Socket Preservation Using Dentin Mixed with Xenograft Materials: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
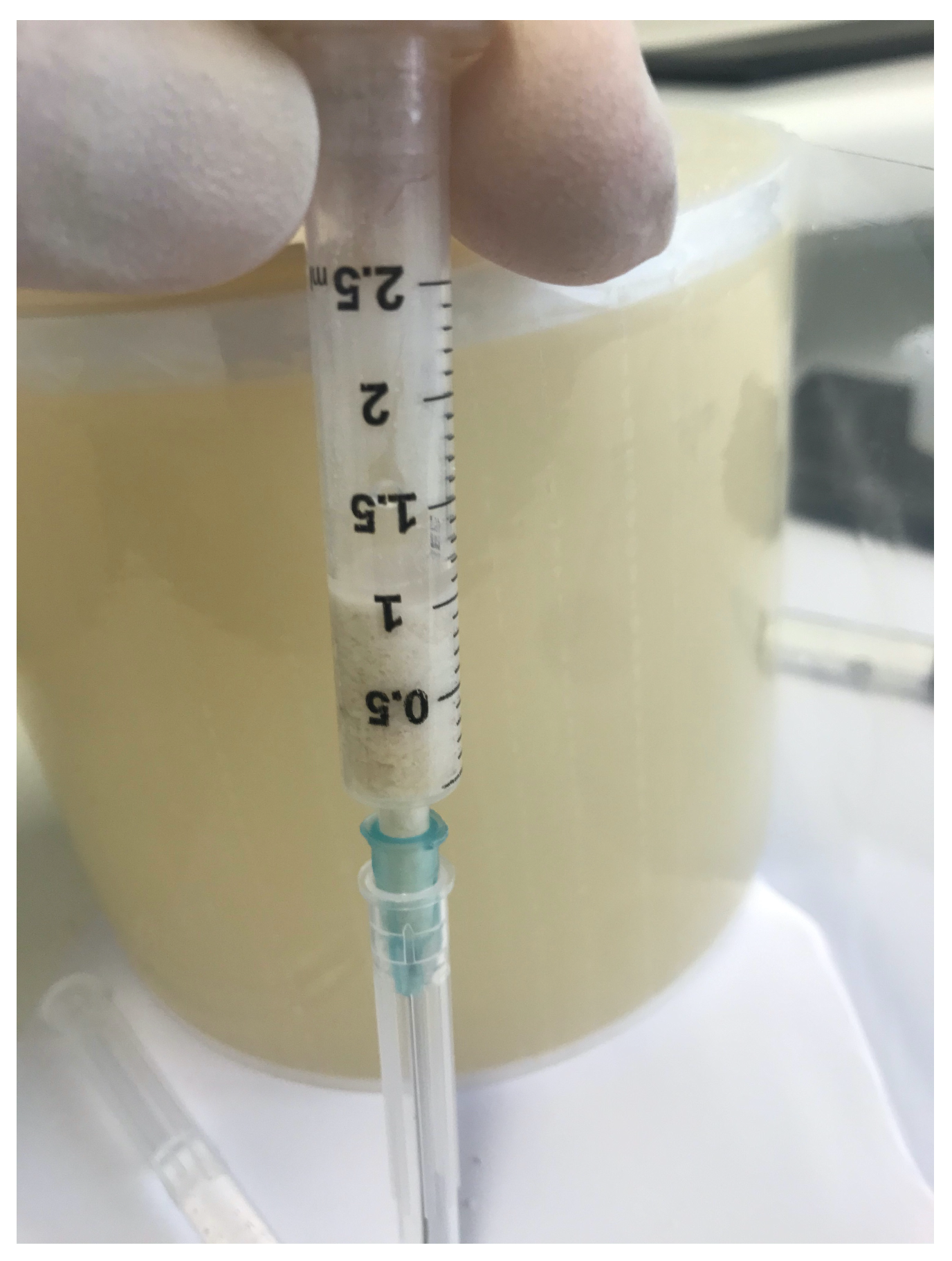
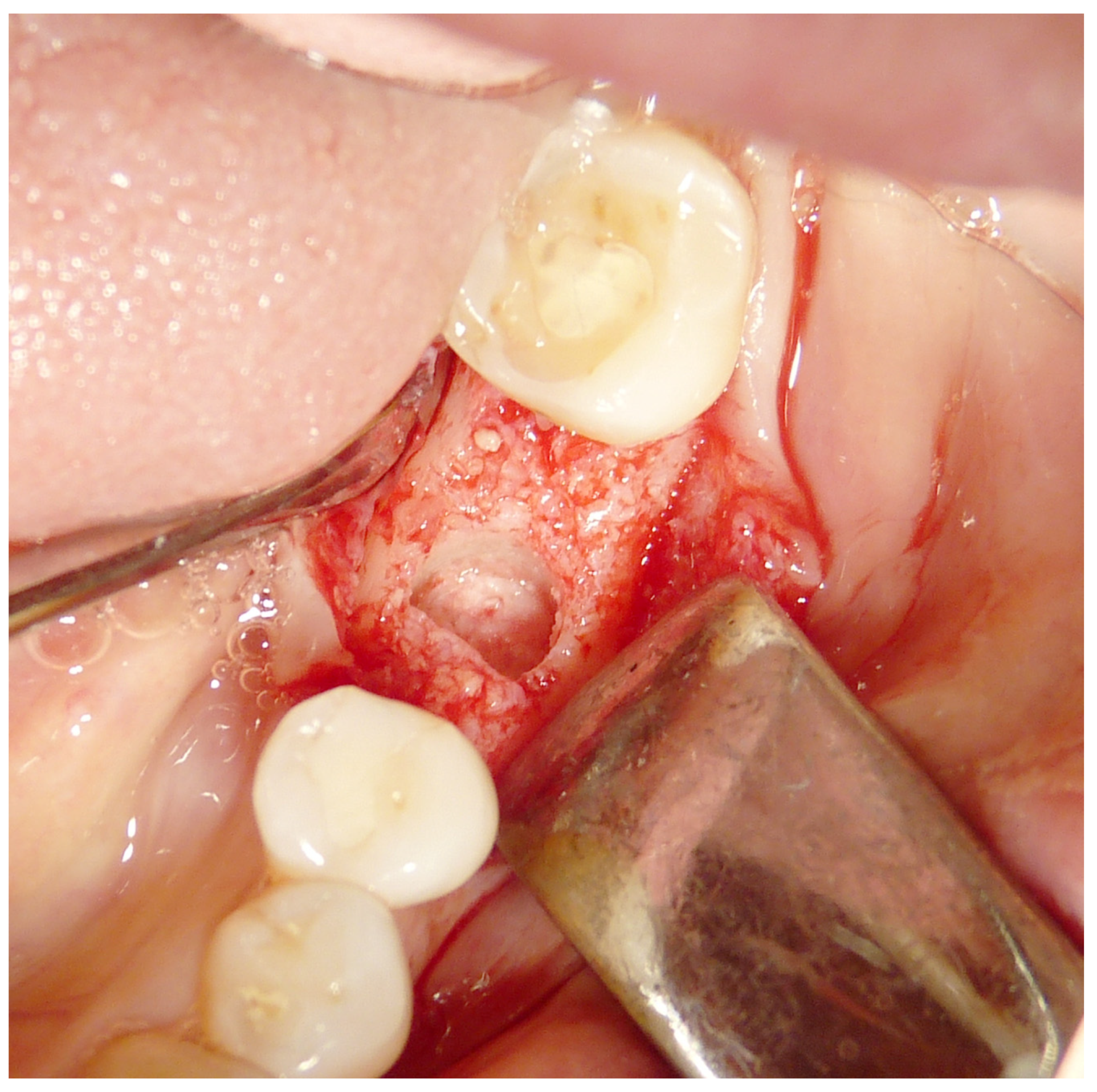
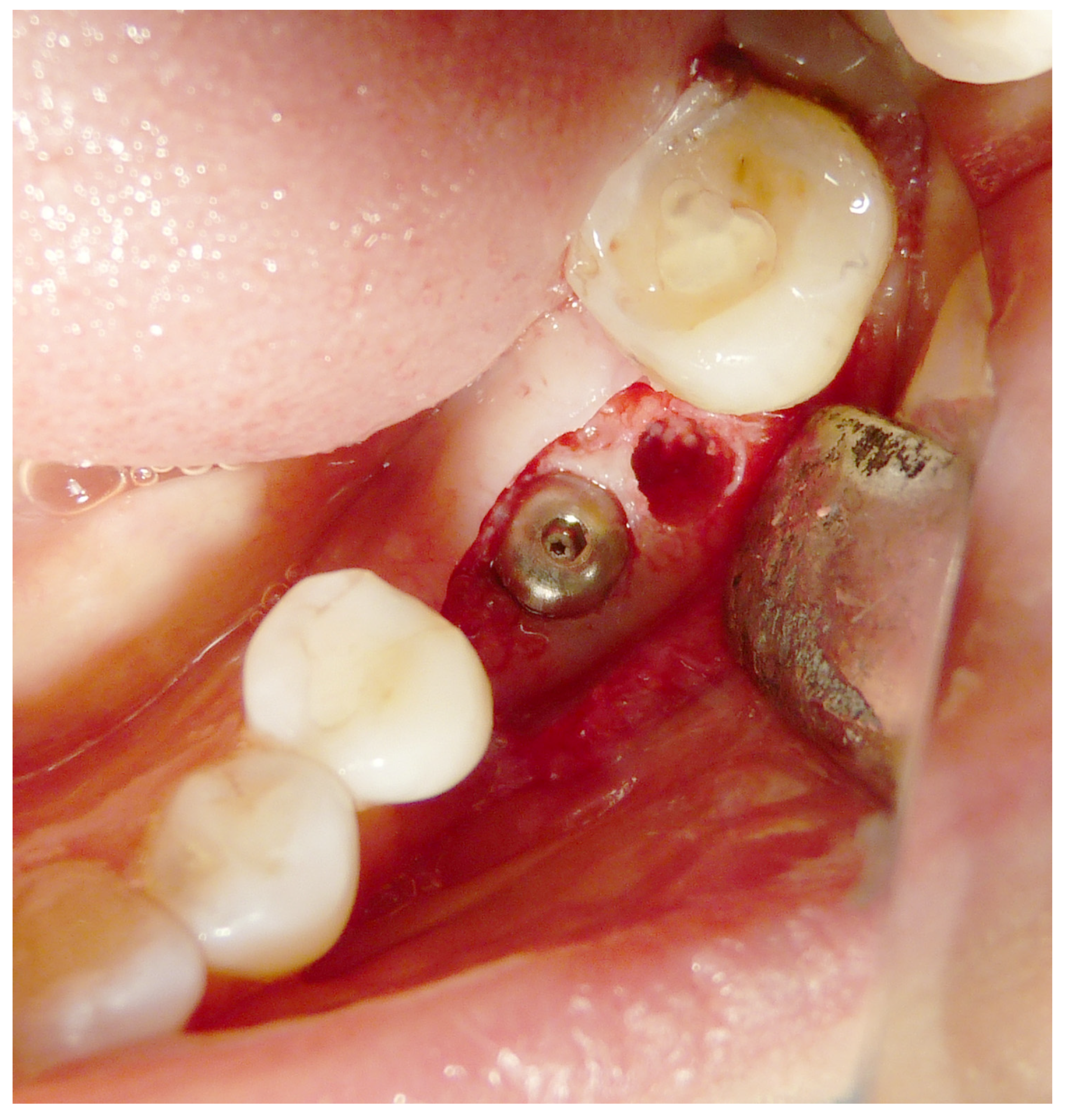
2.4. Surgical Procedures and Follow-Up
2.5. Collection and Statistical Analysis of Data
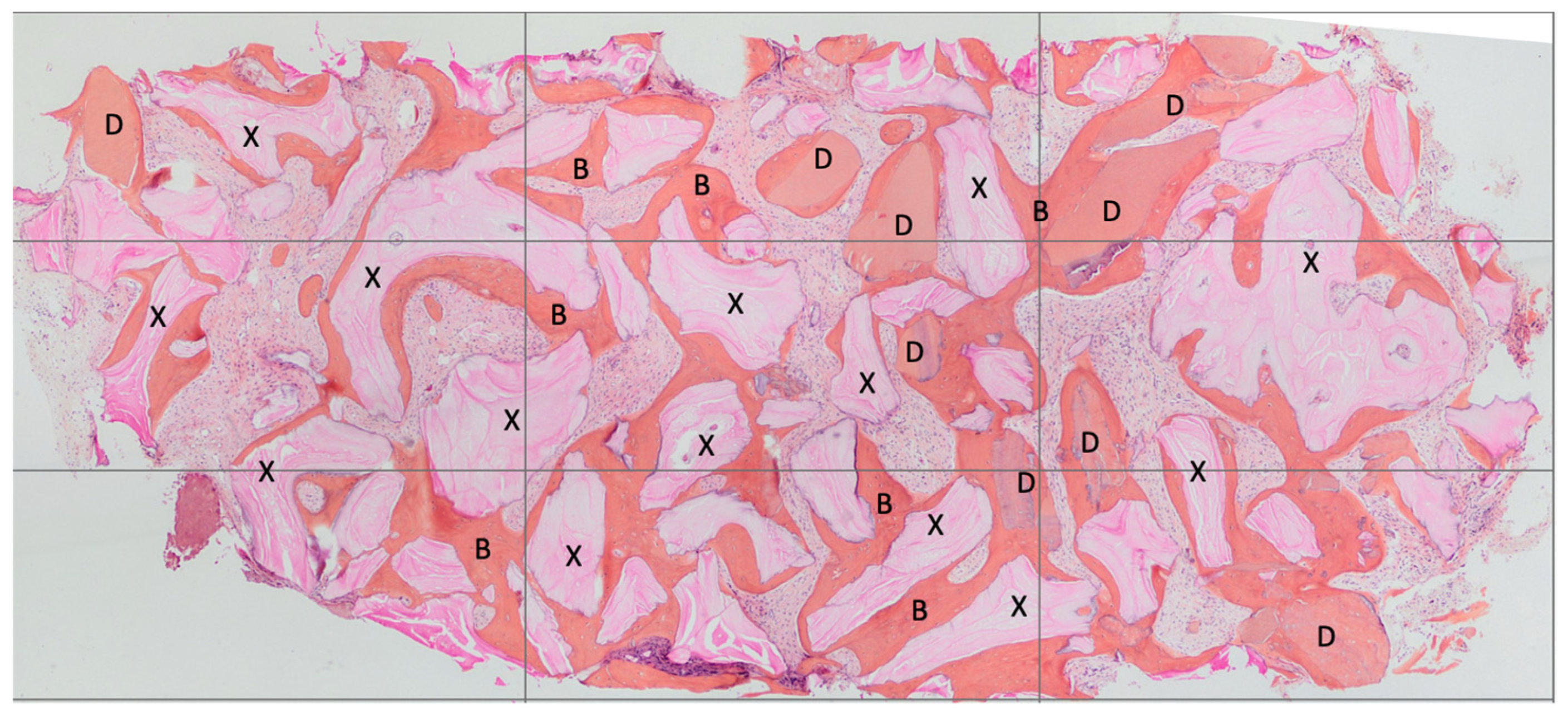
2.6. Histological Technique
- Decalcified:
- BV%: Percentage of mineralized tissue excluding medullary tissues.
- Graft%: Percentage of the volume occupied by the remaining graft or dentin.
- VB%: Percentage of vital bone excluding medullary tissues. The sum of TT% and VB% represented the BV%. Each subsection was measured using the ImageJ 1.52q program.
- Non-decalcified:
- BV% = Percentage of residual bone volume, excluding medullary tissues;
- Graft% = Percentage of the remaining graft, without bone and marrow;
- VB% = Percentage of vital bone, excluding the bone marrow and the residual graft.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMPs | Bone Morphogenetic Proteins |
| ECM | Extracellular Matrix |
| TT | Tooth Transformer |
| BV | Bone Volume |
| NB | New Bone |
References
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar Bone Dimensional Changes of Post-Extraction Sockets in Humans: A Systematic Review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone Healing and Soft Tissue Contour Changes Following Single-Tooth Extraction: A Clinical and Radiographic 12-Month Prospective Study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Vittorini Orgeas, G.; Clementini, M.; De Risi, V.; de Sanctis, M. Surgical Techniques for Alveolar Socket Preservation: A Systematic Review. Int. J. Oral. Maxillofac. Implant. 2013, 28, 1049–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minetti, E.; Taschieri, S.; Corbella, S. Autologous Deciduous Tooth-Derived Material for Alveolar Ridge Preservation: A Clinical and Histological Case Report. Case Rep. Dent. 2020, 2020, 2936878. [Google Scholar] [CrossRef] [PubMed]
- Brugnami, F.; Then, P.R.; Moroi, H.; Leone, C.W. Histologic Evaluation of Human Extraction Sockets Treated with Demineralized Freeze-Dried Bone Allograft (DFDBA) and Cell Occlusive Membrane. J. Periodontol. 1996, 67, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Carmagnola, D.; Adriaens, P.; Berglundh, T. Healing of Human Extraction Sockets Filled with Bio-Oss. Clin. Oral. Implant. Res. 2003, 14, 137–143. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral. Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of Bone Defects by Guided Tissue Regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Lindhe, J.; Cecchinato, D.; Donati, M.; Tomasi, C.; Liljenberg, B. Ridge Preservation with the Use of Deproteinized Bovine Bone Mineral. Clin. Oral. Implant. Res. 2014, 25, 786–790. [Google Scholar] [CrossRef]
- Bernardi, S.; Mummolo, S.; Tecco, S.; Continenza, M.; Marzo, G. Histological Characterization of Sacco’s Concentrated Growth Factors Membrane. Int. J. Morphol. 2017, 35, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Rapone, B.; Inchingolo, A.D.; Trasarti, S.; Ferrara, E.; Qorri, E.; Mancini, A.; Montemurro, N.; Scarano, A.; Inchingolo, A.M.; Dipalma, G.; et al. Long-Term Outcomes of Implants Placed in Maxillary Sinus Floor Augmentation with Porous Fluorohydroxyapatite (Algipore® FRIOS®) in Comparison with Anorganic Bovine Bone (Bio-Oss®) and Platelet Rich Plasma (PRP): A Retrospective Study. J. Clin. Med. 2022, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Odell, E.W.; Thompson, I.D.; Cook, R.J. Efficacy of Bovine Bone Mineral for Alveolar Augmentation: A Human Histologic Study. Clin. Oral. Implant. Res. 2003, 14, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Nagursky, H.; Lozada, J.L.; Nagy, K. Horizontal Ridge Augmentation with a Collagen Membrane and a Combination of Particulated Autogenous Bone and Anorganic Bovine Bone-Derived Mineral: A Prospective Case Series in 25 Patients. Int. J. Periodontics Restor. Dent. 2013, 33, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Stavola, L.; Tunkel, J. A New Approach to Maintenance of Regenerated Autogenous Bone Volume: Delayed Relining with Xenograft and Resorbable Membrane. Int. J. Oral. Maxillofac. Implant. 2013, 28, 1062–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijndert, L.; Raghoebar, G.M.; Schüpbach, P.; Meijer, H.J.A.; Vissink, A. Bone Quality at the Implant Site after Reconstruction of a Local Defect of the Maxillary Anterior Ridge with Chin Bone or Deproteinised Cancellous Bovine Bone. Int. J. Oral. Maxillofac. Surg. 2005, 34, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Von Arx, T.; Hardt, N.; Wallkamm, B. The TIME Technique: A New Method for Localized Alveolar Ridge Augmentation Prior to Placement of Dental Implants. Int. J. Oral. Maxillofac. Implant. 1996, 11, 387–394. [Google Scholar]
- Corbella, S.; Taschieri, S.; Francetti, L.; Weinstein, R.; Del Fabbro, M. Histomorphometric Results After Postextraction Socket Healing with Different Biomaterials: A Systematic Review of the Literature and Meta-Analysis. Int. J. Oral. Maxillofac. Implant. 2017, 32, 1001–1017. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L.; Cardaropoli, G. Socket Preservation Using Bovine Bone Mineral and Collagen Membrane: A Randomized Controlled Clinical Trial with Histologic Analysis. Int. J. Periodontics Restor. Dent. 2012, 32, 421–430. [Google Scholar]
- Scarano, A.; Inchingolo, F.; Murmura, G.; Traini, T.; Piattelli, A.; Lorusso, F. Three-Dimensional Architecture and Mechanical Properties of Bovine Bone Mixed with Autologous Platelet Liquid, Blood, or Physiological Water: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1230. [Google Scholar] [CrossRef] [Green Version]
- Pang, K.-M.; Um, I.-W.; Kim, Y.-K.; Woo, J.-M.; Kim, S.-M.; Lee, J.-H. Autogenous Demineralized Dentin Matrix from Extracted Tooth for the Augmentation of Alveolar Bone Defect: A Prospective Randomized Clinical Trial in Comparison with Anorganic Bovine Bone. Clin. Oral. Implant. Res. 2017, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Yun, P.-Y.; Yeo, I.-S.; Jin, S.-C.; Oh, J.-S.; Kim, H.-J.; Yu, S.-K.; Lee, S.-Y.; Kim, J.-S.; et al. Autogenous Teeth Used for Bone Grafting: A Comparison with Traditional Grafting Materials. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2014, 117, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, A.S.; Bhatavadekar, N.B. Clinical Efficacy of Tooth-Bone Graft: A Systematic Review and Risk of Bias Analysis of Randomized Control Trials and Observational Studies. Implant. Dent. 2018, 27, 119–134. [Google Scholar] [CrossRef]
- Del Canto-Díaz, A.; de Elío-Oliveros, J.; Del Canto-Díaz, M.; Alobera-Gracia, M.-A.; Del Canto-Pingarrón, M.; Martínez-González, J.-M. Use of Autologous Tooth-Derived Graft Material in the Post-Extraction Dental Socket. Pilot Study. Med. Oral. Patol. Oral. Cir. Bucal 2019, 24, e53–e60. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Nevins, M.; Schupbach, P. New Bone Formation Using an Extracted Tooth as a Biomaterial: A Case Report with Histologic Evidence. Int. J. Periodontics Restor. Dent. 2019, 39, 157–163. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Um, I.-W.; Kim, K.-W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-Derived Bone Graft Material. J. Korean Assoc. Oral. Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef]
- Yeomans, J.D.; Urist, M.R. Bone Induction by Decalcified Dentine Implanted into Oral, Osseous and Muscle Tissues. Arch. Oral. Biol. 1967, 12, 999-IN16. [Google Scholar] [CrossRef]
- Bang, G.; Urist, M.R. Bone Induction in Excavation Chambers in Matrix of Decalcified Dentin. Arch. Surg. 1967, 94, 781–789. [Google Scholar] [CrossRef]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human Dentin-Matrix-Derived Bone Morphogenetic Protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A.; Malcangi, G.; Inchingolo, A.D.; Mancini, A.; Dipalma, G.; Inchingolo, F.; Patano, A.; Inchingolo, A.M. Dentin, Dentin Graft, and Bone Graft: Microscopic and Spectroscopic Analysis. J. Funct. Biomater. 2023, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Hazballa, D.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; Minetti, E.; Di Venere, D.; Limongelli, L.; Bordea, I.R.; Scarano, A.; et al. The Effectiveness of Autologous Demineralized Tooth Graft for the Bone Ridge Preservation: A Systematic Review of the Literature. J. Biol. Regul. Homeost. Agents 2021, 35, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Berardini, M.; Trisi, P. A New Tooth Processing Apparatus Allowing to Obtain Dentin Grafts for Bone Augmentation: The Tooth Transformer. Open Dent. J. 2019, 13, 6–14. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Ferrante, F.; Schmitz, J.H.; Lung Ho, H.K.; Dih Hann, S.N.; Giacometti, E.; Gambardella, U.; Contessi, M.; Celko, M.; et al. Autologous Tooth Graft after Endodontical Treated Used for Socket Preservation: A Multicenter Clinical Study. Appl. Sci. 2019, 9, 5396. [Google Scholar] [CrossRef] [Green Version]
- Minetti, E.; Giacometti, E.; Gambardella, U.; Contessi, M.; Ballini, A.; Marenzi, G.; Celko, M.; Mastrangelo, F. Alveolar Socket Preservation with Different Autologous Graft Materials: Preliminary Results of a Multicenter Pilot Study in Human. Materials 2020, 13, 1153. [Google Scholar] [CrossRef] [Green Version]
- Minamizato, T.; Koga, T.; Takashi, I.; Nakatani, Y.; Umebayashi, M.; Sumita, Y.; Ikeda, T.; Asahina, I. Clinical Application of Autogenous Partially Demineralized Dentin Matrix Prepared Immediately after Extraction for Alveolar Bone Regeneration in Implant Dentistry: A Pilot Study. Int. J. Oral. Maxillofac. Surg. 2018, 47, 125–132. [Google Scholar] [CrossRef]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Appl. Sci. 2020, 10, 5084. [Google Scholar] [CrossRef]
- Inchingolo, F.; Dipalma, G.; Paduanelli, G.; De Oliveira, L.A.; Inchingolo, A.M.; Georgakopoulos, P.I.; Inchingolo, A.D.; Malcangi, G.; Athanasiou, E.; Fotopoulou, E.; et al. Computer-Based Quantification of an Atraumatic Sinus Augmentation Technique Using CBCT. J. Biol. Regul. Homeost. Agents 2019, 33, 31–39. [Google Scholar]
- Kim, S.-Y.; Kim, Y.-K.; Park, Y.-H.; Park, J.-C.; Ku, J.-K.; Um, I.-W.; Kim, J.-Y. Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts. Materials 2017, 10, 1049. [Google Scholar] [CrossRef] [Green Version]
- Memè, L.; Strappa, E.M.; Monterubbianesi, R.; Bambini, F.; Mummolo, S. SEM and FT-MIR Analysis of Human Demineralized Dentin Matrix: An In Vitro Study. Appl. Sci. 2022, 12, 1480. [Google Scholar] [CrossRef]
- Rijal, G.; Shin, H.-I. Human Tooth-Derived Biomaterial as a Graft Substitute for Hard Tissue Regeneration. Regen. Med. 2017, 12, 263–273. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, S.-G.; Oh, J.-S.; Jin, S.-C.; Son, J.-S.; Kim, S.-Y.; Lim, S.-Y. Analysis of the Inorganic Component of Autogenous Tooth Bone Graft Material. J. Nanosci. Nanotechnol. 2011, 11, 7442–7445. [Google Scholar] [CrossRef]
- Bono, N.; Tarsini, P.; Candiani, G. Demineralized Dentin and Enamel Matrices as Suitable Substrates for Bone Regeneration. J. Appl. Biomater. Funct. Mater. 2017, 15, e236–e243. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Schultz, T.H.; Schultz, M. Intact Growth Factors Are Conserved in the Extracellular Matrix of Ancient Human Bone and Teeth: A Storehouse for the Study of Human Evolution in Health and Disease. Biol. Chem. 2005, 386, 767–776. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Andreacchio, D.; Köster, G.; Carnes, D.L.; Cochran, D.L.; Dean, D.D.; Boyan, B.D.; Schwartz, Z. Tissue Response and Osteoinduction of Human Bone Grafts in Vivo. Arch. Orthop. Trauma. Surg. 2001, 121, 583–590. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef]
- Memè, L.; Bambini, F.; Gallusi, G.; Sartini, D.; Pozzi, V.; Emanuelli, M.; Strappa, E.M.; Mummolo, S. The Effect and the Potential Use of Magnetic–Dam Barrier in Guided Bone Regeneration: A Laboratory Study. Appl. Sci. 2023, 13, 1625. [Google Scholar] [CrossRef]
- Umebayashi, M.; Ohba, S.; Kurogi, T.; Noda, S.; Asahina, I. Full Regeneration of Maxillary Alveolar Bone Using Autogenous Partially Demineralized Dentin Matrix and Particulate Cancellous Bone and Marrow for Implant-Supported Full Arch Rehabilitation. J. Oral. Implantol. 2020, 46, 122–127. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Wang, F.; Wang, Z. Maxillary Sinus Floor Augmentation and Dental Implant Placement Using Dentin Matrix Protein-1 Gene-Modified Bone Marrow Stromal Cells Mixed with Deproteinized Boving Bone: A Comparative Study in Beagles. Arch. Oral. Biol. 2016, 64, 102–108. [Google Scholar] [CrossRef]
- Park, S.-H.; Choi, H.; Han, J.-S.; Park, Y.-B. Comparative Study of Decalcification versus Nondecalcification for Histological Evaluation of One-Wall Periodontal Intrabony Defects in Dogs. Microsc. Res. Tech. 2015, 78, 94–104. [Google Scholar] [CrossRef]
- Bernardi, S.; Mummolo, S.; Varvara, G.; Marchetti, E.; Continenza, M.A.; Marzo, G.; Macchiarelli, G. Bio-Morphological Evaluation of Periodontal Ligament Fibroblasts on Mineralized Dentin Graft: An in Vitro Study. J. Biol. Regul. Homeost. Agents 2019, 33, 275–280. [Google Scholar]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- MacBeth, N.; Trullenque-Eriksson, A.; Donos, N.; Mardas, N. Hard and Soft Tissue Changes Following Alveolar Ridge Preservation: A Systematic Review. Clin. Oral. Implant. Res. 2017, 28, 982–1004. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.; Payne, A.G.; Duncan, W.; Faggion, C.M.; Esposito, M. Interventions for Replacing Missing Teeth: Alveolar Ridge Preservation Techniques for Dental Implant Site Development. Cochrane Database Syst. Rev. 2015, 2015, CD010176. [Google Scholar] [CrossRef]
- Kim, E.-S.; Lee, I.-K.; Kang, J.-Y.; Lee, E.-Y. Various Autogenous Fresh Demineralized Tooth Forms for Alveolar Socket Preservation in Anterior Tooth Extraction Sites: A Series of 4 Cases. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 27. [Google Scholar] [CrossRef] [Green Version]
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J. Funct. Biomater. 2022, 13, 121. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Scacco, S.; Inchingolo, A.D.; Dipalma, G.; Vermesan, D.; Abbinante, A.; Cagiano, R. Trial with Platelet-Rich Fibrin and Bio-Oss Used as Grafting Materials in the Treatment of the Severe Maxillar Bone Atrophy: Clinical and Radiological Evaluations. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 1075–1084. [Google Scholar]
- Dimonte, M.; Inchingolo, F.; Dipalma, G.; Stefanelli, M. [Maxillary sinus lift in conjunction with endosseous implants. A long-term follow-up scintigraphic study]. Minerva Stomatol. 2002, 51, 161–165. [Google Scholar]
- Wenisch, S.; Stahl, J.-P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In Vivo Mechanisms of Hydroxyapatite Ceramic Degradation by Osteoclasts: Fine Structural Microscopy. J. Biomed. Mater. Res. A 2003, 67, 713–718. [Google Scholar] [CrossRef]
- Detsch, R.; Mayr, H.; Ziegler, G. Formation of Osteoclast-like Cells on HA and TCP Ceramics. Acta Biomater. 2008, 4, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Corbella, S.; Taschieri, S.; Canullo, L. Tooth as Graft Material: Histologic Study. Clin. Implant. Dent. Relat. Res. 2022, 24, 488–496. [Google Scholar] [CrossRef] [PubMed]

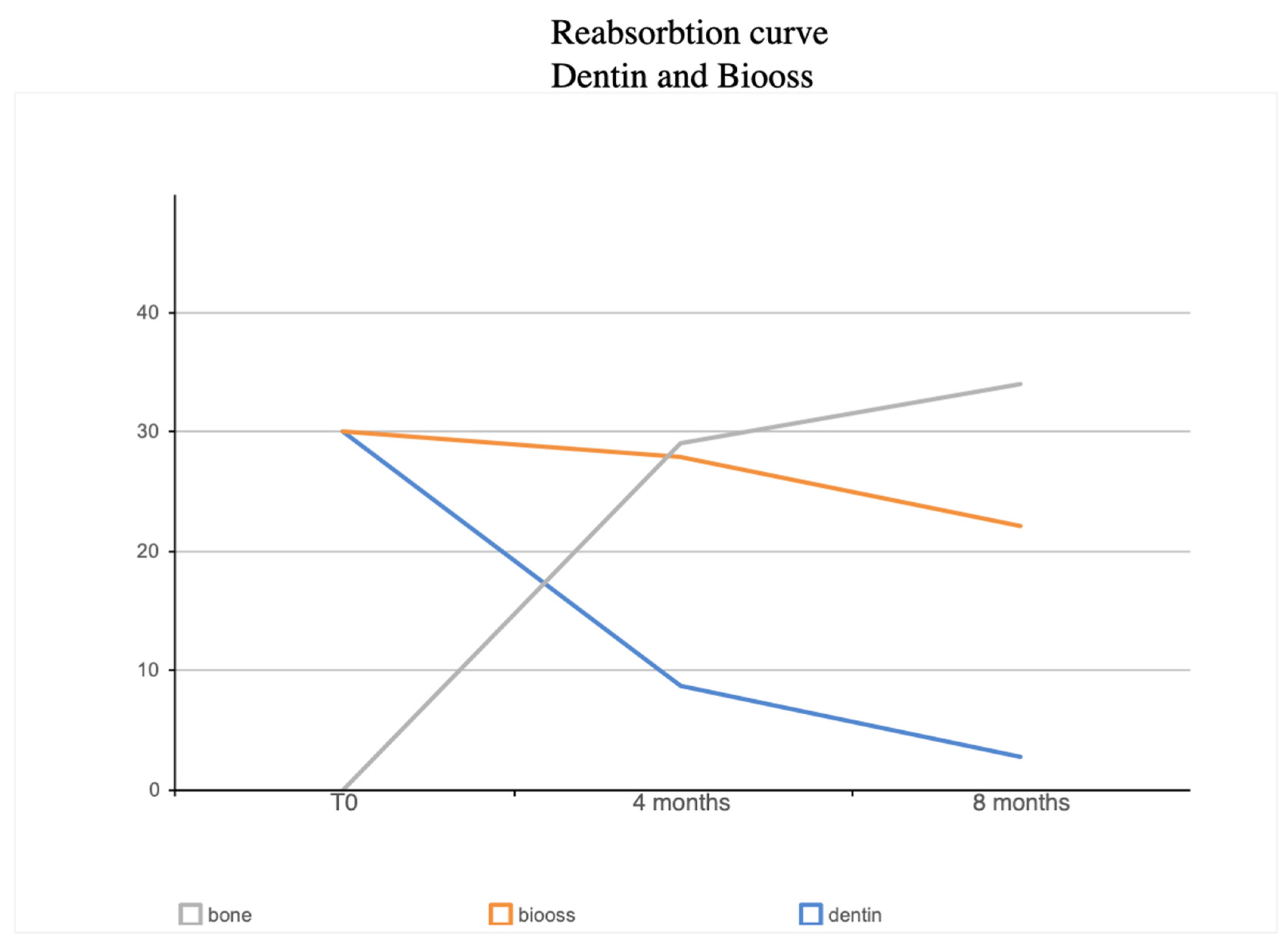
| BV% | NB% | Residual Graft Dentin % | Dentin Loss Percentage | Residual Graft Bio-Oss % | Bio-Oss Loss Percentage | |
|---|---|---|---|---|---|---|
| 4 Months | 65.66% | 29.03± 6.57% | 8.68 ± 3.36% | 71% | 27.95 ± 5.23% | 6.74% |
| 8 Months | 59% | 34.12 ± 5.05 | 2.79 ± 1.48 | 90.71% | 22.09 ± 13.81 | 26.42% |
| Granules | Water | |
|---|---|---|
| Percentage of space 100% | 60% | 40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minetti, E.; Palermo, A.; Savadori, P.; Patano, A.; Inchingolo, A.D.; Rapone, B.; Malcangi, G.; Inchingolo, F.; Dipalma, G.; Tartaglia, F.C.; et al. Socket Preservation Using Dentin Mixed with Xenograft Materials: A Pilot Study. Materials 2023, 16, 4945. https://doi.org/10.3390/ma16144945
Minetti E, Palermo A, Savadori P, Patano A, Inchingolo AD, Rapone B, Malcangi G, Inchingolo F, Dipalma G, Tartaglia FC, et al. Socket Preservation Using Dentin Mixed with Xenograft Materials: A Pilot Study. Materials. 2023; 16(14):4945. https://doi.org/10.3390/ma16144945
Chicago/Turabian StyleMinetti, Elio, Andrea Palermo, Paolo Savadori, Assunta Patano, Alessio Danilo Inchingolo, Biagio Rapone, Giuseppina Malcangi, Francesco Inchingolo, Gianna Dipalma, Francesco Carlo Tartaglia, and et al. 2023. "Socket Preservation Using Dentin Mixed with Xenograft Materials: A Pilot Study" Materials 16, no. 14: 4945. https://doi.org/10.3390/ma16144945










