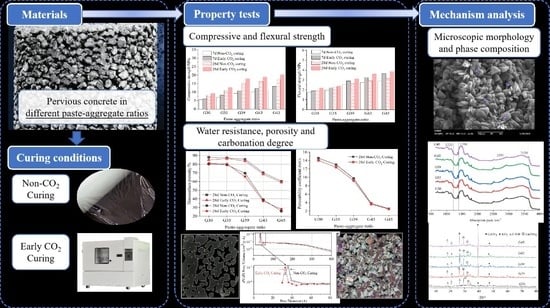
Effects of CO2 Curing on the Properties of Pervious Concrete in Different Paste–Aggregate Ratios
Abstract
:1. Introduction
2. Material and Experiments
2.1. Raw Materials
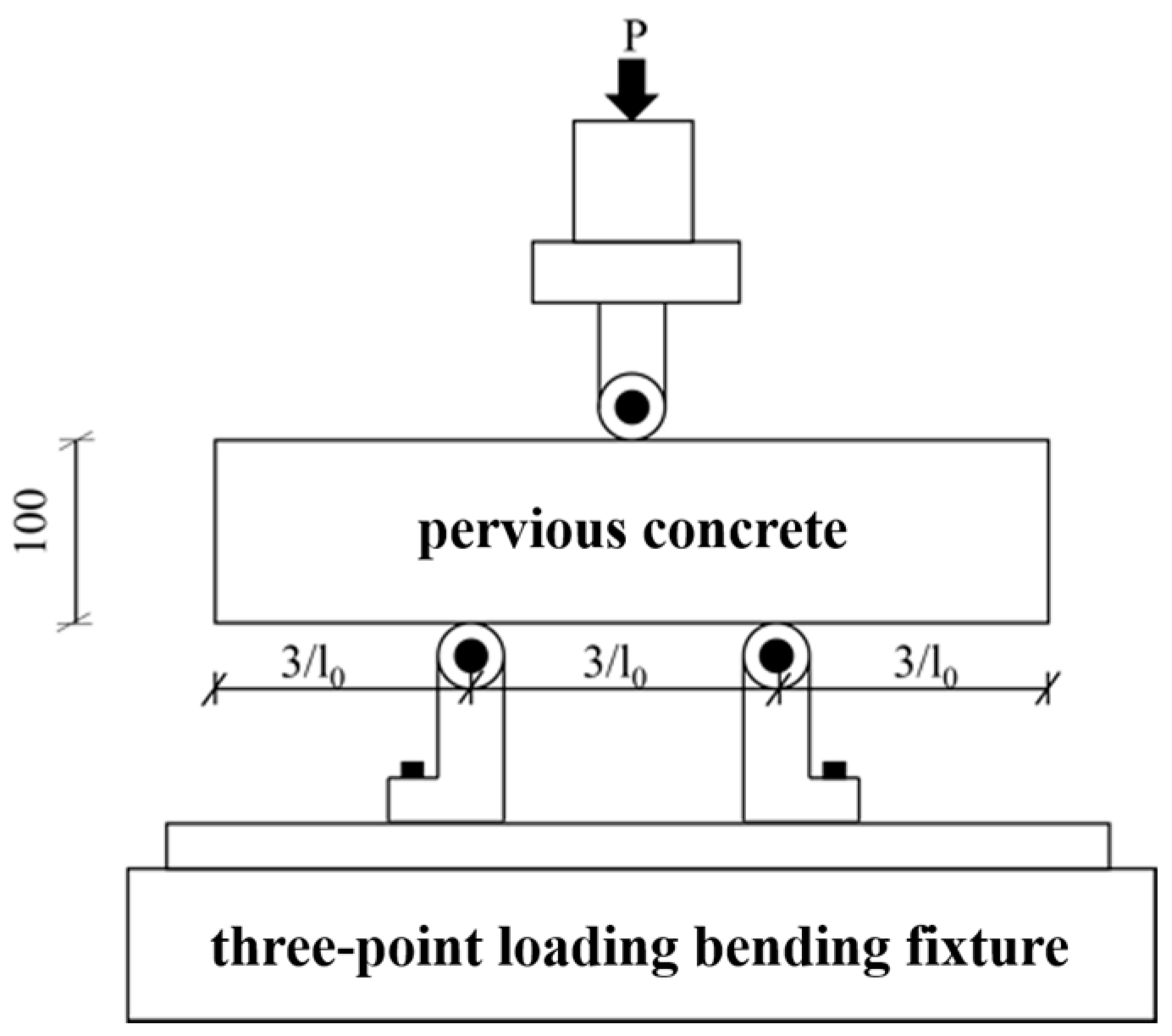
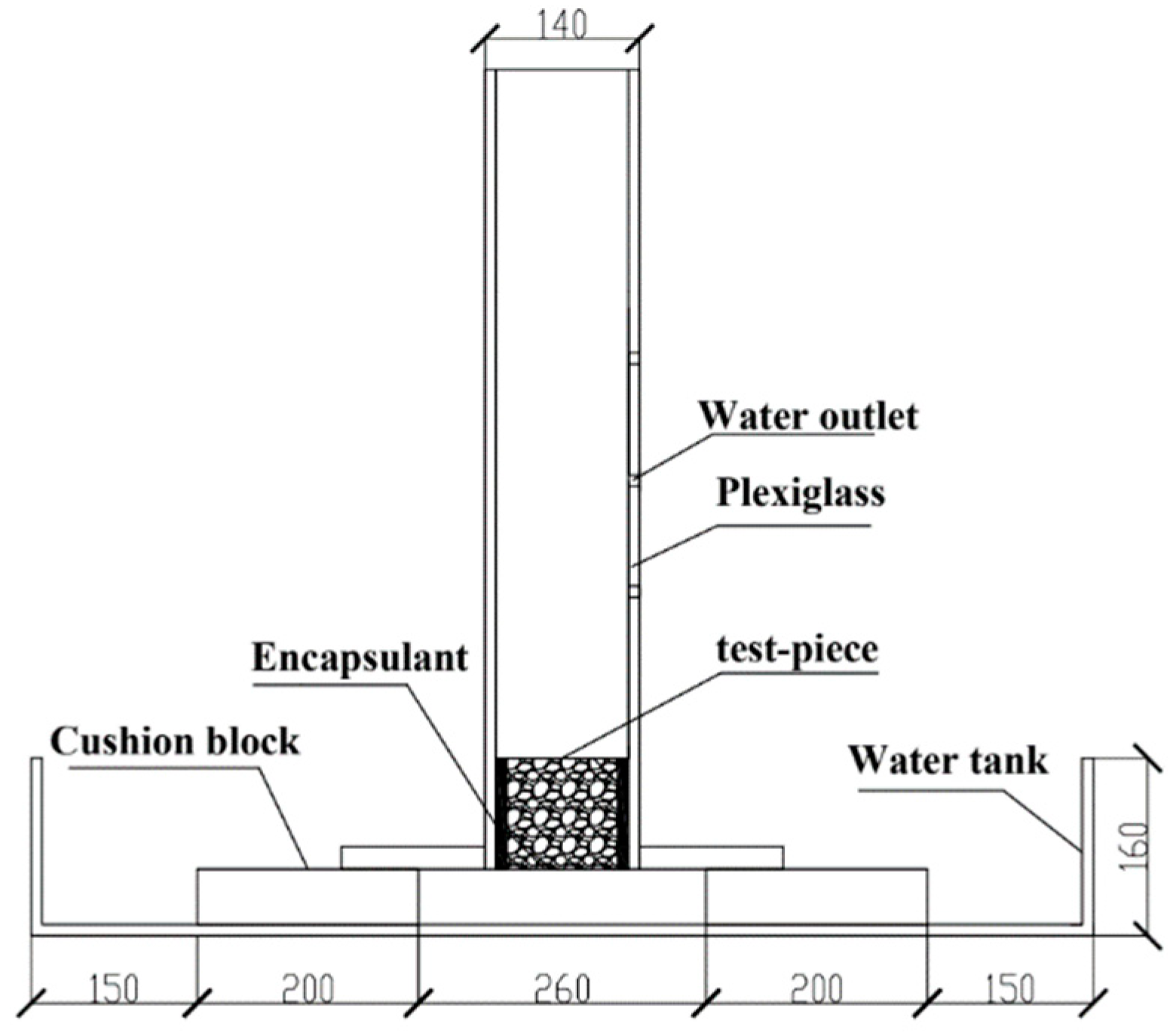
2.2. Sample Preparation and Experiments
3. Results and Analysis
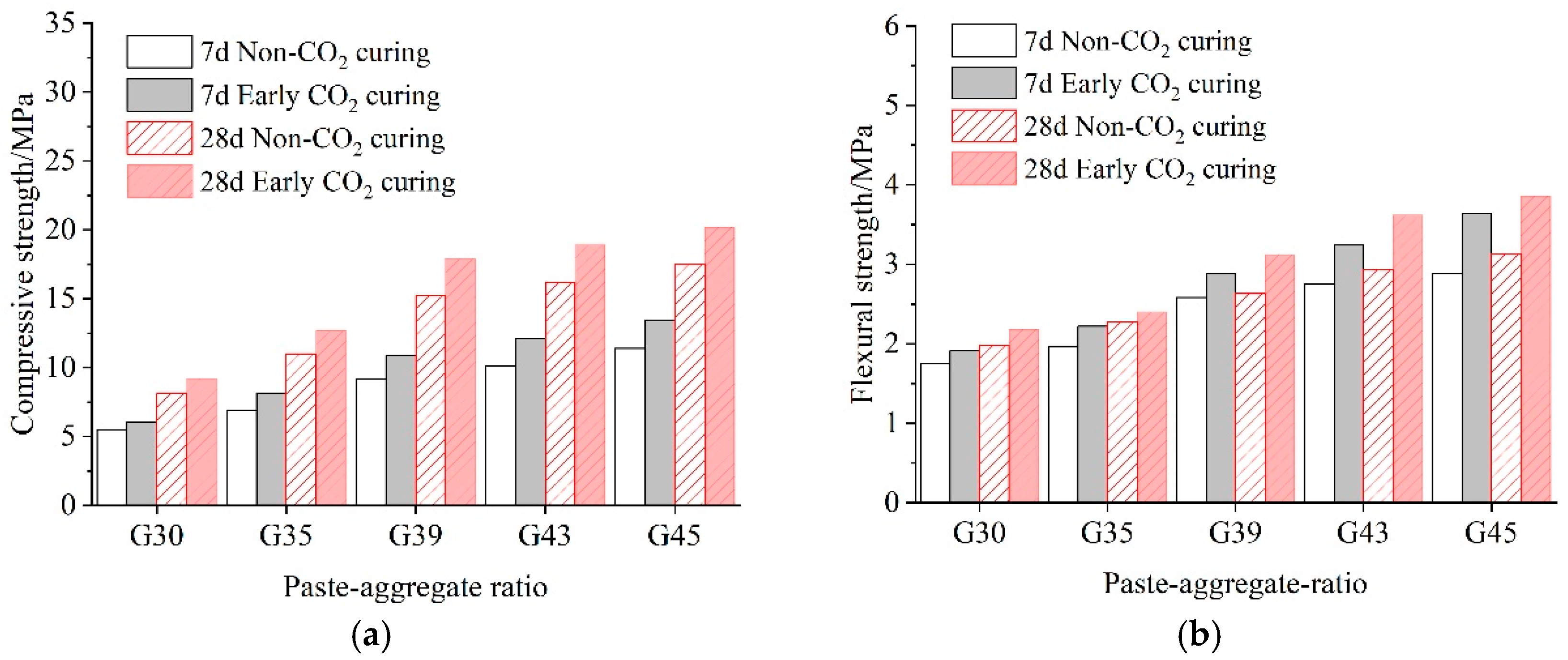
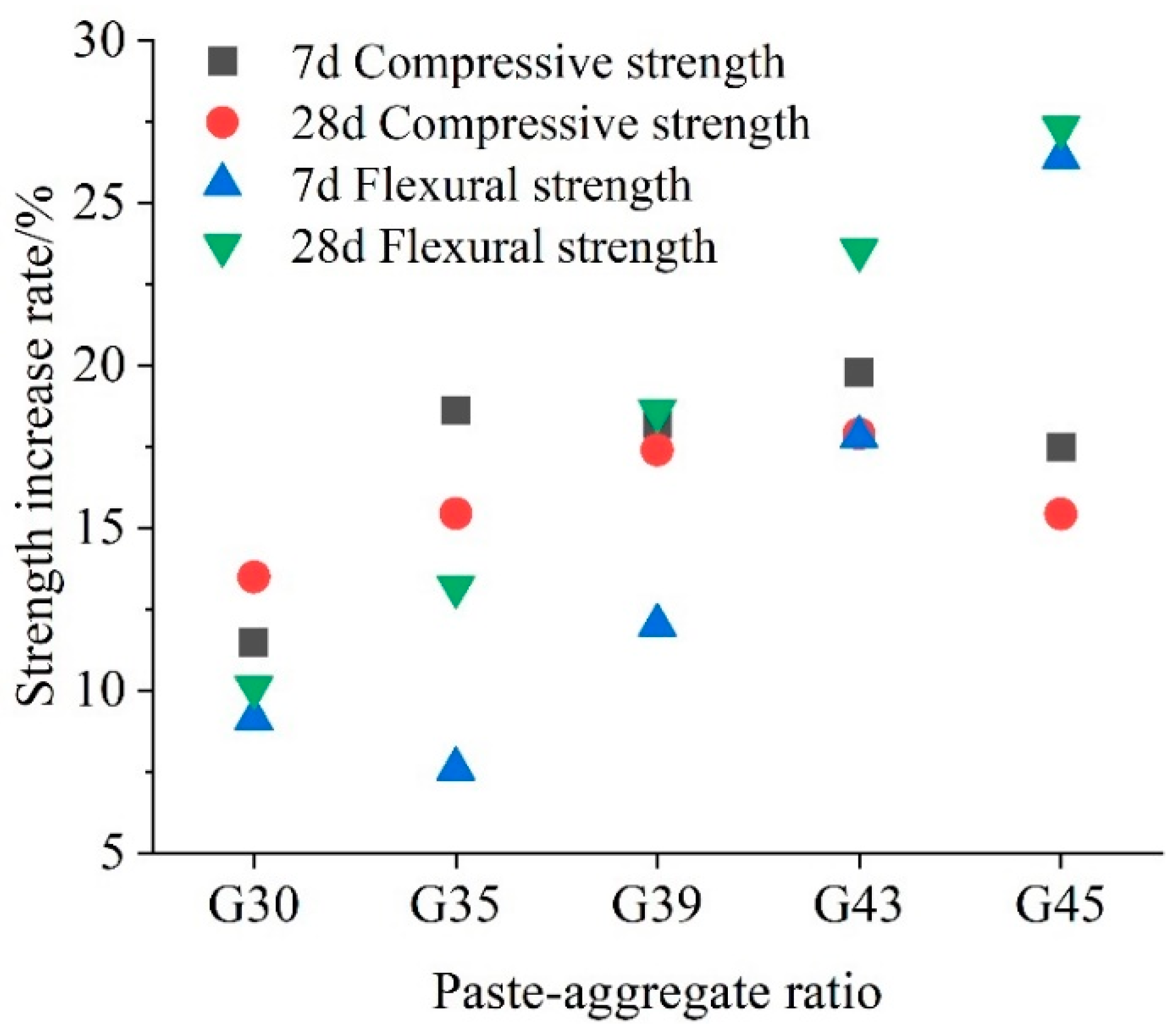
3.1. Effects of Carbonation on Mechanical Performance
3.1.1. Compressive and Flexural Strength
3.1.2. Compressive and Flexural Toughness
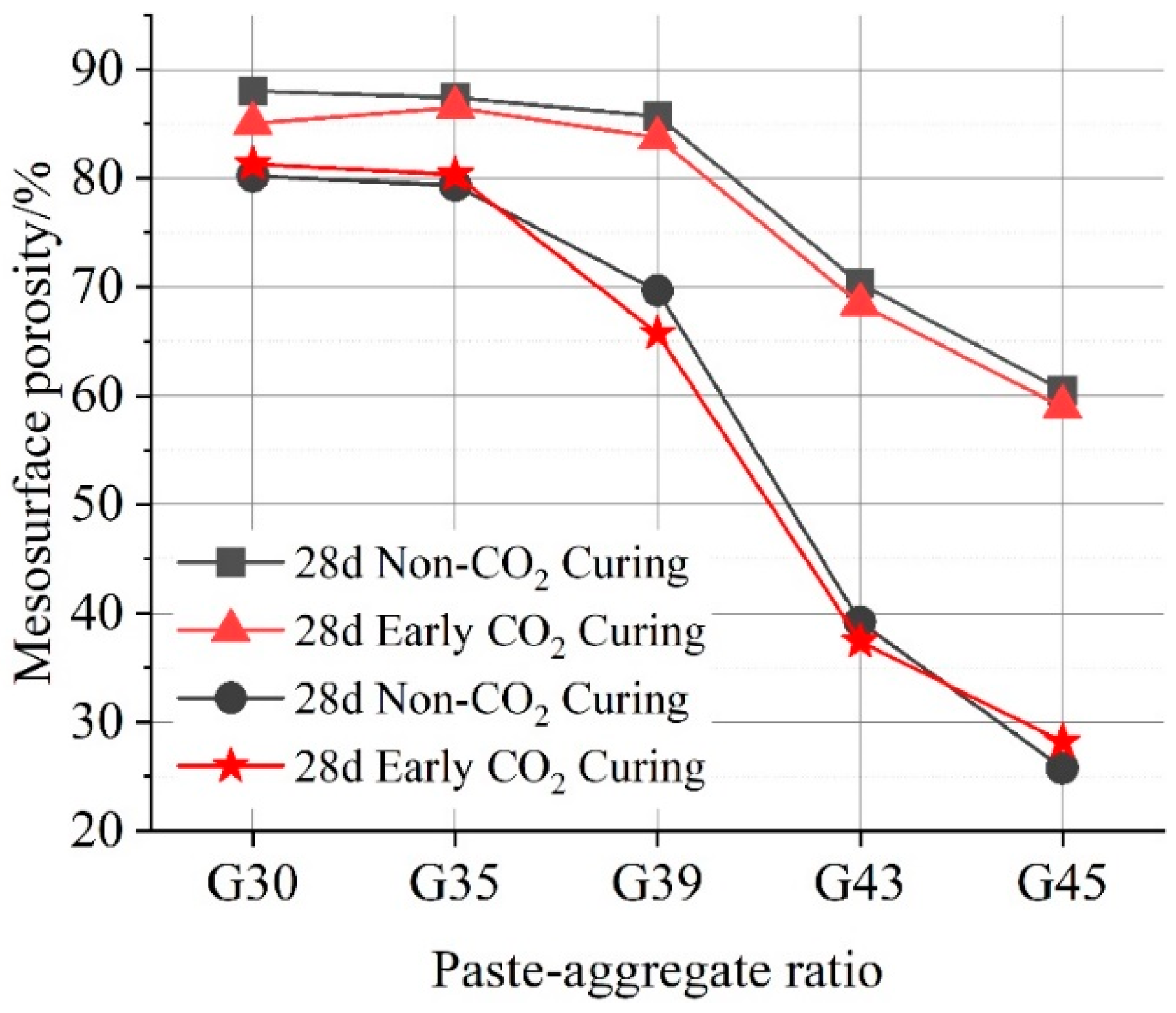
3.2. Analysis of Effective Porosity and Water Permeability
3.2.1. Water Permeability and Effective Porosity
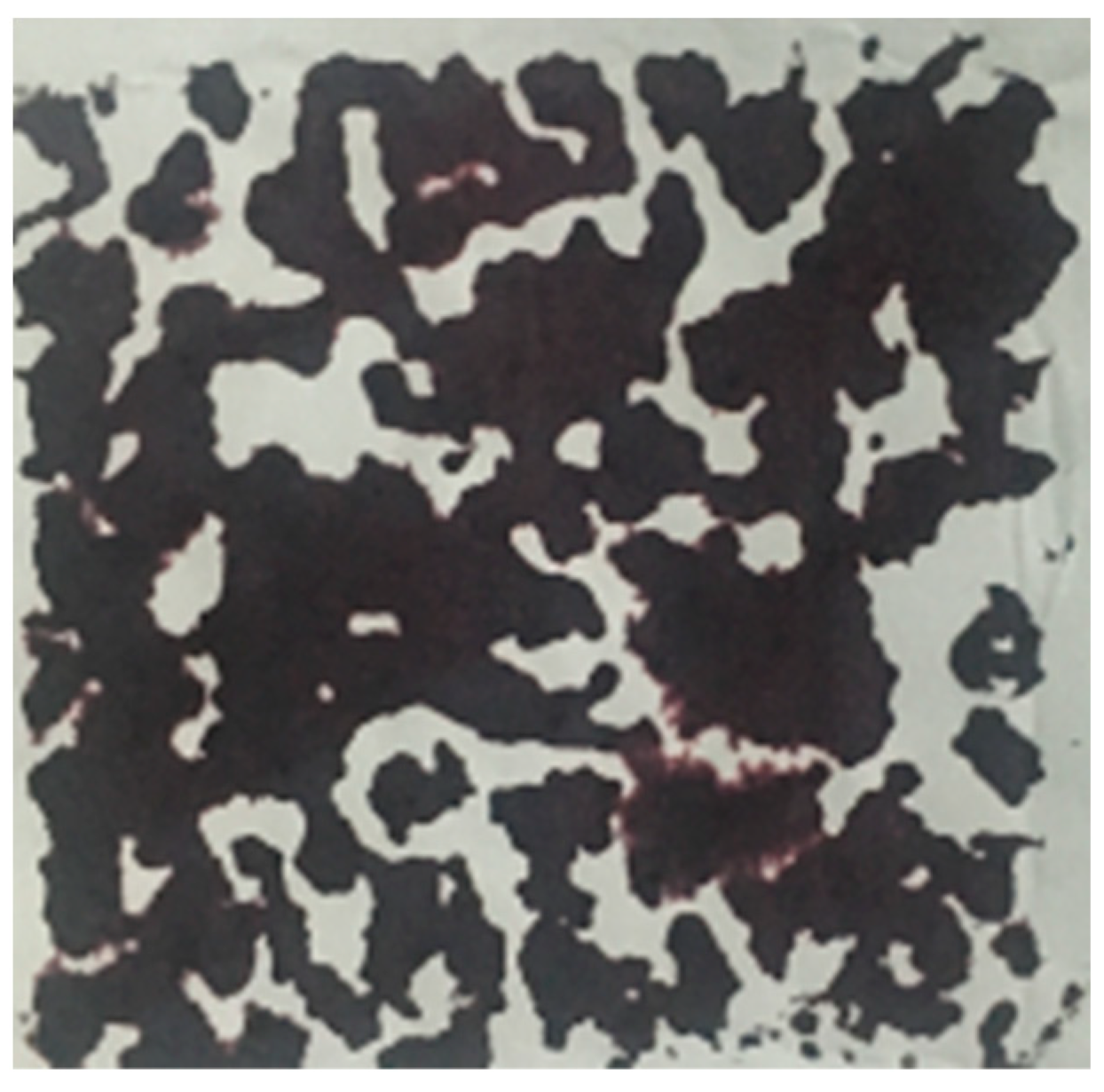
3.2.2. Macro-Surface Porosity
3.3. Carbonation Degree
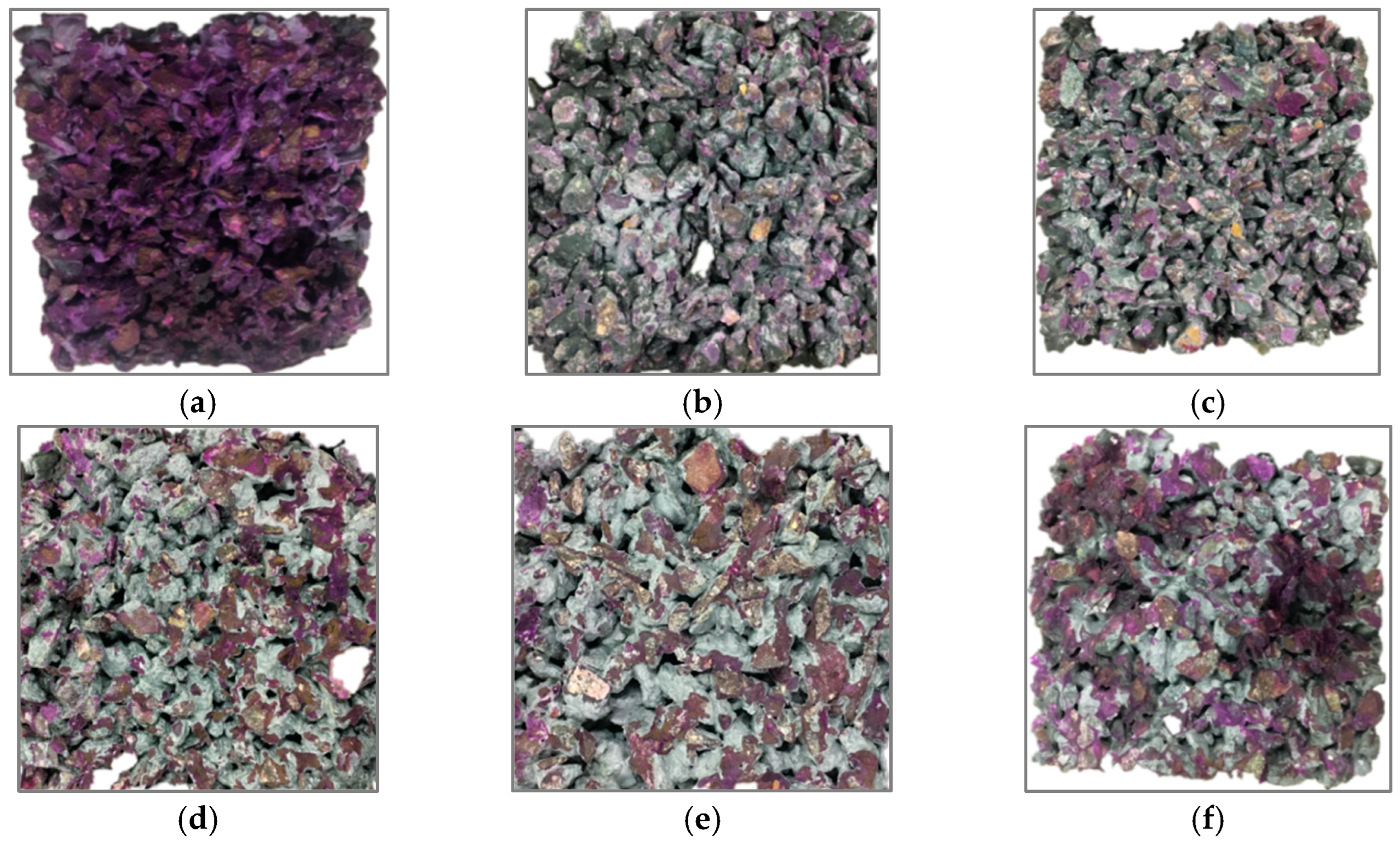
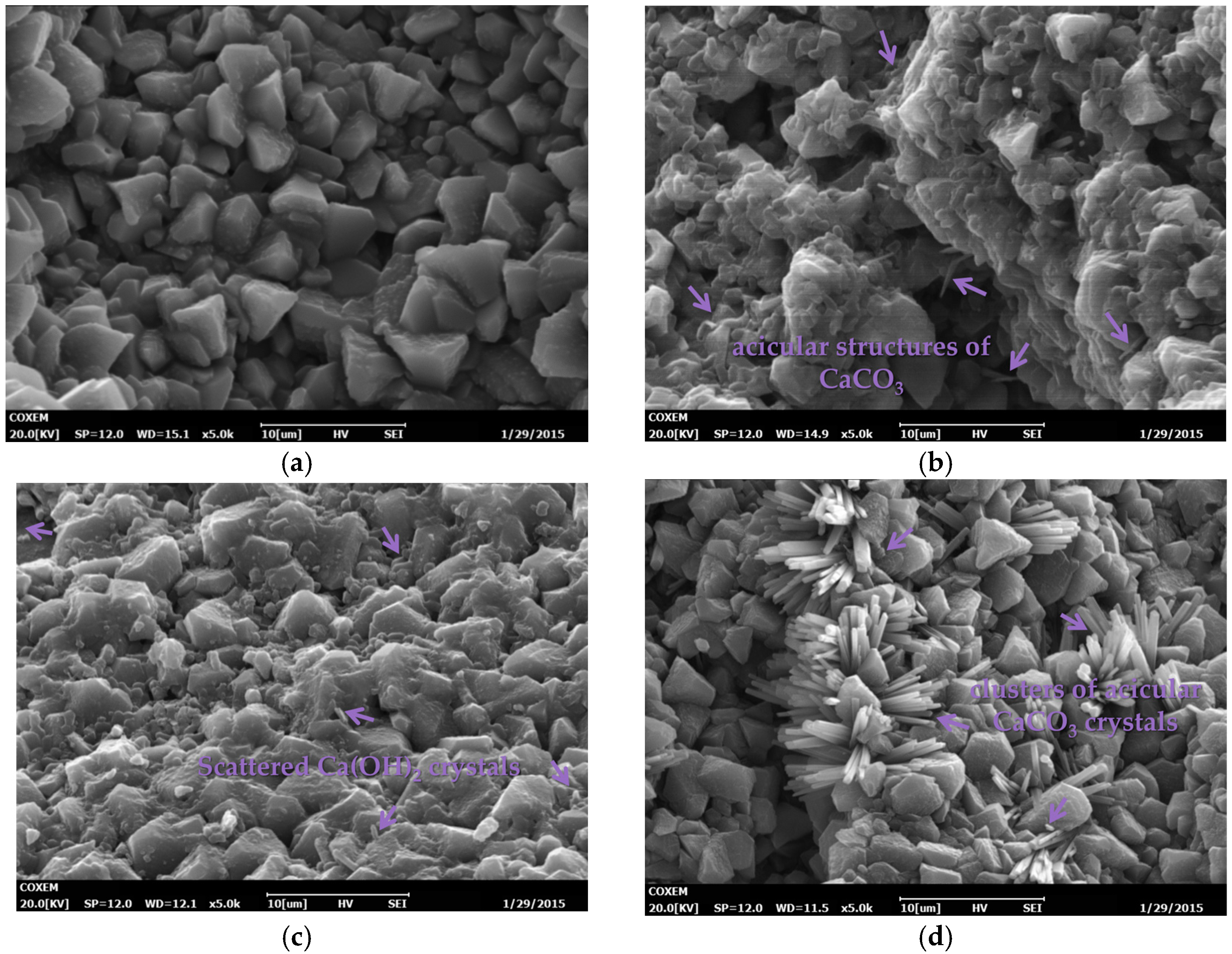
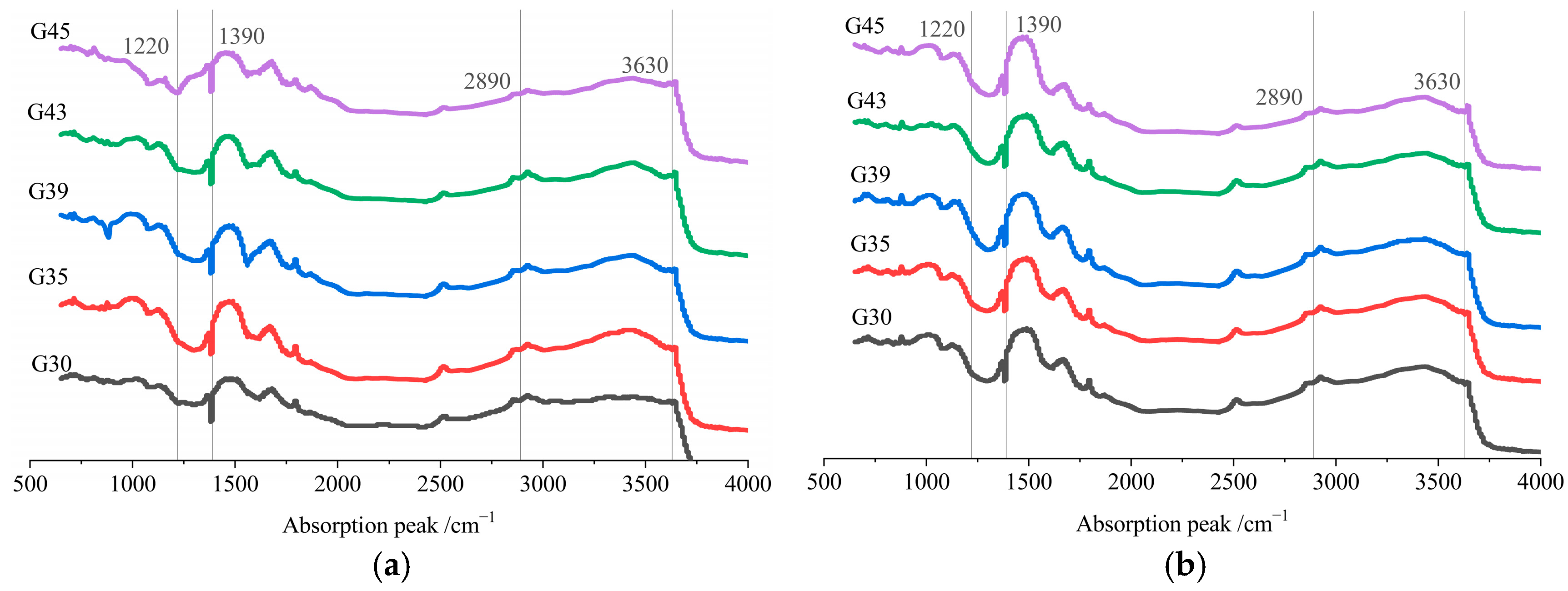
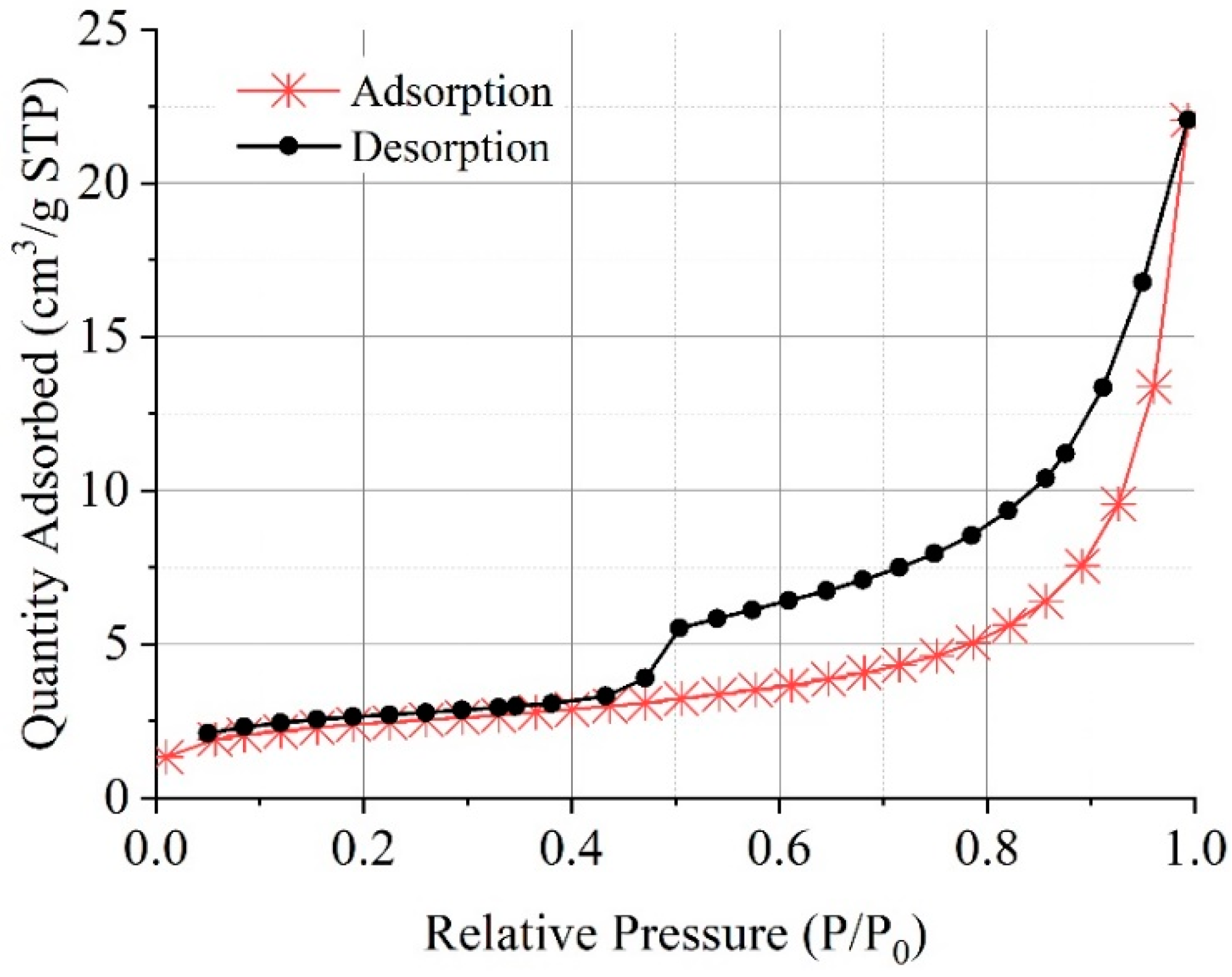
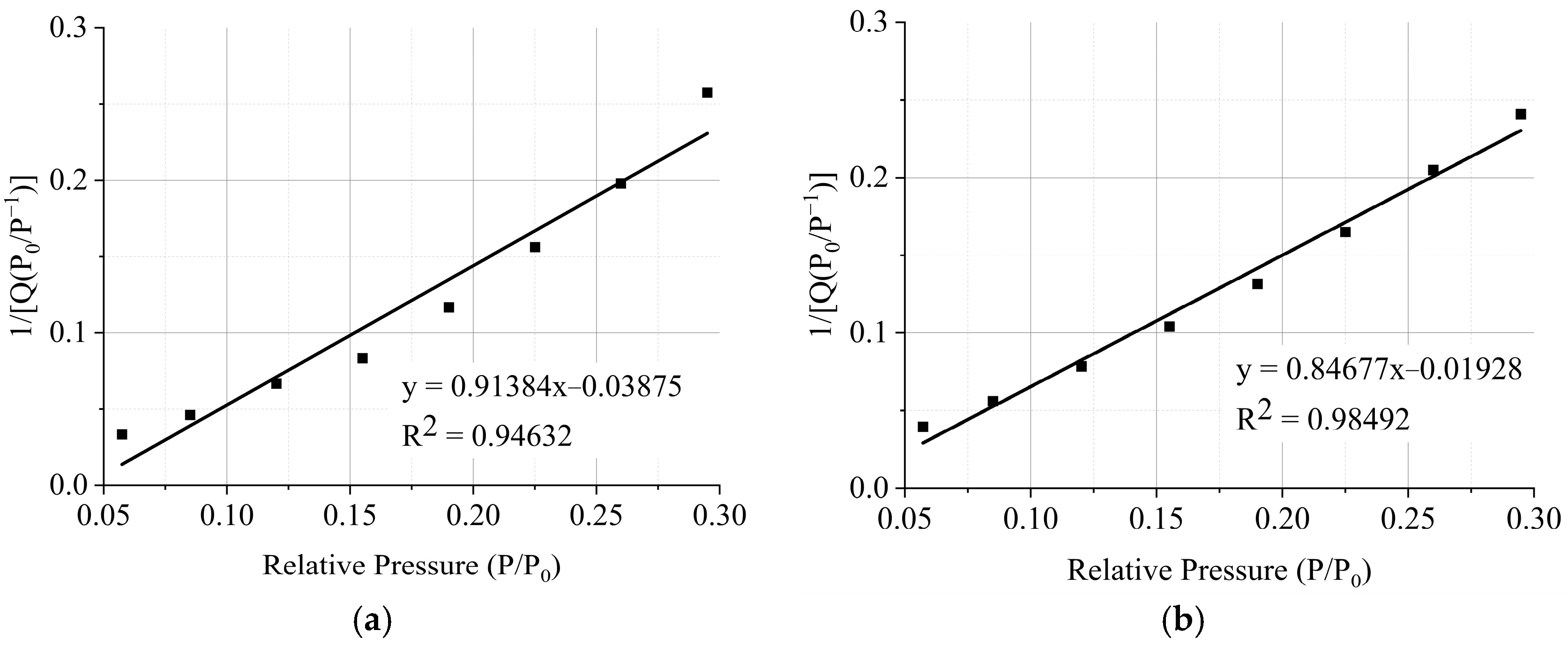
3.4. Effects on Microstructure and Mechanism Analysis
3.4.1. Microscopic Morphology
3.4.2. Phase Composition
3.4.3. Microscopic Pore Structure
4. Conclusions
- (1)
- With the increase in the paste–aggregate ratio and curing time, the mechanical properties of pervious concrete are improved to some extent under both curing conditions. In addition, the mechanical properties of pervious concrete after early CO2 curing are higher than those without CO2 curing at all paste–aggregate ratios.
- (2)
- Early CO2 curing can improve the compressive toughness of pervious concrete but reduces its flexural toughness. However, its influence on flexural toughness decreases with the increase in the paste–aggregate ratio.
- (3)
- The permeability and effective porosity of pervious concrete decrease significantly with the increase in the paste–aggregate ratio. Additionally, pervious concrete cured with early carbonization has poor water permeability and smaller porosity due to its denser structure.
- (4)
- Under early CO2 curing conditions, the degree of carbonization in pervious concrete gradually decreases with the increase in the paste–aggregate ratio.
- (5)
- In the research on micro mechanisms, it was found that CO2 in pervious concrete directly reacts with C-S-H gel under early CO2 curing treatment, and CaCO3 mainly exists in the form of calcite. With the increase in the paste–aggregate ratio, the formation of CaCO3 is inhibited due to the decrease in carbonation degree in pervious concrete. At the same time, early CO2 curing condition will reduce the pore size by less than 40 nm in pervious concrete and increase the pore size by greater than 40 nm. It is worth mentioning that the overall pore volume is greater in pervious concrete.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrappa, A.K.; Biligiri, K.P. Pervious concrete as a sustainable pavement material-Research findings and future prospects: A state-of-the-art review. Constr. Build. Mater. 2016, 111, 262–274. [Google Scholar] [CrossRef]
- AlShareedah, O.; Somayeh, N. Pervious concrete mixture optimization, physical, and mechanical properties and pavement design: A review. J. Clean. Prod. 2021, 288, 125095. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Hatanaka, S.; Chareerat, T.; Mishima, N.; Yuasa, Y. Cement paste characteristics and porous concrete properties. Constr. Build. Mater. 2008, 22, 894–901. [Google Scholar] [CrossRef]
- ACI Committee 522. ACI 522R-2010, Report on Pervious Concrete; American Concrete Institute: Farmington Hills, MI, USA, 2010. [Google Scholar]
- Ćosić, K.; Korat, L.; Ducman, V.; Netinger, I. Influence of aggregate type and size on properties of pervious concrete. Constr. Build. Mater. 2015, 78, 69–76. [Google Scholar] [CrossRef]
- Akkaya, A.; Çağatay, İ.H. Investigation of the density, porosity, and permeability properties of pervious concrete with different methods. Constr. Build. Mater. 2021, 294, 123539. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, G.L. Experimental Study on properties of pervious concrete pavement materials. Cem. Concr. Res. 2003, 33, 381–386. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Boutouil, M.; Sebaibi, N.; Leleyter, L.; Baraud, F. Valorization of seashell by-products in pervious concrete pavers. Constr. Build. Mater. 2013, 49, 151–160. [Google Scholar] [CrossRef]
- Hertzberg, M.; Schreuder, H. Role of atmospheric carbon dioxide in climate change. Energy Environ. 2016, 27, 785–797. [Google Scholar] [CrossRef]
- Zhang, D.; Zaid, G.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J.; He, Z. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 2012, 42, 186–193. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, M.W. Fundamental understanding of carbonation curing and durability of carbonation-cured cement-based composites: A review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation curing versus steam curing for precast concrete production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Yang, Y.; Peng, H.; Huang, J.; Zhou, Y.; Yu, M.; Gao, H. The Relationship Between the Concrete Strength Values of Curing Specimens Using Microwave and Standard Methods. In Hydraulic and Civil Engineering Technology VI; IOS Press: Amsterdam, The Netherlands, 2021; pp. 61–65. [Google Scholar]
- Chen, T.F.; Gao, X.J. Use of Carbonation Curing to Improve Mechanical Strength and Durability of Pervious Concrete. ACS Sustain. Chem. Eng. 2020, 8, 3872–3884. [Google Scholar] [CrossRef]
- Macmaster, D.; Tavares, O. Carbon Sequestration of concrete masonry units. ACI Mater. J. 2015, 112, 775–780. [Google Scholar] [CrossRef]
- Tanaka, R.; Habuchi, T.; Amino, T.; Fukute, T. A study on improvement and its evaluation for the surface layer of concrete placed with pervious form. Int. J. Mod. Phys. Conf. Ser. 2012, 6, 664–669. [Google Scholar] [CrossRef]
- Hasegawa, L.I.I. Carbonation Curing and Performance of Pervious Concrete Using Portland Limestone Cement; McGill University: Montreal, QC, Canada, 2011. [Google Scholar]
- Chen, T.F. Carbonization Curing of Cement-Based Materials and Its Modification Mechanism on Pervious Concrete. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2020. [Google Scholar]
- Fang, Y.F.; Wang, Y.Q.; Zhou, M.; Li, Y.; Kong, J.X. Experimental study on the preparation of pervious concrete by carbonization strengthening of steel slag. Sintered Pellets 2022, 47, 139–145. [Google Scholar]
- Wu, H.Z.; Xu, D.Y. Preparation of permeable concrete using carbonized steel slag aggregate. Cem. Eng. 2020, 3, 90–92. [Google Scholar]
- Liu, Y. Preparation and Research of Carbon Activated Steel Slag Ecological Pervious Concrete. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2018. [Google Scholar]
- Zhang, C.H.; Wang, Q.F.; Yang, J.J.; Liao, J.Q.; Yang, J. Study and design on pervious concrete mix proportion. Concrete 2008, 6, 120–122. [Google Scholar]
- Kevern, J.T.; Nowasell, Q.C. Internal curing of pervious concrete using lightweight aggregate. Constr. Build. Mater. 2018, 161, 229–235. [Google Scholar] [CrossRef]
- Ministry of Housing and Urban-Rural Construction of the People’s Republic of China. Technical Specification for Pervious Cement Concrete Pavement; Ministry of Housing and Urban-Rural Construction: Beijing, China, 2009. [Google Scholar]
- Ba, M.F.; Huang, G.Y.; Liu, J.Z. Mix Design Method of Pervious Concrete Based on Optimum Volume Paste-Aggregate Ratio. Zhejiang Province. China Patent CN111116136A, 8 May 2020. [Google Scholar]
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Academy of Building Research: Beijing, China, 2002.
- GB/T 50081-2002; Standard for Test Method of Mechanical Properties on Ordinary Concrete. National Standard of the People’s Republic of China: Beijing, China, 2002.
- Johnston, C.D.; Skarendahl, A. Comparative flexural performance evaluation of steel fibre-reinforced concretes according to ASTM C1018 shows importance of fibre parameters. Mater. Struct. 1992, 25, 191–200. [Google Scholar] [CrossRef]
- JC/T 945-2005; Water Permeable Brick. National Development and Reform Commission: Beijing, China, 2005.
- Yang, J.; Wang, F.Z.; He, X.Y.; Su, Y. Pore structure of affected zone around saturated and large superabsorbent polymers in cement paste. Cem. Concr. Compos. 2019, 97, 54–67. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, C.F.; Yang, J. Research on factors affecting the strength and permeability of pervious concrete. Concrete 2008, 3, 7–9. [Google Scholar]
- Zhang, X.C. Research on Mix Design of High-Performance Permeable Concrete and Its Life Cycle Environmental Assessment System. Master’s Thesis, Central South University, Changsha, China, 2012. [Google Scholar]
- Qin, L. Kinetics and Long-Term Properties of Early Carbonization of Cement-Based Materials. Ph.D. Thesis, Harbin University of Technology, Harbin, China, 2021. [Google Scholar] [CrossRef]
- Aldea, C.M.; Shah, S.P.; Karr, A. Permeability of cracked concrete. Mater. Struct. 1999, 32, 370–376. [Google Scholar] [CrossRef]
- Sircar, S. Comments on practical use of Langmuir gas adsorption isotherm model. Adsorption 2017, 23, 121–130. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H. The Use of Low Temperature van der Waals Adsorption Isotherms in Determining the Surface Areas of Various Adsorbents. J. Am. Chem. Soc. 1937, 59, 2682–2689. [Google Scholar] [CrossRef]
- Panesar, D.K.; Francis, J. Influence of limestone and slag on the pore structure of cement paste based on mercury intrusion porosimetry and water vapour sorption measurements. Constr. Build. Mater. 2014, 52, 52–58. [Google Scholar] [CrossRef]








| Mass Fraction (%) | ||||||
|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Others |
| 16.23 | 2.81 | 4.30 | 69.52 | 1.33 | 4.17 | 1.64 |
| Density (g/cm3) | Flexural Strength (MPa) | Compressive Strength (MPa) | ||
|---|---|---|---|---|
| 3d | 28d | 3d | 28d | |
| 3.3 | 5.73 | 11.48 | 26.86 | 44.2 |
| Aggregate Gradation (mm) | Apparent Density (kg/m3) | Compacted Bulk Density (kg/m3) | Specific Surface Area (Quality) S1 (cm2/g) | Percentage of Mud Content (%) |
|---|---|---|---|---|
| 4.75–9.5 | 2648 | 1470 | 2.99 | <1 |
| No. | Paste–Aggregate Ratio | Aggregate | P·O42.5 | Water | Superplasticizer |
|---|---|---|---|---|---|
| G45 a | 0.45 | 1512 | 494.71 | 98.94 | 9.89 |
| G43 a | 0.43 | 1476 | 472.72 | 94.54 | 9.45 |
| G39 a | 0.39 | 1400 | 438.11 | 87.62 | 8.76 |
| G35 a | 0.35 | 1355 | 396.15 | 79.23 | 7.92 |
| G30 a | 0.30 | 1307 | 337.54 | 67.51 | 6.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ba, M.; Fang, S.; Cheng, W.; Zhao, Y. Effects of CO2 Curing on the Properties of Pervious Concrete in Different Paste–Aggregate Ratios. Materials 2023, 16, 4581. https://doi.org/10.3390/ma16134581
Ba M, Fang S, Cheng W, Zhao Y. Effects of CO2 Curing on the Properties of Pervious Concrete in Different Paste–Aggregate Ratios. Materials. 2023; 16(13):4581. https://doi.org/10.3390/ma16134581
Chicago/Turabian StyleBa, Mingfang, Siyi Fang, Wei Cheng, and Yawen Zhao. 2023. "Effects of CO2 Curing on the Properties of Pervious Concrete in Different Paste–Aggregate Ratios" Materials 16, no. 13: 4581. https://doi.org/10.3390/ma16134581