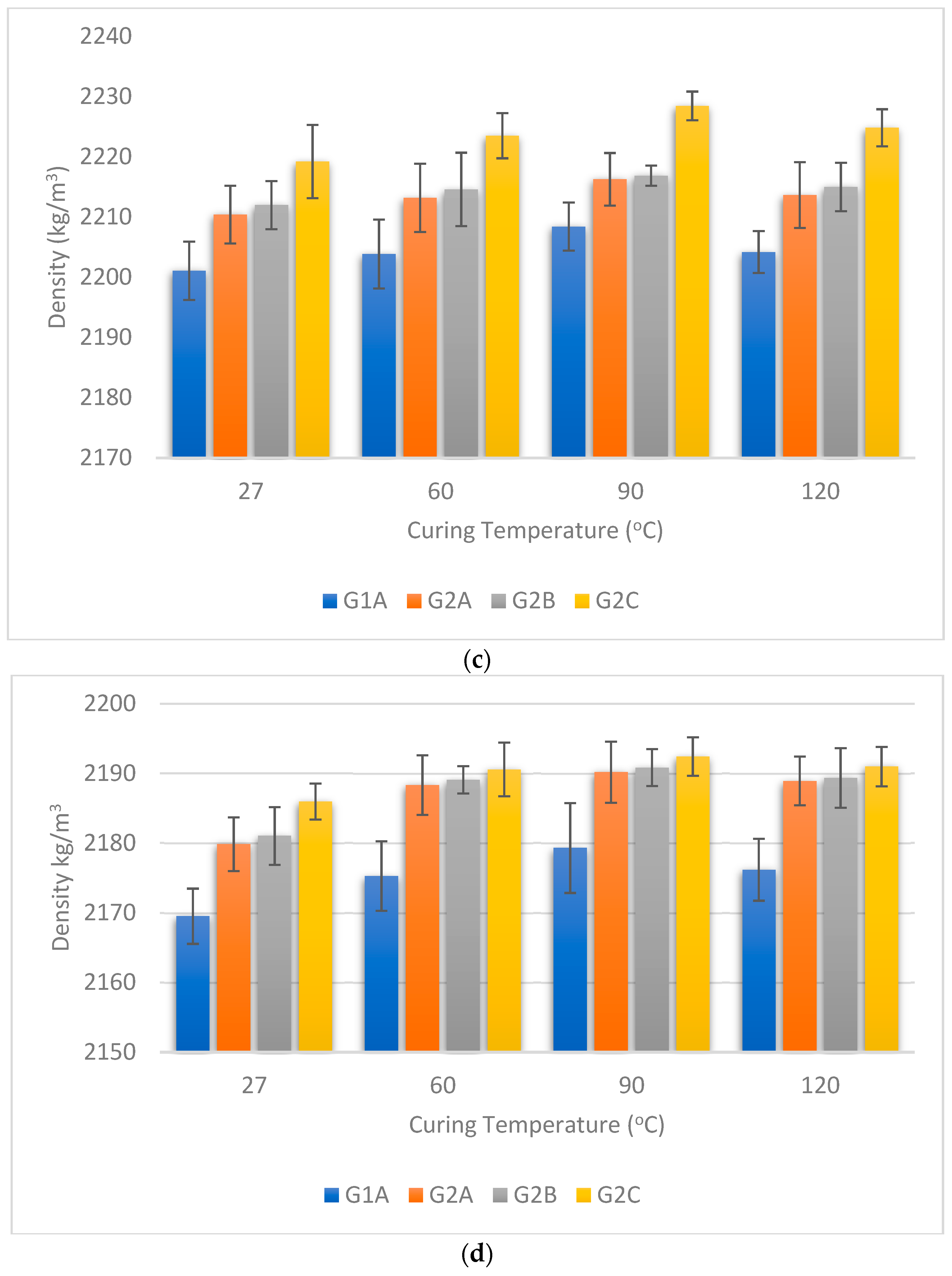
3.2. Density of GPC
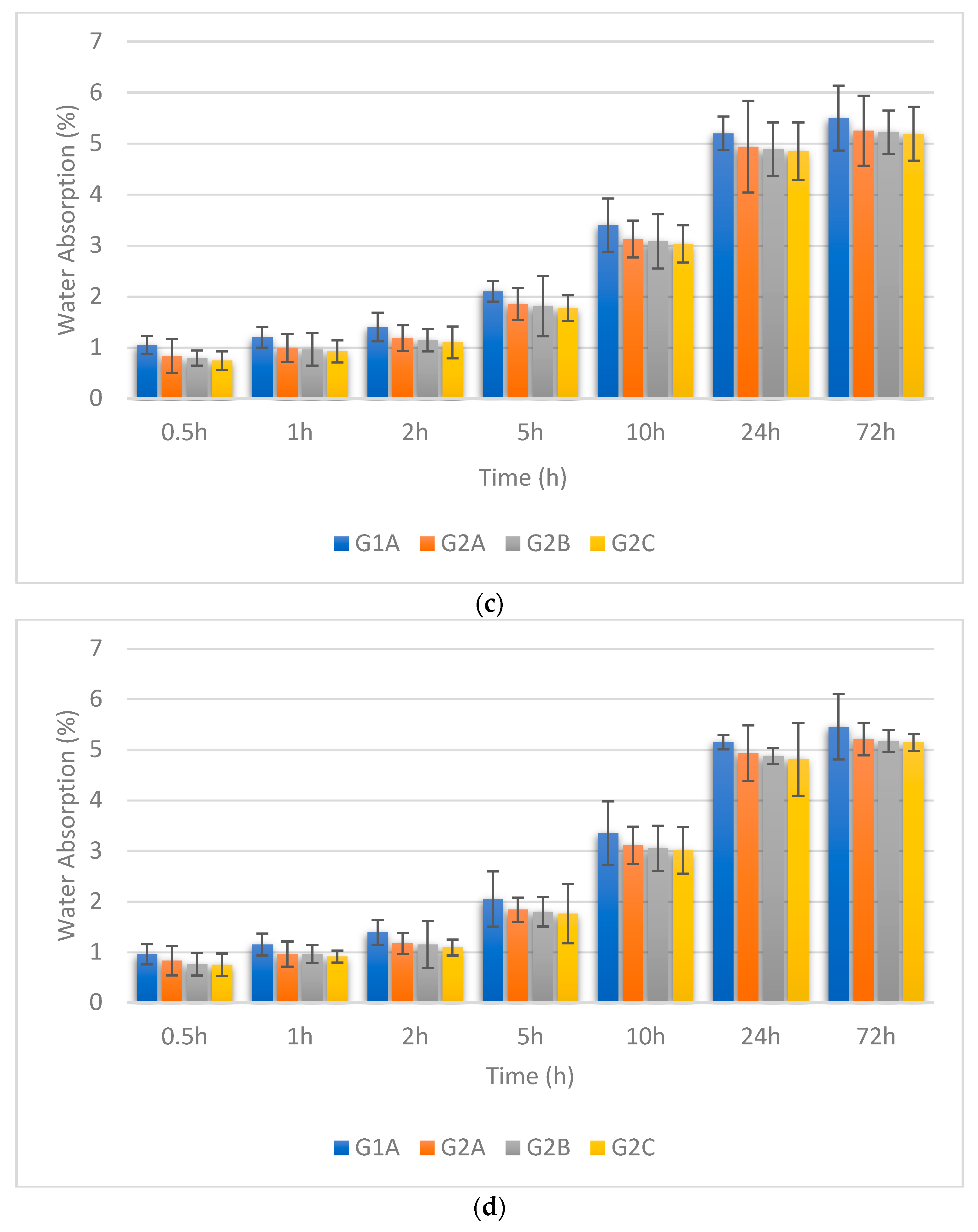
3.3. Water Absorption
3.4. Sorptivity
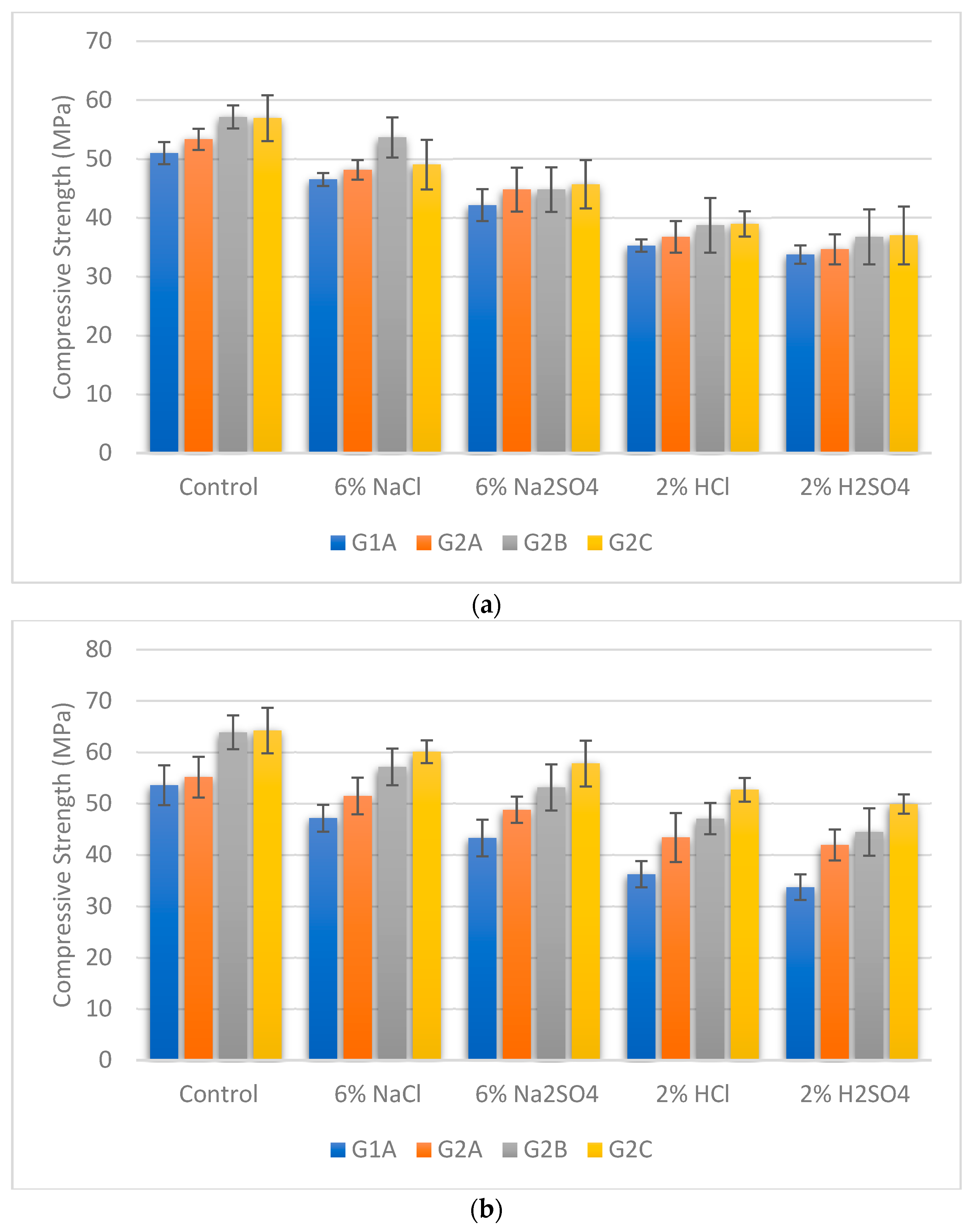
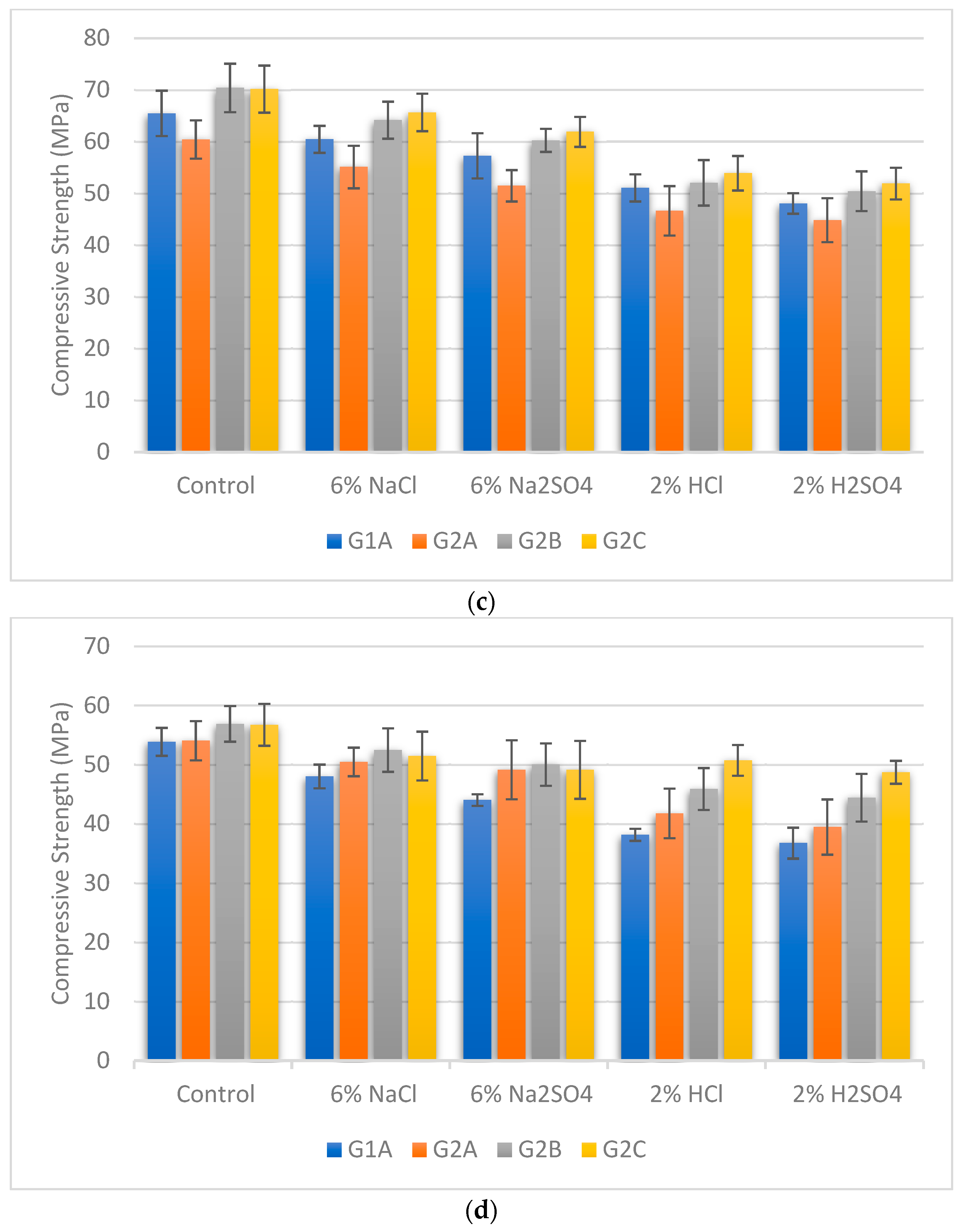
3.5. Compressive Strength under Exposure to the Chemicals
4. Cost Analysis of GPC
5. Conclusions and Future Prospects
- The addition of the NS + SF combination exhibited a consistent influence on the density of GPC specimens across different curing temperatures and chemical exposures. An upward trend in density was observed with the introduction of the NS + SF combination, indicating a potential to enhance the material’s compactness.
- The density response of GPC to different curing temperatures was notable. The density of GPC demonstrated an incremental trend with increasing curing temperature up to 90 °C, followed by a subsequent decrease. This indicates a complex interplay between temperature and material density.
- The density behaviour of GPC under various chemical exposures—NaCl, Na2SO4, HCl, and H2SO4—was consistent. Chemical attacks led to a reduction in density, indicating material deterioration. The magnitude of density decrease was observed to be in the range of 8.20% and 12.42% across the different curing temperatures.
- The reduction in water absorption, consistent across all NS + SF combinations at higher curing temperatures, accentuates the capacity of elevated curing temperatures to promote non-porous characteristics within the GPC matrix. Decline reflection in the water absorption as the curing temperature elevates to 90 °C and 120 °C, highlighting the consistent trend of improved moisture resistance.
- Quantifying the magnitude of impact, the data illustrates the percentage decrease in water absorption for various curing temperatures and NS + SF combination percentages. It is evident that higher NS + SF proportions and elevated curing temperatures yielded more pronounced reductions in water absorption percentages, reinforcing the moisture-resistant attributes of GPC.
- A noteworthy trend emerges with sorptivity decreasing over time. It is particularly striking that the impact of NS and SF additives consistently contributes to reducing sorptivity across all curing temperatures. It becomes evident that the effect of sorptivity reduction is more pronounced with increased curing duration.
- At an early age, the disparity in sorptivity across GPC specimens cured at varying temperatures is less pronounced due to the predominant influence of capillary forces. However, the divergence becomes more pronounced over time. The secondary age difference in sorptivity is attributed to the additive-driven filling of pores with NS and SF, a phenomenon consistent across different curing conditions.
- Adding NS and SF combined proportions contributes to enhanced compressive strength across all curing temperatures. Furthermore, it is evident that GPC’s compressive strength is more resilient against exposure to NaCl and Na2SO4 compared to the corrosive effects of acids (HCl and H2SO4). Among the chemicals tested, H2SO4 emerges as the most detrimental, leading to significant compressive strength deterioration.
- The role of NS and SF combined proportions in augmenting compressive strength across curing temperatures highlights the potential for improved structural integrity. These findings offer valuable insights into developing concrete structures that can effectively withstand chemical challenges, fostering the advancement of durable and sustainable construction practices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, L.S. Durability performance of geopolymer concrete: A review. Polymers 2022, 14, 868. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, A.E.; Alzeebaree, R.; Aljumaili, O.; Nis, A.; Gulsan, M.E.; Humur, G.; Cevik, A. Mechanical and durability properties of fly ash and slag based geopolymer concrete. Adv. Concr. Constr. 2018, 6, 345. [Google Scholar]
- Karthik, A.; Sudalaimani, K.; Vijayakumar, C. Durability study on coal fly ash-blast furnace slag geopolymer concretes with bio-additives. Ceram. Int. 2017, 43, 11935–11943. [Google Scholar] [CrossRef]
- Karakoc, M.B.; Türkmen, İ.; Maraş, M.M.; Kantarci, F.; Demirboğa, R. Sulfate resistance of ferrochrome slag based geopolymer concrete. Ceram. Int. 2016, 42, 1254–1260. [Google Scholar] [CrossRef]
- Paruthi, S.; Khan, A.H.; Kumar, A.; Kumar, F.; Hasan, M.A.; Magbool, H.M.; Manzar, M.S. Sustainable cement replacement using waste eggshells: A review on mechanical properties of eggshell concrete and strength prediction using artificial neural network. Case Stud. Constr. Mater. 2023, 18, e02160. [Google Scholar] [CrossRef]
- Valencia Saavedra, W.G.; Angulo, D.E.; Mejía de Gutiérrez, R. Fly ash slag geopolymer concrete: Resistance to sodium and magnesium sulfate attack. J. Mater. Civ. Eng. 2016, 28, 04016148. [Google Scholar] [CrossRef]
- Okoye, F.N.; Prakash, S.; Singh, N.B. Durability of fly ash based geopolymer concrete in the presence of silica fume. J. Clean. Prod. 2017, 149, 1062–1067. [Google Scholar] [CrossRef]
- Paruthi, S.; Husain, A.; Alam, P.; Khan, A.H.; Hasan, M.A.; Magbool, H.M. A review on material mix proportion and strength influence parameters of geopolymer concrete: Application of ANN model for GPC strength prediction. Constr. Build. Mater. 2022, 356, 129253. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.A.; Rafiq, S.; Mohammed, A.S.; Mosavi, A.; Sor, N.H.; Qaidi, S.M. Compressive strength of sustainable geopolymer concrete composites: A state-of-the-art review. Sustainability 2021, 13, 13502. [Google Scholar] [CrossRef]
- Kabir, S.; Alengaram, U.J.; Jumaat, M.Z.; Sharmin, A.; Islam, A. Influence of molarity and chemical composition on the development of compressive strength in POFA based geopolymer mortar. Adv. Mater. Sci. Eng. 2015, 2015, 647071. [Google Scholar] [CrossRef]
- Albitar, M.; Visintin, P.; Mohamed Ali, M.; Drechsler, M. Assessing behaviour of fresh and hardened geopolymer concrete mixed with class-F fly ash. KSCE J. Civ. Eng. 2015, 19, 1445–1455. [Google Scholar] [CrossRef]
- AL-Kharabsheh, B.N.; Moafak Arbili, M.; Majdi, A.; Ahmad, J.; Deifalla, A.F.; Hakamy, A.; Majed Alqawasmeh, H. Feasibility study on concrete made with substitution of quarry dust: A review. Sustainability 2022, 14, 15304. [Google Scholar] [CrossRef]
- Alraddadi, S.; Assaedi, H. Characterization and potential applications of different powder volcanic ash. J. King Saud Univ. Sci. 2020, 32, 2969–2975. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Khan, A.H.; Tayeh, B.A. Durability and strength characteristics of high-strength concrete incorporated with volcanic pumice powder and polypropylene fibers. J. Mater. Res. Technol. 2020, 9, 806–818. [Google Scholar] [CrossRef]
- Topark-Ngarm, P.; Chindaprasirt, P.; Sata, V. Setting time, strength, and bond of high-calcium fly ash geopolymer concrete. J. Mater. Civ. Eng. 2015, 27, 04014198. [Google Scholar] [CrossRef]
- Nuruddin, M.F.; Demie, S.; Ahmed, M.F.; Shafiq, N. Effect of superplasticizer and NaOH molarity on workability, compressive strength and microstructure properties of self-compacting geopolymer concrete. Int. J. Geol. Environ. Eng. 2011, 5, 187–194. [Google Scholar]
- Haustein, E.; Kuryłowicz-Cudowska, A.; Łuczkiewicz, A.; Fudala-Książek, S.; Cieślik, B.M. Influence of cement replacement with sewage sludge ash (SSA) on the heat of hydration of cement mortar. Materials 2022, 15, 1547. [Google Scholar] [CrossRef]
- Imtiaz, L.; Rehman, S.K.U.; Ali Memon, S.; Khizar Khan, M.; Faisal Javed, M. A review of recent developments and advances in eco-friendly geopolymer concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Parathi, S.; Nagarajan, P.; Pallikkara, S.A. Ecofriendly geopolymer concrete: A comprehensive review. Clean Technol. Environ. Policy 2021, 23, 1701–1713. [Google Scholar] [CrossRef]
- Allaoui, D.; Nadi, M.; Hattani, F.; Majdoubi, H.; Haddaji, Y.; Mansouri, S.; Oumam, M.; Hannache, H.; Manoun, B. Eco-friendly geopolymer concrete based on metakaolin and ceramics sanitaryware wastes. Ceram. Int. 2022, 48, 34793–34802. [Google Scholar] [CrossRef]
- Reddy, D.V.; Edouard, J.-B.; Sobhan, K. Durability of fly ash–based geopolymer structural concrete in the marine environment. J. Mater. Civ. Eng. 2013, 25, 781–787. [Google Scholar] [CrossRef]
- Pasupathy, K.; Sanjayan, J.; Rajeev, P.; Law, D.W. The effect of chloride ingress in reinforced geopolymer concrete exposed in the marine environment. J. Build. Eng. 2021, 39, 102281. [Google Scholar] [CrossRef]
- Rajamane, N.; Nataraja, M.; Lakshmanan, N.; Dattatreya, J. Rapid chloride permeability test on geopolymer and Portland cement. Indian Concr. J. 2011, 21–26. [Google Scholar]
- Sathia, R.; Babu, K.G.; Santhanam, M. Durability study of low calcium fly ash geopolymer concrete. In Proceedings of the 3rd ACF International Conference-ACF/VCA, Ho Chi Minh City, Vietnam, 11–13 November 2008; Indian Institute of Technology Madras Chennai: Chennai, India, 2008. [Google Scholar]
- Goriparthi, M.R.; TD, G.R. Effect of fly ash and GGBS combination on mechanical and durability properties of GPC. Adv. Concr. Constr. 2017, 5, 313. [Google Scholar]
- Prusty, J.K.; Pradhan, B. Effect of GGBS and chloride on compressive strength and corrosion performance of steel in fly ash-GGBS based geopolymer concrete. Mater. Today Proc. 2020, 32, 850–855. [Google Scholar] [CrossRef]
- Okoye, F.; Durgaprasad, J.; Singh, N. Effect of silica fume on the mechanical properties of fly ash based-geopolymer concrete. Ceram. Int. 2016, 42, 3000–3006. [Google Scholar] [CrossRef]
- Memon, F.A.; Nuruddin, M.F.; Shafiq, N. Effect of silica fume on the fresh and hardened properties of fly ash-based self-compacting geopolymer concrete. Int. J. Miner. Metall. Mater. 2013, 20, 205–213. [Google Scholar] [CrossRef]
- Alanazi, H.; Yang, M.; Zhang, D.; Gao, Z. Early strength and durability of metakaolin-based geopolymer concrete. Mag. Concr. Res. 2017, 69, 46–54. [Google Scholar] [CrossRef]
- Esparham, A. Factors influencing compressive strength of metakaolin-based geopolymer concrete. Modares Civ. Eng. J. 2020, 20, 77–90. [Google Scholar]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of metakaolin-based geopolymer concrete for different mix design parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Jena, S.; Panigrahi, R.; Sahu, P. Mechanical and durability properties of Fly ash Geopolymer concrete with silica fume. J. Inst. Eng. Ser. A 2019, 100, 697–705. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.; Low, I.M. Characterizations of flax fabric reinforced nanoclay-geopolymer composites. Compos. Part B Eng. 2016, 95, 412–422. [Google Scholar] [CrossRef]
- Assaedi, H.S.; Olawale, M.D. Impact of nano-alumina on the mechanical characterization of PVA fibre-reinforced geopolymer composites. J. Taibah Univ. Sci. 2022, 16, 828–835. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R.; Eddy, N.O.; Khan, M.A.; Khan, A.H.; Alomayri, T.; Berwal, P. Mechanical strength and durability analysis of mortars prepared with fly ash and nano-metakaolin. Case Stud. Constr. Mater. 2023, 18, e01796. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Hakamy, A.; Amin, M.; Zeyad, A.M.; Agwa, I.S. Effect of air agent on mechanical properties and microstructure of lightweight geopolymer concrete under high temperature. Case Stud. Constr. Mater. 2022, 16, e00951. [Google Scholar] [CrossRef]
- Thokchom, S.; Dutta, D.; Ghosh, S. Effect of incorporating silica fume in fly ash geopolymers. Int. J. Civ. Environ. Eng. 2011, 5, 750–754. [Google Scholar]
- Saba, A.M.; Khan, A.H.; Akhtar, M.N.; Khan, N.A.; Koloor, S.S.R.; Petrů, M.; Radwan, N. Strength and flexural behavior of steel fiber and silica fume incorporated self-compacting concrete. J. Mater. Res. Technol. 2021, 12, 1380–1390. [Google Scholar] [CrossRef]
- Wani, A.Y.; Bhandari, M. Effect of Ground Granulated Blast Furnace Slag, Silica Fume and Nano Silica on the Strength & Durability Properties of Concrete: A Contemporary Review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surakarta, Indonesia, 24–25 August 2021; IOP Publishing: Bristol, UK, 2021; p. 012007. [Google Scholar]
- Çevik, A.; Alzeebaree, R.; Humur, G.; Niş, A.; Gülşan, M.E. Effect of nano-silica on the chemical durability and mechanical performance of fly ash based geopolymer concrete. Ceram. Int. 2018, 44, 12253–12264. [Google Scholar] [CrossRef]
- Jaradat, Y.; Matalkah, F. Effects of micro silica on the compressive strength and absorption characteristics of olive biomass ash-based geopolymer. Case Stud. Constr. Mater. 2023, 18, e01870. [Google Scholar] [CrossRef]
- Sikder, A.; Saha, P. Effect of different types of Waste as Binder on Durability Properties of Geopolymer Concrete: A Review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surakarta, Indonesia, 24–25 August 2021; IOP Publishing: Bristol, UK, 2021; p. 012018. [Google Scholar]
- Bagchi, S.; Ghuleand, S.; Jadhav, R. Fly ash fineness–Comparing residue on 45 micron sieve with Blaine’s surface area. IndIan ConCreTe J. 2012, 40, 39–42. [Google Scholar]
- IS 12089:1987; Specification of Granulated Slag for the Manufacture of Portland Slag Cement. Indian Standard: Mirzapu, India, 1987.
- IS 15388:2003; Specification for Silica Fume [CED 2: Cement and Concrete]. Indian Standard: Mirzapu, India, 2003.
- IS 383:1970; Specification for Coarse and Fine Aggregates from Natural Sources for Concrete [CED 2: Cement and Concrete]. Indian Standard: Mirzapu, India, 1970.
- Albitar, M.; Ali, M.M.; Visintin, P.; Drechsler, M. Effect of granulated lead smelter slag on strength of fly ash-based geopolymer concrete. Constr. Build. Mater. 2015, 83, 128–135. [Google Scholar] [CrossRef]
- Sivakumar, V.; Kavitha, O.; Arulraj, G.P.; Srisanthi, V. An experimental study on combined effects of glass fiber and Metakaolin on the rheological, mechanical, and durability properties of self-compacting concrete. Appl. Clay Sci. 2017, 147, 123–127. [Google Scholar] [CrossRef]
- Bellum, R.R.; Muniraj, K.; Madduru, S.R.C. Influence of slag on mechanical and durability properties of fly ash-based geopolymer concrete. J. Korean Ceram. Soc. 2020, 57, 530–545. [Google Scholar] [CrossRef]
- Siddique, R. Effect of fine aggregate replacement with Class F fly ash on the abrasion resistance of concrete. Cem. Concr. Res. 2003, 33, 1877–1881. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Singh, N.; Vyas, S.; Pathak, R.; Sharma, P.; Mahure, N.; Gupta, S. Effect of aggressive chemical environment on durability of green geopolymer concrete. Int. J. Eng. Innov. Technol. 2013, 3, 277–284. [Google Scholar]
- Al-Dulaijan, S.; Macphee, D.; Maslehuddin, M.; Al-Zahrani, M.; Ali, M. Performance of plain and blended cements exposed to high sulphate concentrations. Adv. Cem. Res. 2007, 19, 167–175. [Google Scholar] [CrossRef]
- Thokchom, S. Fly ash geopolymer pastes in sulphuric acid. Int. J. Eng. Innov. Res. 2014, 3, 943–947. [Google Scholar]
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I.; Wardhono, A. Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 2015, 48, 721–731. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, N.; Saxena, K.K. Mechanical and durability characteristics assessment of geopolymer composite (Gpc) at varying silica fume content. J. Compos. Sci. 2021, 5, 237. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Wongsa, A.; Srinavin, K.; Chindaprasirt, P. Recycled aggregate high calcium fly ash geopolymer concrete with inclusion of OPC and nano-SiO2. Constr. Build. Mater. 2018, 174, 244–252. [Google Scholar] [CrossRef]
- Jalal, M.; Pouladkhan, A.; Harandi, O.F.; Jafari, D. Comparative study on effects of Class F fly ash, nano silica and silica fume on properties of high performance self compacting concrete. Constr. Build. Mater. 2015, 94, 104. [Google Scholar] [CrossRef]
- Adak, D.; Sarkar, M.; Mandal, S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Constr. Build. Mater. 2014, 70, 453–459. [Google Scholar] [CrossRef]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and acid resistance of ambient-cured geopolymer mortars containing nano-silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef]
- Their, J.M.; Özakça, M. Developing geopolymer concrete by using cold-bonded fly ash aggregate, nano-silica, and steel fiber. Constr. Build. Mater. 2018, 180, 12–22. [Google Scholar] [CrossRef]
- Saini, G.; Vattipalli, U. Assessing properties of alkali activated GGBS based self-compacting geopolymer concrete using nano-silica. Case Stud. Constr. Mater. 2020, 12, e00352. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Taebuanhuad, S. Resistance to acid and sulfate solutions of microwave-assisted high calcium fly ash geopolymer. Mater. Struct. 2013, 46, 375–381. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Sothornchaiwit, K.; Dokduea, W.; Tangchirapat, W.; Keawsawasvong, S.; Thongchom, C.; Jaturapitakkul, C. Influences of silica fume on compressive strength and chemical resistances of high calcium fly ash-based alkali-activated mortar. Sustainability 2022, 14, 2652. [Google Scholar] [CrossRef]
- Aygörmez, Y.; Canpolat, O. Long-term sulfuric and hydrochloric acid resistance of silica fume and colemanite waste reinforced metakaolin-based geopolymers. Rev. Constr. 2021, 20, 291–307. [Google Scholar] [CrossRef]
- Abbas, R.; Khereby, M.A.; Ghorab, H.Y.; Elkhoshkhany, N. Preparation of geopolymer concrete using Egyptian kaolin clay and the study of its environmental effects and economic cost. Clean Technol. Environ. Policy 2020, 22, 669–687. [Google Scholar] [CrossRef]
- Shaikh, F. Deflection hardening behaviour of short fibre reinforced fly ash based geopolymer composites. Mater. Des. 2013, 50, 674–682. [Google Scholar] [CrossRef]
- Thaarrini, J.; Dhivya, S. Comparative study on the production cost of geopolymer and conventional concretes. Int. J. Civ. Eng. Res. 2016, 7, 117–124. [Google Scholar]
- Akhtar, N.; Ahmad, T.; Husain, D.; Majdi, A.; Alam, M.T.; Husain, N.; Wayal, A.K.S. Ecological footprint and economic assessment of conventional and geopolymer concrete for sustainable construction. J. Clean. Prod. 2022, 380, 134910. [Google Scholar] [CrossRef]
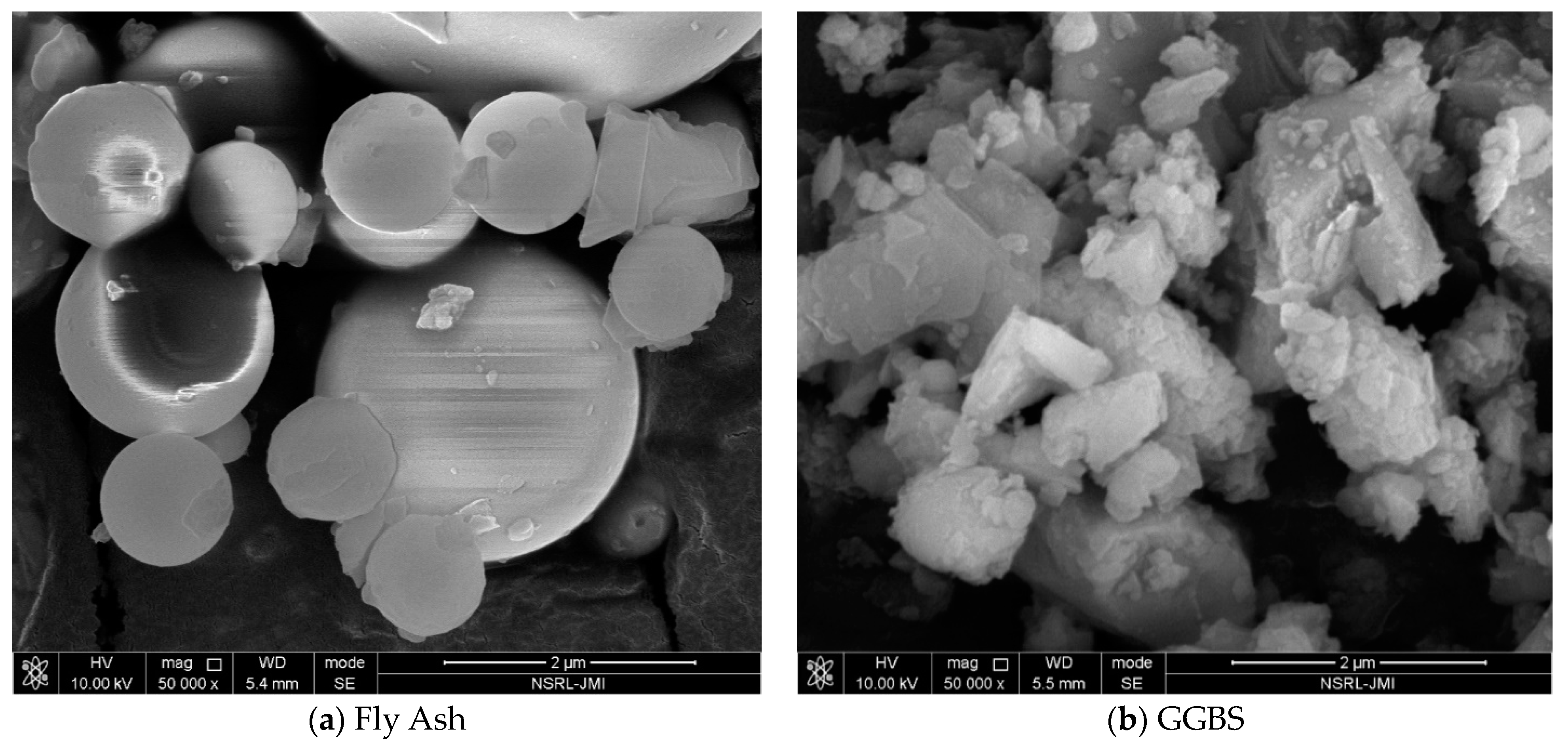
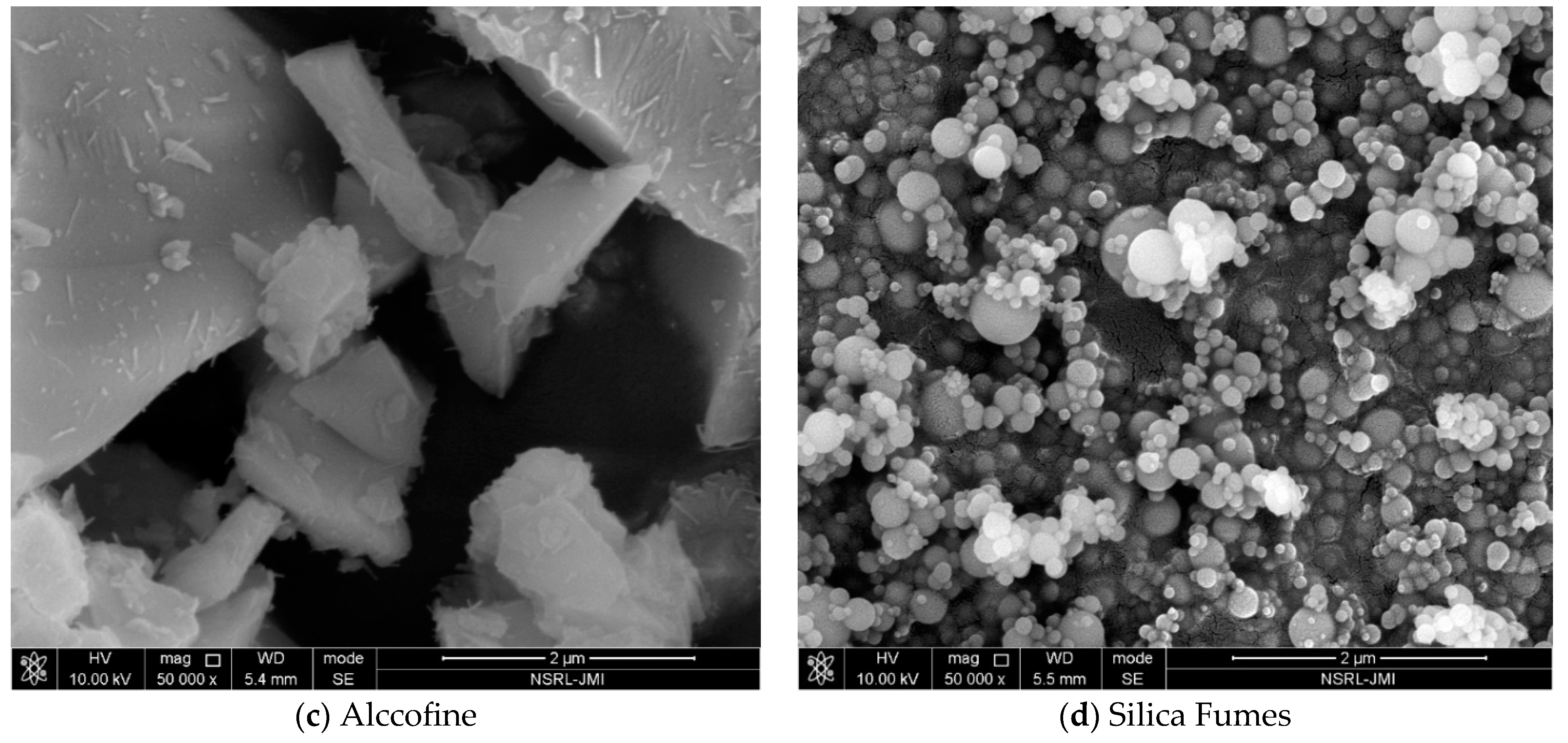





| Sample (%) | SiO2 | Fe2O3 | CaO | Al2O3 | MgO | K2O | Na2O | SO3 | P2O5 | TiO2 | LOI a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 49 | 12.5 | 2.79 | 27.25 | 0.89 | 0.46 | 0.32 | 0.38 | 0.98 | 1.54 | 0.64 |
| GGBS | 32.46 | 0.61 | 43.1 | 14.3 | 3.94 | 0.33 | 0.24 | 4.58 | 0.02 | 0.55 | 0.09 |
| SF | 85 | 1.5 | 0.8 | 1.1 | 2.5 | 0.9 | 1.3 | 1.3 | - | - | 2.8 |
| AF | 35.3 | 2.20 | 35.20 | 26.4 | - | - | - | 0.90 | - | - | - |
| NS | 92 | - | 0.70 | - | - | 0.25 | - | 0.80 | - | - | 2.42 |
| Property | FA | GGBS | AF | SF | CoAg | FiAg |
|---|---|---|---|---|---|---|
| Specific gravity | 2.41 | 3.12 | 2.89 | 2.20 | 2.89 | 2.61 |
| Water Absorption (%) | - | - | - | - | 0.98 | 1.35 |
| Fineness Modulus | - | - | - | - | - | 3.017 |
| Specific surface area (m2/kg) | 412 | 407 | 1200 | - | - | - |
| Mix | FA | GGBS | AF | NaOH | Na2SiO3 | SP | CoAg | FiAg |
|---|---|---|---|---|---|---|---|---|
| GPC | 149.17 | 213.1 | 63.93 | 42.6 | 106.6 | 6 | 1276.8 | 547.8 |
| S. No. | Material | Rate in Rs./Kg | GPC G40 | IS M40 | ACI M40 | DOE M40 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quantity | Cost | Quantity | Cost | Quantity | Cost | Quantity | Cost | |||
| 1 | OPC | 6.56 | 0 | 0 | 400 | 2624 | 356 | 2335.36 | 264 | 1731.84 |
| 2 | Fly Ash | 1.45 | 426.2 | 216.29 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | Coarse Aggregate | 0.6 | 1276.8 | 766.08 | 1230 | 738 | 1170.8 | 1943.52 | 1359.8 | 2257.26 |
| 4 | Fine Aggregate | 1.4 | 547.8 | 766.92 | 410 | 574 | 714.8 | 1000.72 | 698.8 | 978.32 |
| 5 | Sodium hydroxide | 25.5 | 42.6 | 1086.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | Sodium Silicate | 10 | 106.6 | 1066 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | Super Plasticizer | 100 | 6 | 600 | 1.5 | 150 | 1 | 100 | 1.5 | 150 |
| Total cost (Rs.) | 4501.59 | 4086 | 5379.6 | 5117.42 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paruthi, S.; Rahman, I.; Husain, A.; Hasan, M.A.; Khan, A.H. Effects of Chemicals Exposure on the Durability of Geopolymer Concrete Incorporated with Silica Fumes and Nano-Sized Silica at Varying Curing Temperatures. Materials 2023, 16, 6332. https://doi.org/10.3390/ma16186332
Paruthi S, Rahman I, Husain A, Hasan MA, Khan AH. Effects of Chemicals Exposure on the Durability of Geopolymer Concrete Incorporated with Silica Fumes and Nano-Sized Silica at Varying Curing Temperatures. Materials. 2023; 16(18):6332. https://doi.org/10.3390/ma16186332
Chicago/Turabian StyleParuthi, Sagar, Ibadur Rahman, Asif Husain, Mohd Abul Hasan, and Afzal Husain Khan. 2023. "Effects of Chemicals Exposure on the Durability of Geopolymer Concrete Incorporated with Silica Fumes and Nano-Sized Silica at Varying Curing Temperatures" Materials 16, no. 18: 6332. https://doi.org/10.3390/ma16186332