Effect of the Sintering Mechanism on the Crystallization Kinetics of Geopolymer-Based Ceramics
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
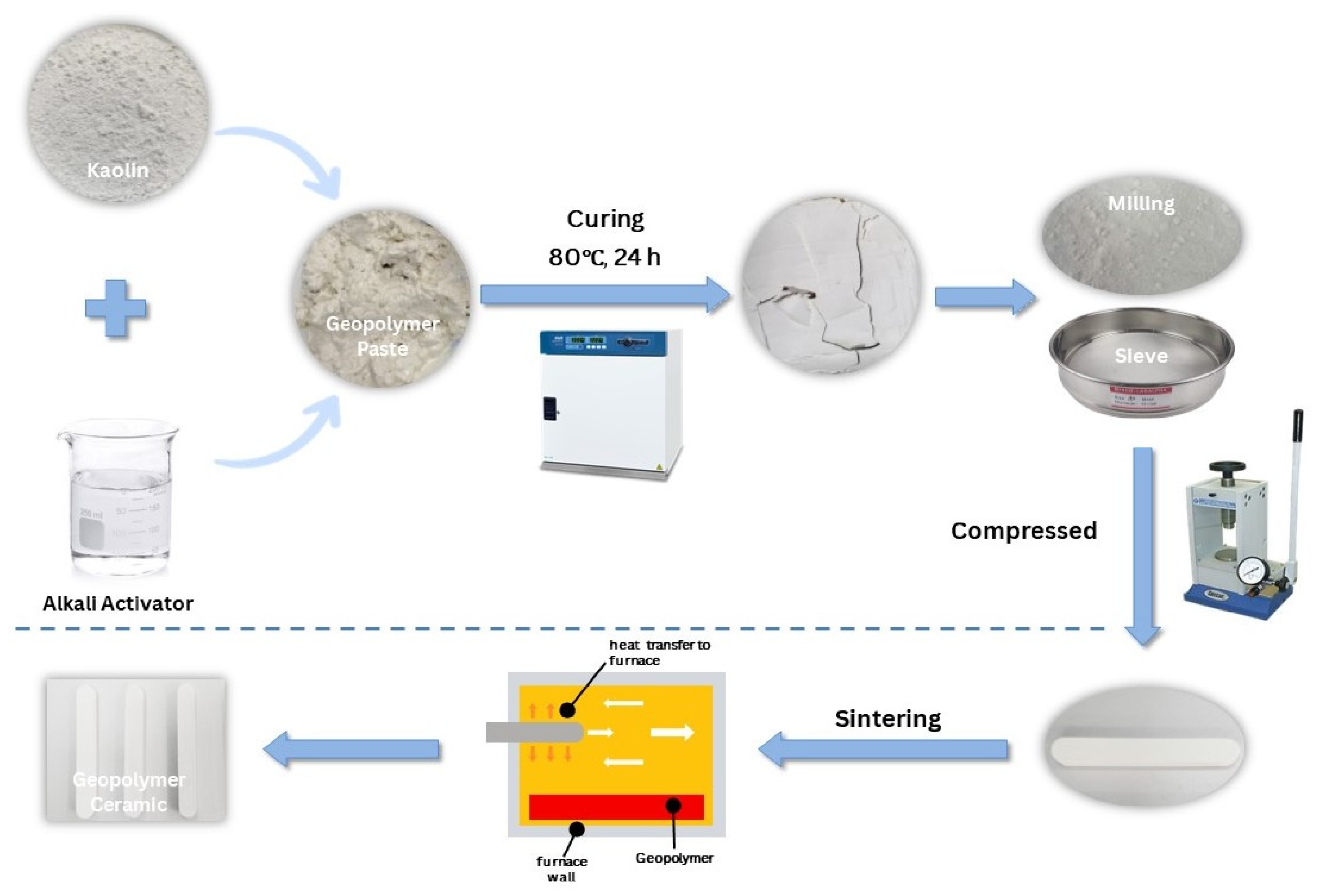
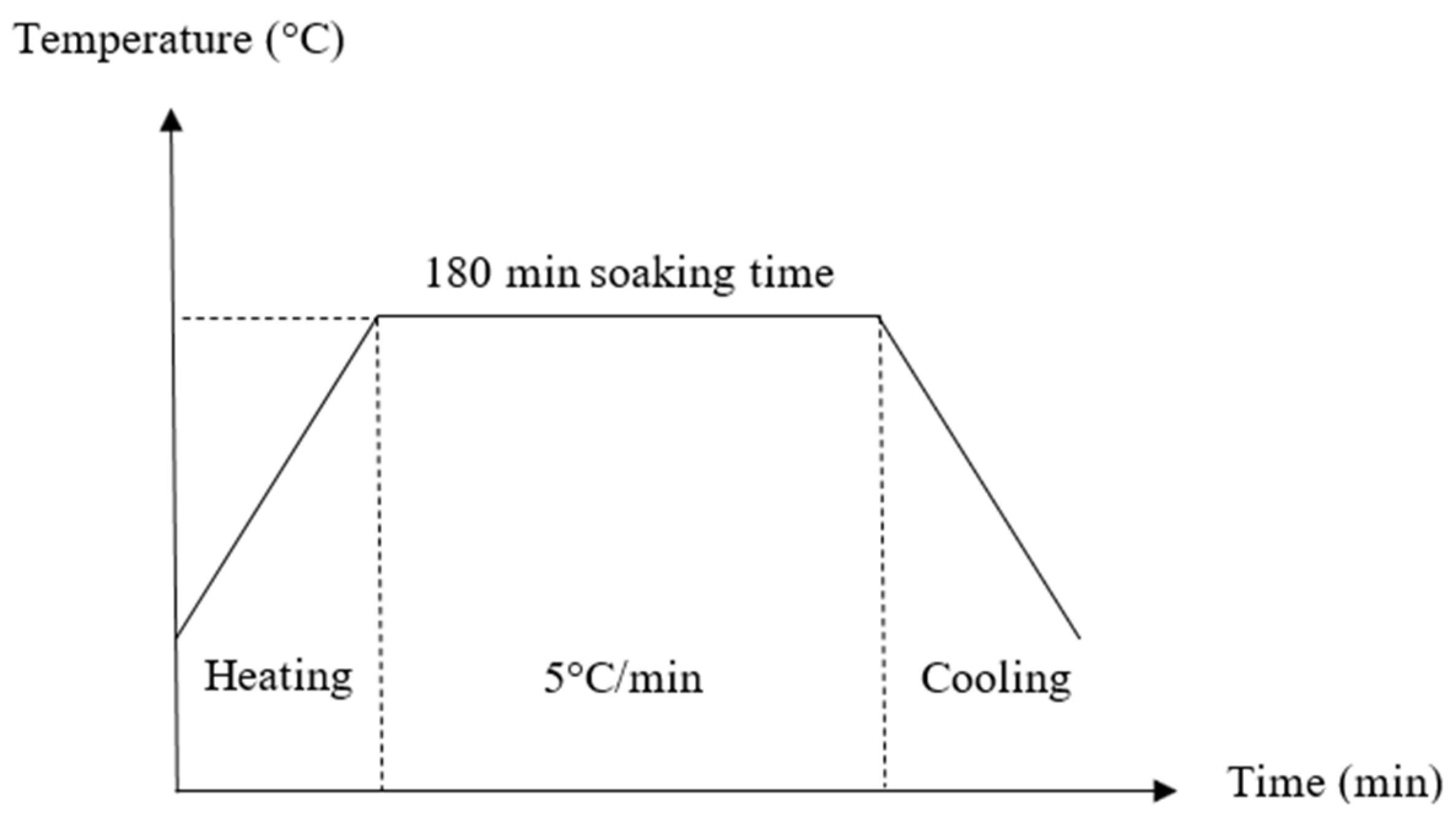
2.2. Sample Preparation
3. Results and Discussions
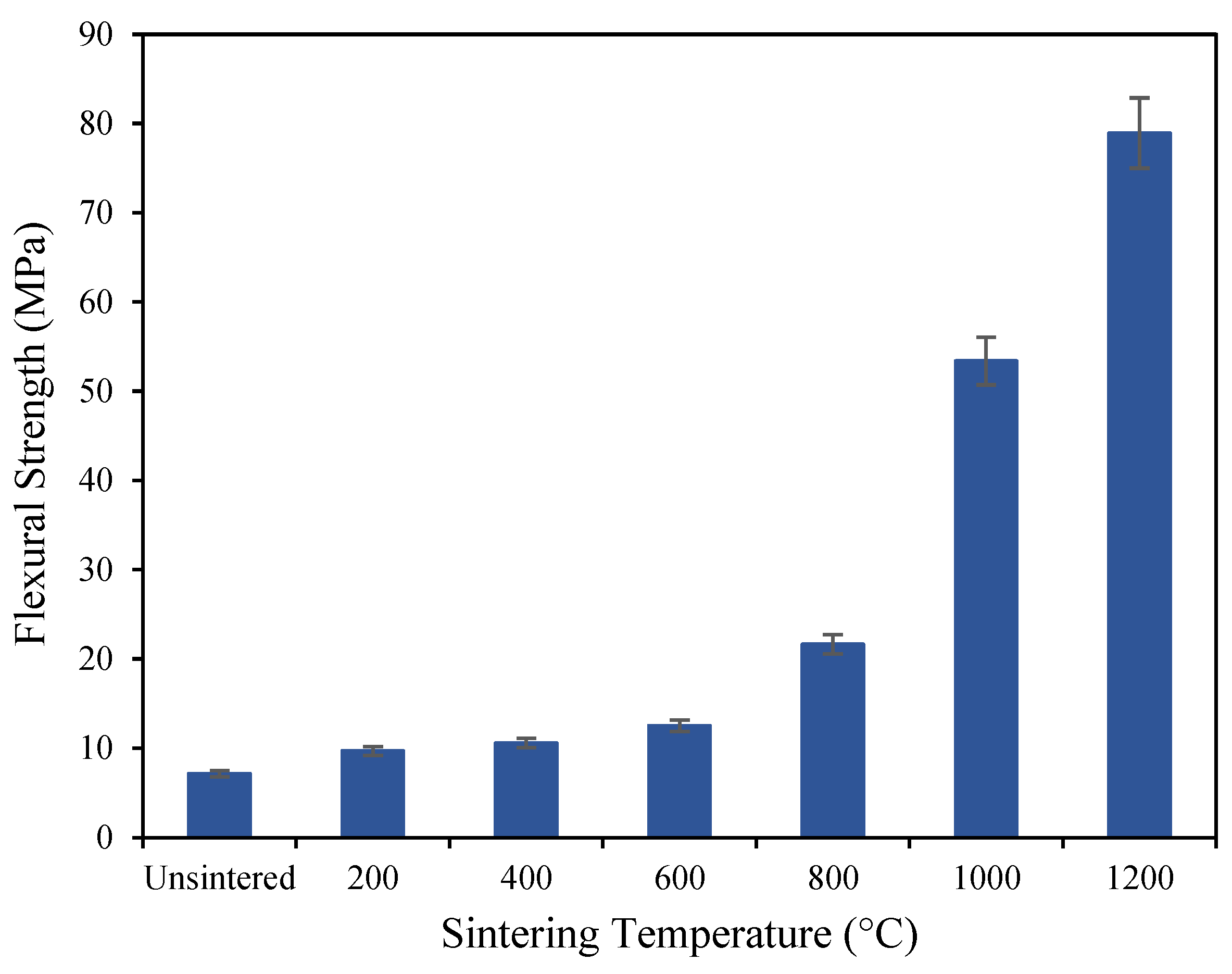
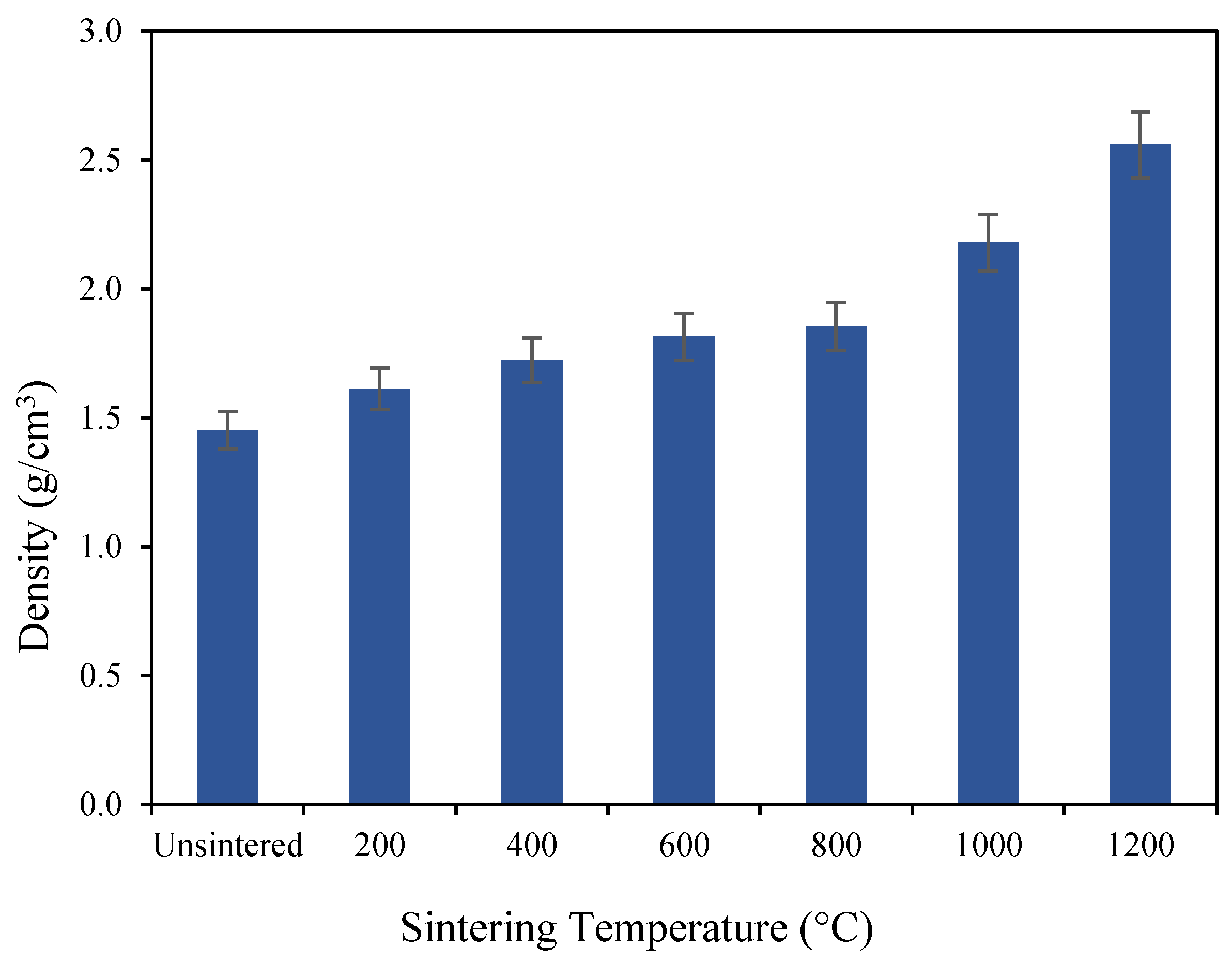
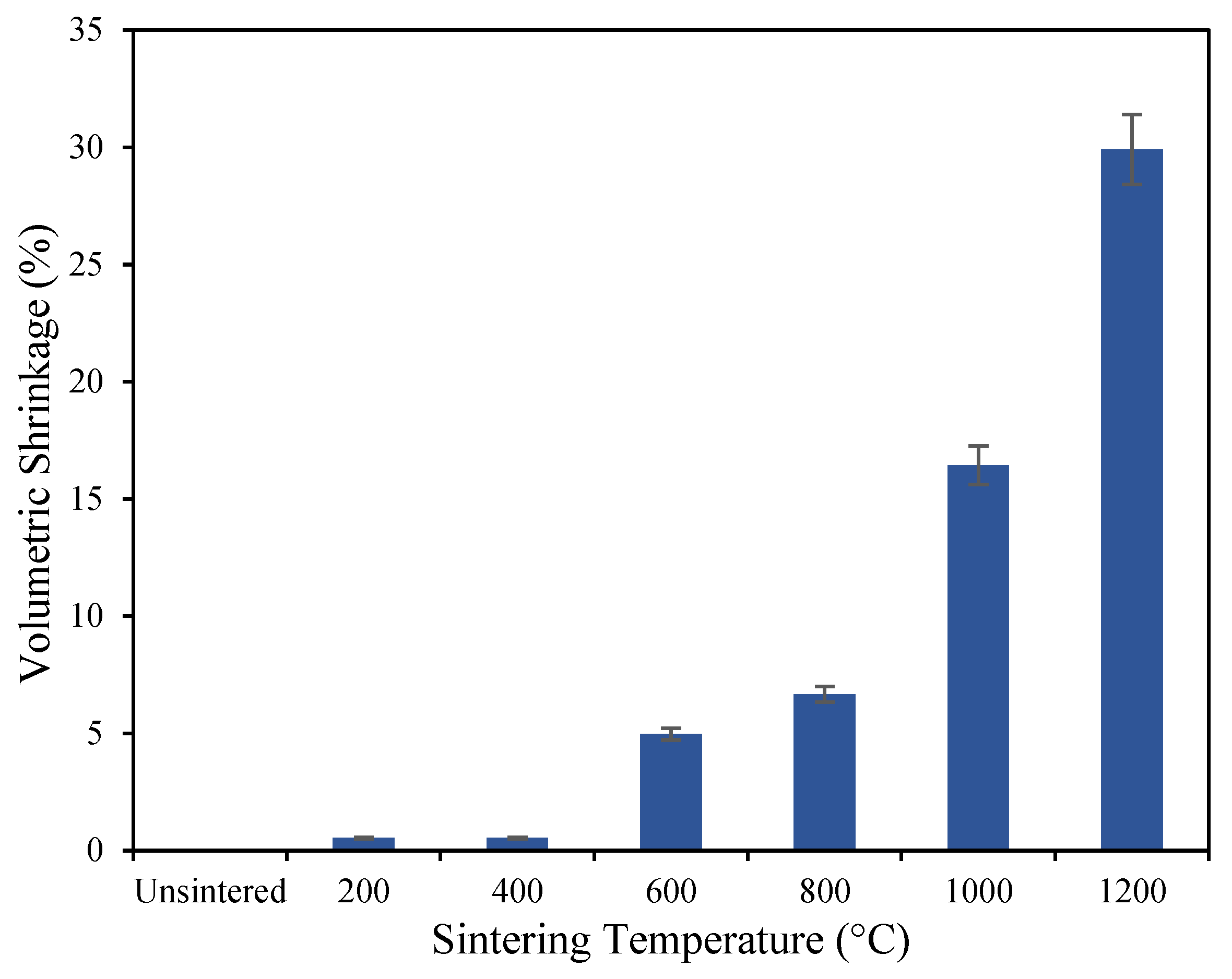
3.1. Mechanical Properties of Geopolymer-Based Ceramics
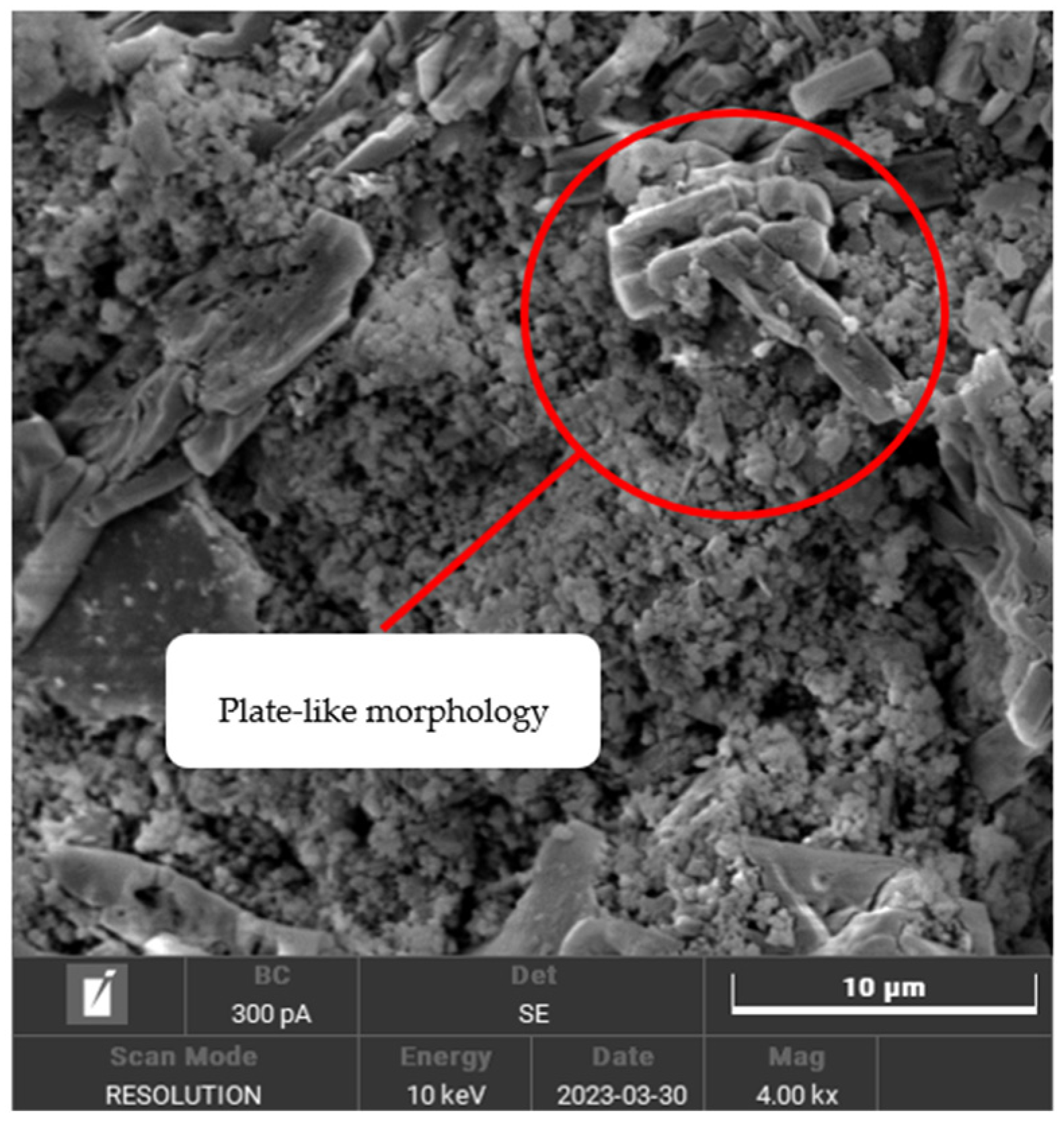
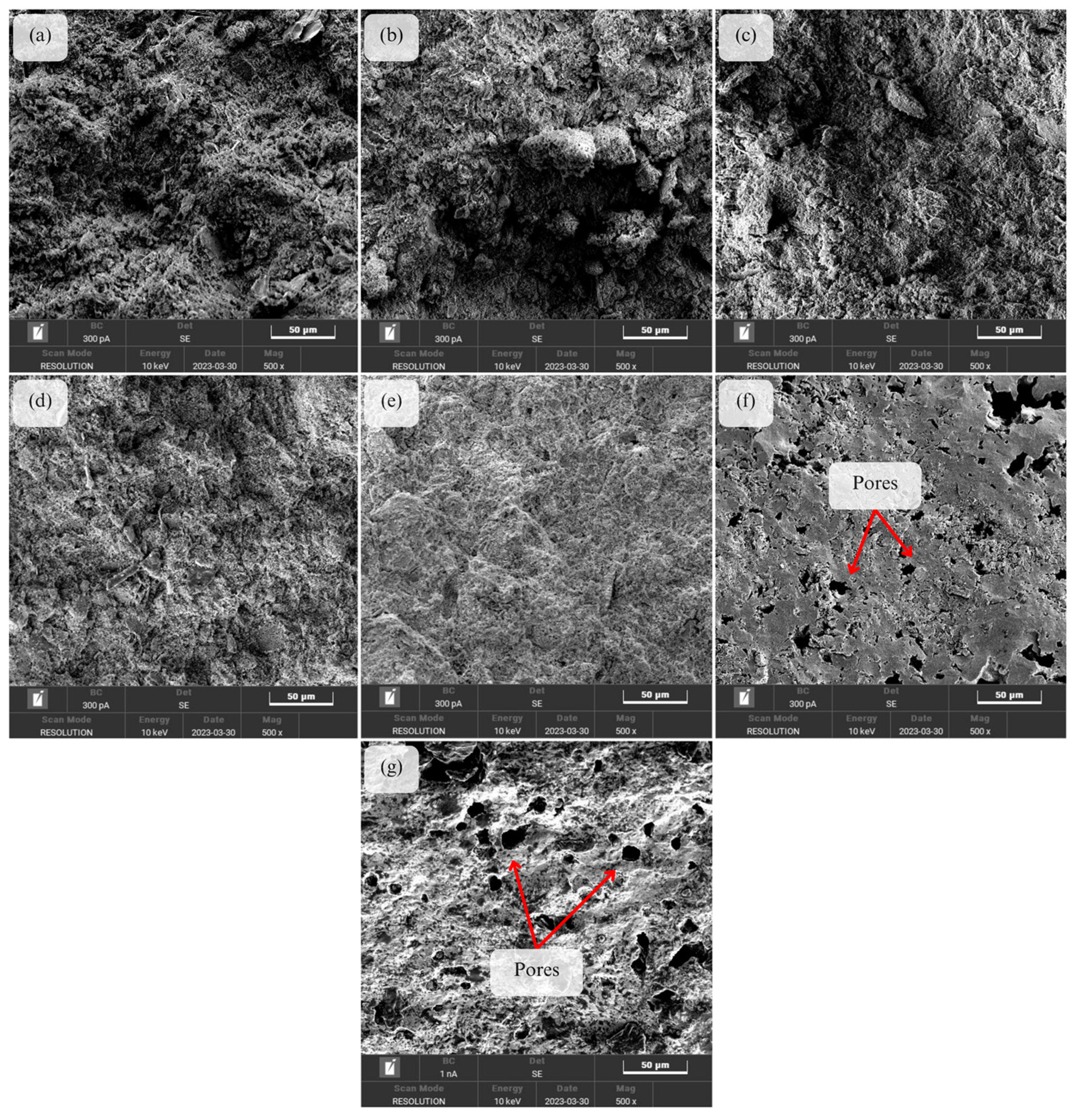
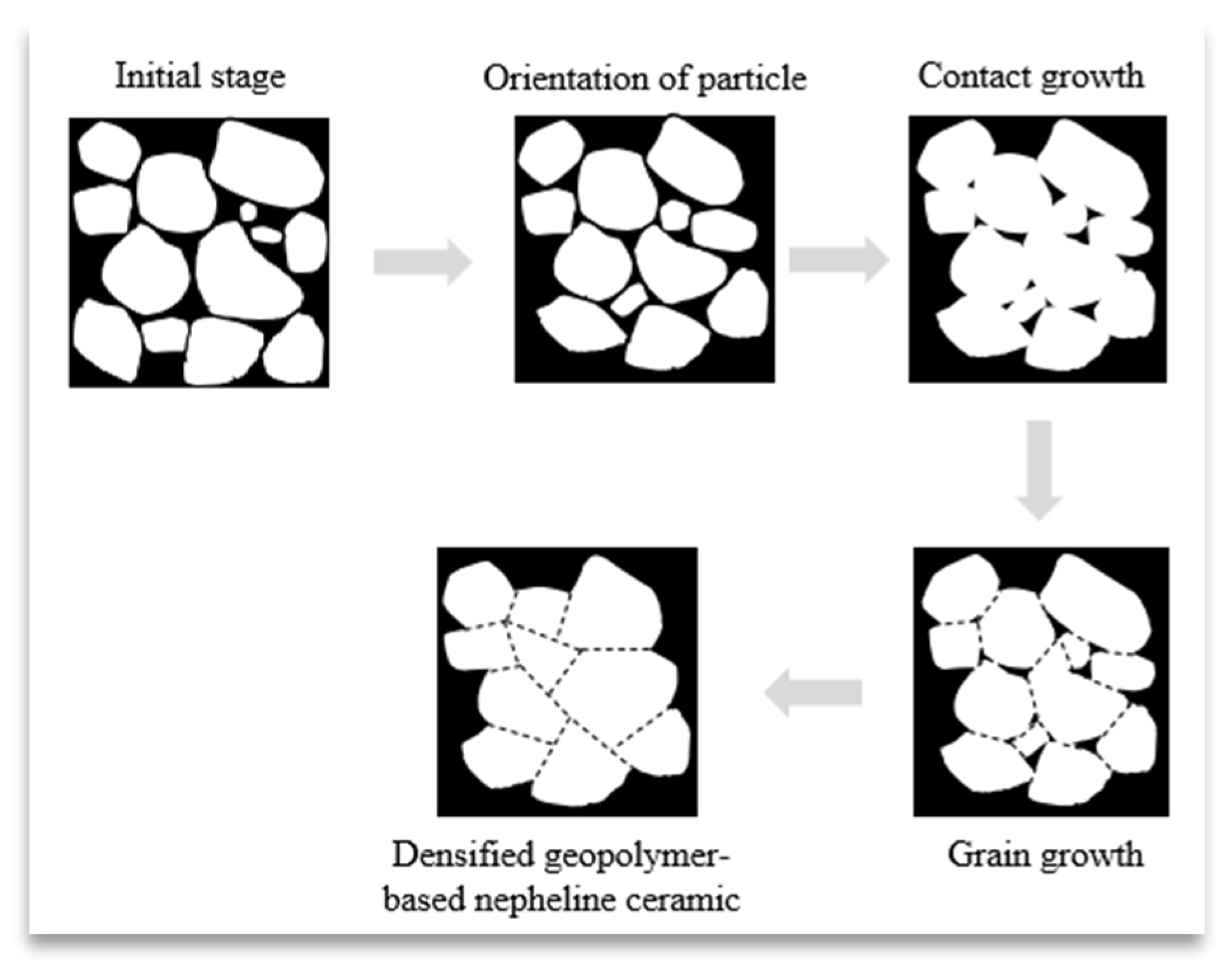
3.2. Morphology Analysis and Porosity of Geopolymer-Based Ceramics
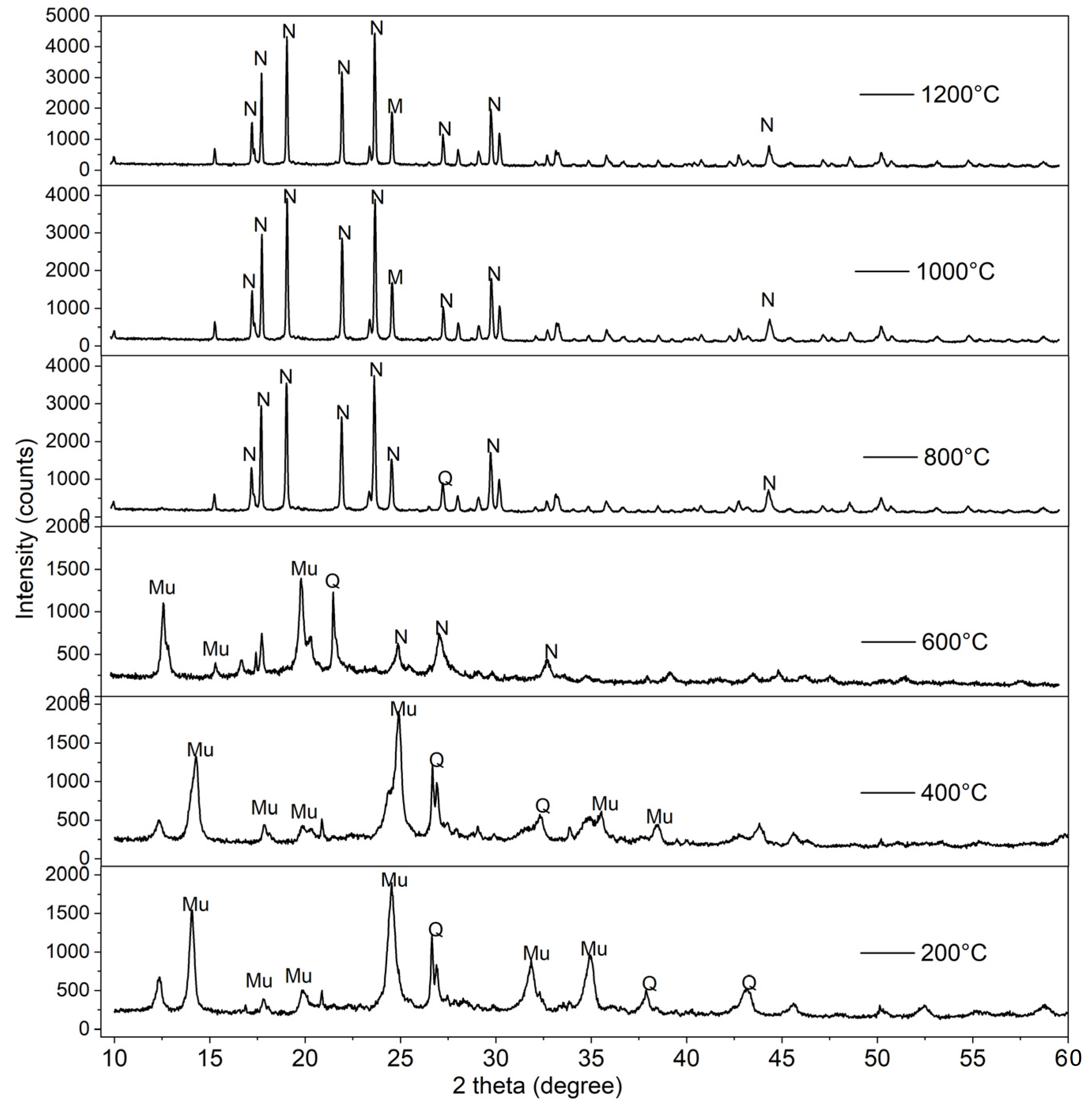
3.3. Phase Analysis of Geopolymer-Based Ceramics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Ren, Q.; Wu, X.; Zheng, J.; Hai, O. Mechanism of low temperature sintered high-strength ferric-rich ceramics using bauxite tailings. Mater. Chem. Phys. 2019, 238, 121929. [Google Scholar] [CrossRef]
- Hofer, A.-K.; Kocjan, A.; Bermejo, R. High-strength lithography-based additive manufacturing of ceramic components with rapid sintering. Addit. Manuf. 2022, 59, 103141. [Google Scholar] [CrossRef]
- Allaoui, D.; Nadi, M.; Hattani, F.; Majdoubi, H.; Haddaji, Y.; Mansouri, S.; Oumam, M.; Hannache, H.; Manoun, B. Eco-friendly geopolymer concrete based on metakaolin and ceramics sanitaryware wastes. Ceram. Int. 2022, 48, 34793–34802. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Lupescu, S.; Burduhos Nergis, D.P.; Perju, M.C.; Sandu, A.V. Microstructural Analysis of Ambient Cured Phosphate Based-Geopolymers with Coal-Ash as Precursor. Arch. Metall. Mater. 2022, 67, 595–600. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers—Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Kovářík, T.; Hájek, J.; Pola, M.; Rieger, D.; Svoboda, M.; Beneš, J.; Šutta, P.; Deshmukh, K.; Jandová, V. Cellular ceramic foam derived from potassium-based geopolymer composite: Thermal, mechanical and structural properties. Mater. Des. 2021, 198, 109355. [Google Scholar] [CrossRef]
- Bykov, Y.V.; Egorov, S.; Eremeev, A.; Holoptsev, V.; Plotnikov, I.; Rybakov, K.; Semenov, V.; Sorokin, A. Temperature profile optimization for microwave sintering of bulk Ni-Al2O3functionally graded materials. J. Mater. Process Technol. 2014, 214, 210–216. [Google Scholar] [CrossRef]
- Yuan, J.; Li, L.; He, P.; Chen, Z.; Lao, C.; Jia, D.; Zhou, Y. Effects of kinds of alkali-activated ions on geopolymerization process of geopolymer cement pastes. Constr. Build. Mater. 2021, 293, 123536. [Google Scholar] [CrossRef]
- Lecomte, I.; Liégeois, M.; Rulmont, A.; Cloots, R.; Maseri, F. Synthesis and characterization of new inorganic polymeric composites based on kaolin or white clay and on ground-granulated blast furnace slag. J. Mater. Res. 2003, 18, 2571–2579. [Google Scholar] [CrossRef]
- Tchadjie, L.N.; Ekolu, S.O. Enhancing the reactivity of aluminosilicate materials toward geopolymer synthesis. J. Mater. Sci. 2017, 53, 4709–4733. [Google Scholar] [CrossRef]
- Taki, K.; Mukherjee, S.; Patel, A.K.; Kumar, M. Reappraisal review on geopolymer: A new era of aluminosilicate binder for metal immobilization. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100345. [Google Scholar] [CrossRef]
- Hamdane, H.; Tamraoui, Y.; Mansouri, S.; Oumam, M.; Bouih, A.; El Ghailassi, T.; Boulif, R.; Manoun, B.; Hannache, H. Effect of alkali-mixed content and thermally untreated phosphate sludge dosages on some properties of metakaolin based geopolymer material. Mater. Chem. Phys. 2020, 248, 122938. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.A.A.M.; Yoriya, S.; Razak, R.A.; Mohamed, R.; Baltatu, M.S. The investigation of ground granulated blast furnace slag geopolymer at high temperature by using electron backscatter diffraction analysis. Arch. Metall. Mater. 2022, 67, 227–231. [Google Scholar] [CrossRef]
- Kohout, J.; Koutník, P.; Hájková, P.; Kohoutová, E.; Soukup, A. Effect of Different Types of Aluminosilicates on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Polymers 2022, 14, 4838. [Google Scholar] [CrossRef] [PubMed]
- Tuyan, M.; Andiç-Çakir, O.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, X.; Yu, Q. Effect of curing conditions on freeze-thaw resistance of geopolymer mortars containing various calcium resources. Constr. Build. Mater. 2021, 313, 125507. [Google Scholar] [CrossRef]
- Luukkonen, T.; Olsen, E.; Turkki, A.; Muurinen, E. Ceramic-like membranes without sintering via alkali activation of metakaolin, blast furnace slag, or their mixture: Characterization and cation-exchange properties. Ceram. Int. 2023, 49, 10645–10651. [Google Scholar] [CrossRef]
- Ahmad, R.; Abdullah, M.M.A.B.; Ibrahim, W.M.W.; Hussin, K.; Zaidi, F.H.A.; Chaiprapa, J.; Wysłocki, J.J.; Błoch, K.; Nabiałek, M. Role of sintering temperature in production of nepheline ceramics-based geopolymer with addition of ultra-high molecular weight polyethylene. Materials 2021, 14, 1077. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Jia, D. Interface evolution of the Cf/leucite composites derived from Cf/geopolymer composites. Ceram. Int. 2013, 39, 1203–1208. [Google Scholar] [CrossRef]
- Mashhadi, M.; Taheri-Nassaj, E.; Sglavo, V.M. Pressureless sintering of boron carbide. Ceram. Int. 2010, 36, 151–159. [Google Scholar] [CrossRef]
- Khattab, R.M.; Wahsh, M.M.S.; Khalil, N.M. Preparation and characterization of porous alumina ceramics through starch consolidation casting technique. Ceram. Int. 2012, 38, 4723–4728. [Google Scholar] [CrossRef]
- Jaya, N.A.; Abdullah, M.M.A.B.; Ghazali, C.M.R.; Hussain, M.; Hussin, K.; Ahmad, R. Characterization and Microstructure of Kaolin-Based Ceramic Using Geopolymerization. Key Eng. Mater. 2016, 700, 3–11. [Google Scholar]
- Ahmad, R.; Ibrahim, W.M.W.; Abdullah, M.M.A.B.; Pakawanit, P.; Vizureanu, P.; Abdullah, A.S.; Sandu, A.V.; Zaidi, F.H.A. Geopolymer-Based Nepheline Ceramics: Effect of Sintering Profile on Morphological Characteristics and Flexural Strength. Crystals 2022, 12, 1313. [Google Scholar]
- Ramli, M.I.I.; Salleh, M.A.A.M.; Abdullah, M.M.A.B.; Aziz, I.H.; Ying, T.C.; Shahedan, N.F.; Kockelmann, W.; Fedrigo, A.; Sandu, A.V.; Vizureanu, P.; et al. The Influence of Sintering Temperature on the Pore Structure of an Alkali-Activated Kaolin-Based Geopolymer Ceramic. Materials 2022, 15, 2667. [Google Scholar] [CrossRef]
- Azevedo, A.R.G.; Vieira, C.M.F.; Ferreira, W.M.; Faria, K.C.P.; Pedroti, L.G.; Mendes, B.C. Potential use of ceramic waste as precursor in the geopolymerization reaction for the production of ceramic roof tiles. J. Build. Eng. 2020, 29, 101156. [Google Scholar]
- He, P.; Jia, D.; Wang, M.; Zhou, Y. Effect of cesium substitution on the thermal evolution and ceramics formation of potassium-based geopolymer. Ceram. Int. 2010, 36, 2395–2400. [Google Scholar] [CrossRef]
- Zhu, Z.; Xiao, J.; He, W.; Wang, T.; Wei, Z.; Dong, Y. A phase-inversion casting process for preparation of tubular porous alumina ceramic membranes. J. Eur. Ceram. Soc. 2015, 35, 3187–3194. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Liu, Y.; Zeng, Q.; Hu, K.; Lu, Z.; Liang, J. Effect of sintering temperature in argon atmosphere on microstructure and properties of 3D printed alumina ceramic cores. J. Adv. Ceram. 2020, 9, 220–231. [Google Scholar] [CrossRef]
- Chen, W.; Garofalo, A.C.; Geng, H.; Liu, Y.; Wang, D.; Li, Q. Effect of high temperature heating on the microstructure and performance of cesium-based geopolymer reinforced by cordierite. Cem. Concr. Compos. 2022, 129, 104474. [Google Scholar] [CrossRef]
- Arriagada, C.; Navarrete, I.; Lopez, M. Understanding the effect of porosity on the mechanical and thermal performance of glass foam lightweight aggregates and the influence of production factors. Constr. Build. Mater. 2019, 228, 116746. [Google Scholar] [CrossRef]
- Payakaniti, P.; Chuewangkam, N.; Yensano, R.; Pinitsoontorn, S.; Chindaprasirt, P. Changes in compressive strength, microstructure and magnetic properties of a high-calcium fly ash geopolymer subjected to high temperatures. Constr. Build. Mater. 2020, 265, 120650. [Google Scholar] [CrossRef]
- Bih, N.L.; Mahamat, A.A.; Hounkpè, J.B.; Onwualu, P.A.; Boakye, E.E. The Effect of Polymer Waste Addition on the Compressive Strength and Water Absorption of Geopolymer Ceramics. Appl. Sci. 2021, 11, 3540. [Google Scholar] [CrossRef]



| Element | SiO2 | Al2O3 | K2O | Fe2O3 | TiO2 | MnO2 | ZrO2 | LOI |
|---|---|---|---|---|---|---|---|---|
| Wt. (%) | 54.0 | 31.7 | 6.05 | 4.89 | 1.14 | 0.11 | 0.10 | 1.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapa, N.B.; Ahmad, R.; Al Bakri Abdullah, M.M.; Ibrahim, W.M.W.; Sandu, A.V.; Nemes, O.; Vizureanu, P.; Kartikowati, C.W.; Risdanareni, P. Effect of the Sintering Mechanism on the Crystallization Kinetics of Geopolymer-Based Ceramics. Materials 2023, 16, 5853. https://doi.org/10.3390/ma16175853
Mustapa NB, Ahmad R, Al Bakri Abdullah MM, Ibrahim WMW, Sandu AV, Nemes O, Vizureanu P, Kartikowati CW, Risdanareni P. Effect of the Sintering Mechanism on the Crystallization Kinetics of Geopolymer-Based Ceramics. Materials. 2023; 16(17):5853. https://doi.org/10.3390/ma16175853
Chicago/Turabian StyleMustapa, Nur Bahijah, Romisuhani Ahmad, Mohd Mustafa Al Bakri Abdullah, Wan Mastura Wan Ibrahim, Andrei Victor Sandu, Ovidiu Nemes, Petrica Vizureanu, Christina W. Kartikowati, and Puput Risdanareni. 2023. "Effect of the Sintering Mechanism on the Crystallization Kinetics of Geopolymer-Based Ceramics" Materials 16, no. 17: 5853. https://doi.org/10.3390/ma16175853











