Vinylated Modification of Biophytic Acid and Flame-Retardant/Crease-Proofing Finishing of Cotton Fabrics via In Situ Copolymerization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
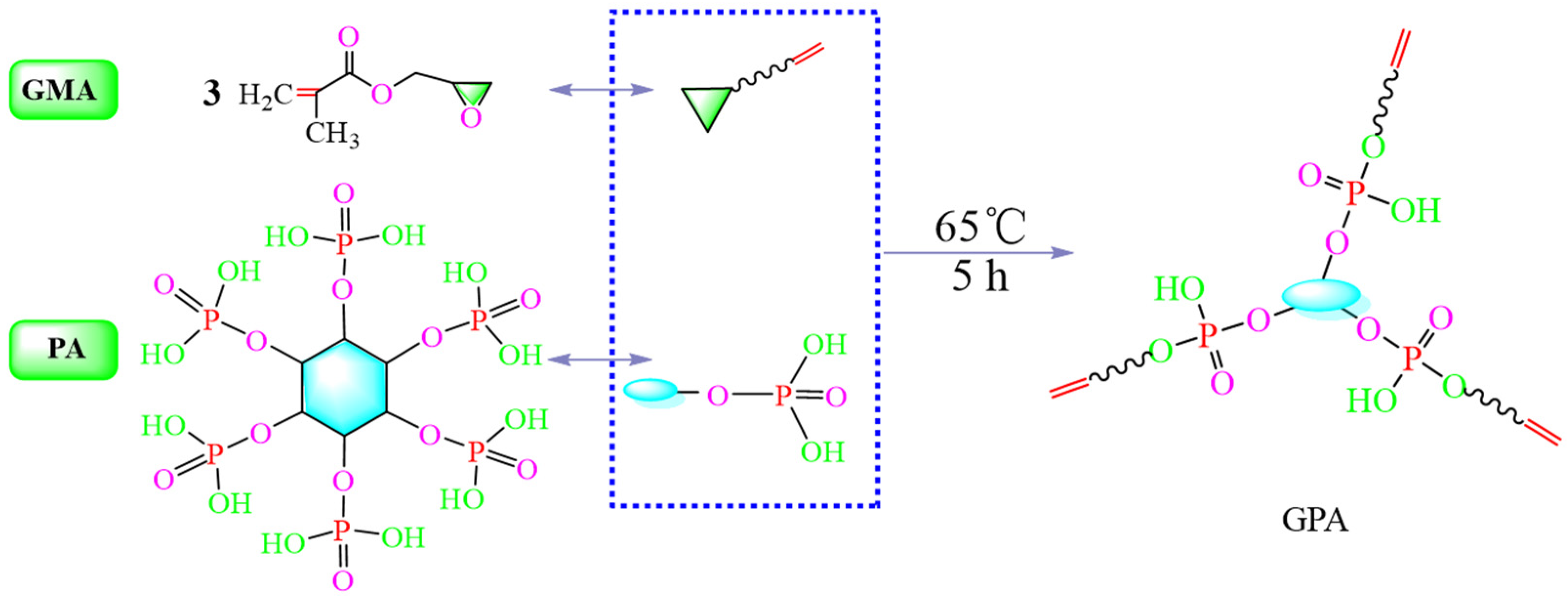
2.2. Preparation of GPA
2.3. Flame-Retardant and Crease-Proofing Finishing of Cotton Fabrics
2.4. Characterization
3. Results and Discussion
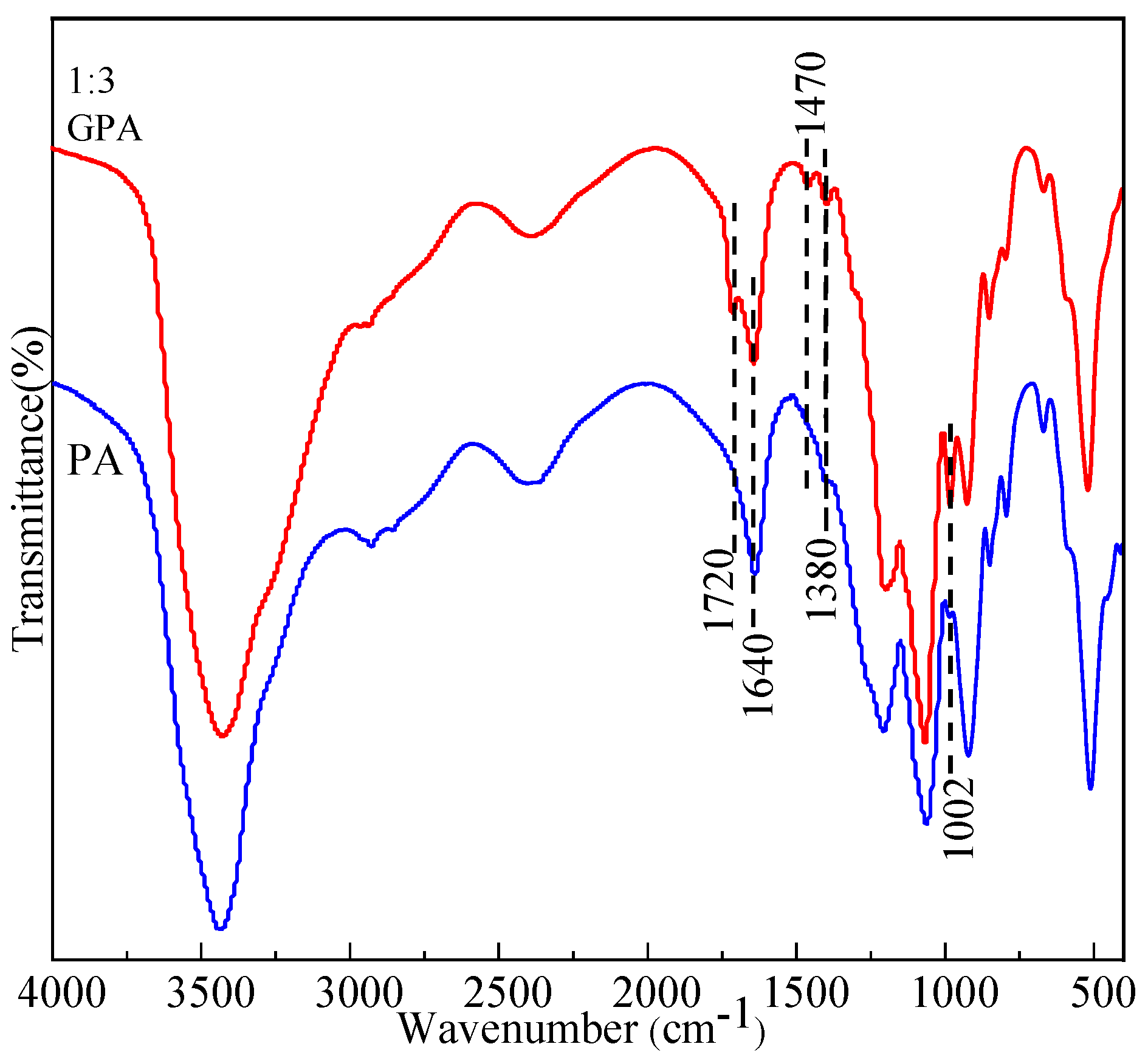
3.1. ATR-FTIR Analysis of GPA
3.2. Preparation of GPA and Graft Reaction Principle of Cotton Fabrics
3.3. Optimization of Finishing Process
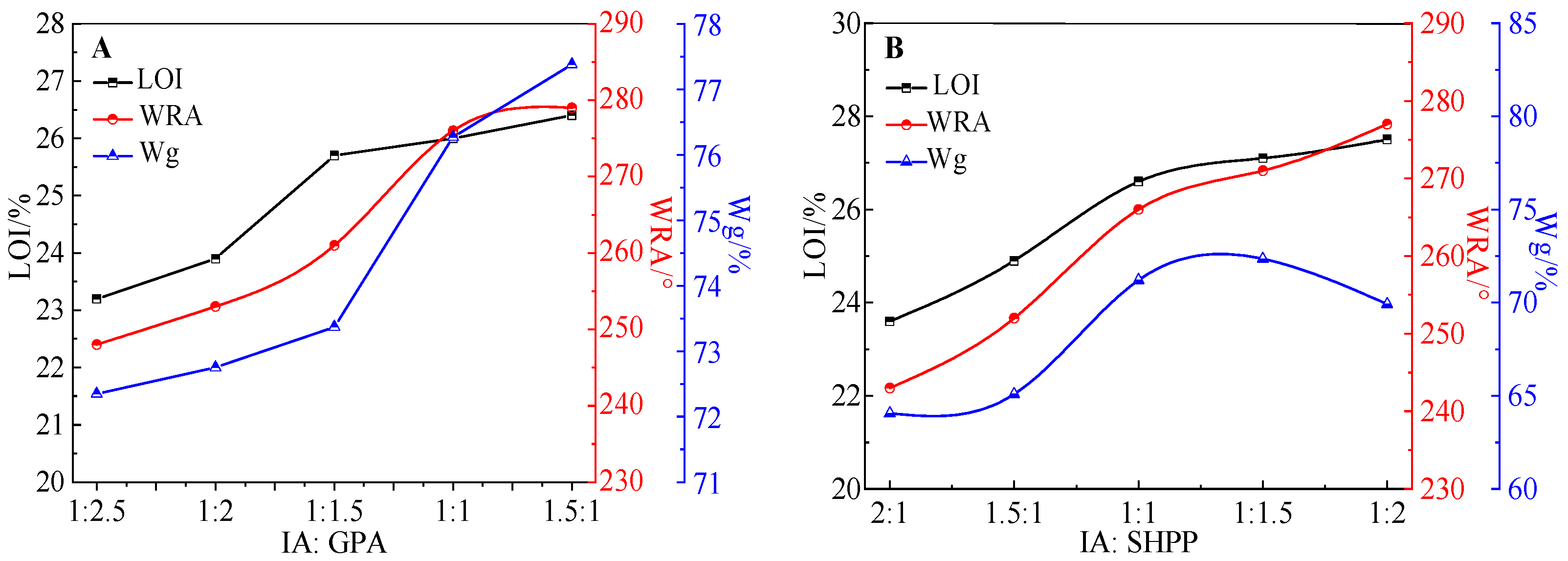
3.3.1. Effect of IA: GPA on the Cotton Fabrics
3.3.2. Effect of IA: SHPP on the Cotton Fabrics
3.3.3. Effect of APS on the Cotton Fabrics
3.3.4. Effect of GPA on the Cotton Fabrics
3.4. Fabric Performance
3.4.1. Basic Properties of Finished Fabrics
3.4.2. SEM and EDX Analysis
3.4.3. XPS Analysis
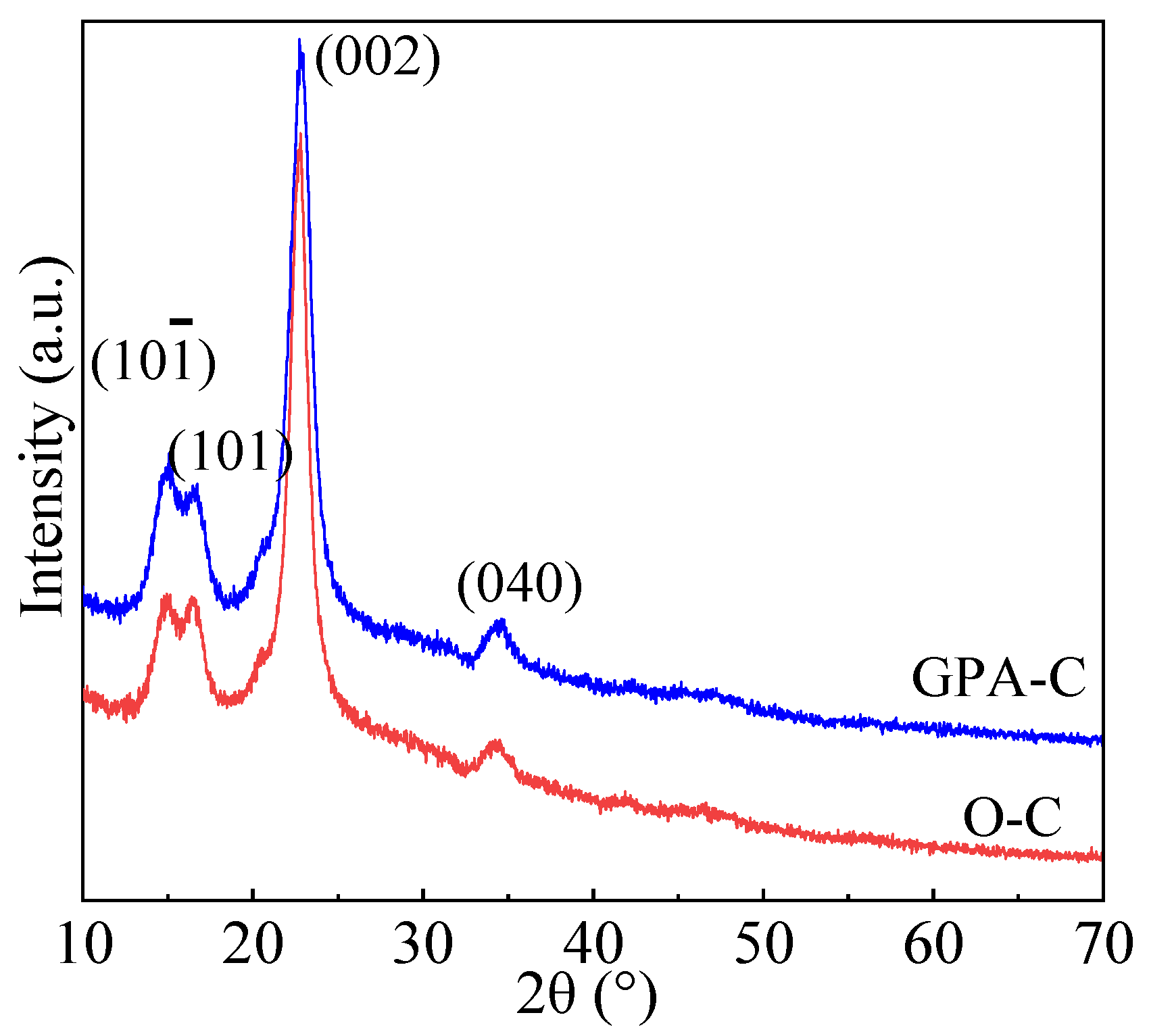
3.4.4. XRD Analysis
3.4.5. TG Analysis
3.4.6. TG-IR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mohsin, M.; Ahmad, S.W.; Khatri, A.; Zahid, B. Performance enhancement of fire-retardant finish with environment friendly bio cross-linker for cotton. J. Clean. Prod. 2013, 51, 191–195. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Wolkoff, P. Cancer effects of formaldehyde: A proposal for an indoor air guideline value. Arch. Toxicol. 2010, 84, 423–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, T.; Mahdi, H. Durable flame retardant finishing of cotton fabrics with poly(amidoamine) dendrimer using citric acid. Mater. Chem. Phys. 2018, 219, 425–432. [Google Scholar]
- Saha, S.; Pandey, A.K.; Gopinath, K.A.; Bhattacharaya, R.; Kundu, S.; Gupta, H.S. Nutritional quality of organic rice grown on organic composts. Agron. Sustain. Dev. 2007, 27, 223–229. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zhou, X.Y.; Cheng, X.W.; Tang, R.C. Phytic acid as an eco-friendly flame retardant for silk/wool blend: A comparative study with fluorotitanate and fluorozirconate. J. Clean. Prod. 2018, 198, 1044–1052. [Google Scholar] [CrossRef]
- Cheng, X.W.; Tang, R.C.; Yao, F.; Yang, X.H. Flame retardant coating of wool fabric with phytic acid/polyethyleneimine polyelectrolyte complex. Prog. Org. Coat. 2019, 132, 336–342. [Google Scholar] [CrossRef]
- Cheng, X.W.; Guan, J.P.; Kiekens, P.; Yang, X.H.; Tang, R.C. Preparation and evaluation of an eco-friendly, reactive, and phytic acid-based flame retardant for wool. React. Funct. Polym. 2019, 134, 58–66. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wei, Y.; Deng, H.B.; Yu, R.Q.; Zhu, J.J.; Yang, Y.B. Synergistic flame retardant effect of barium phytate and intumescent flame retardant for epoxy resin. Polymers 2021, 13, 2900. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.G.; Fan, M.; Luo, J.; Liang, J.; Meng, X. Effect of nickel phytate on flame retardancy of intumescent flame retardant polylactic acid. Polym. Adv. Technol. 2021, 32, 1548–1559. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, Q.Y.; Peng, B.; Ren, Y.L.; Cheng, B.W.; Ding, C.; Su, X.W.; He, J.; Lin, S.G. Flame retardant cellulosic fabrics via layer-by-layer self-assembly double coating with egg white protein and phytic acid. J. Clean. Prod. 2020, 243, 118641–118650. [Google Scholar] [CrossRef]
- Carosio, F.; Laufer, G.; Alongi, J.; Camino, G.; Grunlan, J.C. Layer-by-layer assembly of silica-based flame-retardant thin film on PET fabric. Polym. Degrad. Stabil. 2011, 96, 745–750. [Google Scholar] [CrossRef]
- Carosio, F.; Di, B.A.; Cuttica, F.; Alongi, J.; Frache, A.; Malucelli, G. Flame retardancy of polyester fabrics treated by spray-assisted layer-by-layer silica architectures. Ind. Eng. Chem. Res. 2013, 52, 9544–9550. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Y.; Li, D. A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohydr. Polym. 2017, 175, 636–644. [Google Scholar] [CrossRef]
- Wen, J.Z.; Shuai, S.H.; Ming, Y.Y. A synergistic flame retardant of glycosyl cross-linking boron acid and ammonium salt of phytic acid to enhance durable flame retardancy of cotton fabrics. Cellulose 2020, 27, 9699–9710. [Google Scholar]
- Li, P.; Liu, C.; Wang, B. Eco-friendly coating based on an intumescent flame-retardant system for viscose fabrics with multi-function properties: Flame retardancy, smoke suppression, and antibacterial properties. Prog. Org. Coat. 2021, 159, 106400–106411. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, J.; Tao, Y. Flame-retardant and crease-proofing finishing of cotton fabrics via in-situ copolymerization of ethylene phytic acid and itaconic acid, part I: Fabrication process. Therm. Sci. 2021, 26, 2801–2809. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.L.; Zhou, Y.; Zou, J.; Zhang, G.X.; Zhang, F.X. Straightforward one-step solvent-free synthesis of the flame retardant for cotton with excellent efficiency and durability. Carbohydr. Polym. 2018, 201, 438–445. [Google Scholar] [CrossRef]
- Zhang, X. Research on the Synthesis of Glycidyl Methacrylate. Master’s Thesis, Dalian Polytechnic University, Dalian, China, 2016. [Google Scholar]
- Li, S.F.; Zhou, X.; Zhang, W.L. Formaldehyde-free durable press finishing of cotton fabrics via in-situ copolymerization of MA/IA. Dye. Finish. 2016, 7, 1–6. [Google Scholar]
- Liu, X.H.; Zhang, Q.Y.; Cheng, B.W.; Ren, Y.L.; Zhang, Y.G.; Ding, C. Durable flame-retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 2017, 25, 799–811. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Liu, B.; Wang, N. Preparation of a novel phosphorus-and nitrogen-containing flame retardant and its synergistic effect in the intumescent flame-retarding polypropylene system. Polym. Compos. 2015, 36, 1606–1619. [Google Scholar] [CrossRef]
- Ke, C.H.; Li, J.; Fang, K.Y.; Zhu, Q.L.; Zhu, J.; Yan, Q.; Wang, Y.Z. Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide. Polym. Degrad. Stabil. 2010, 95, 763–770. [Google Scholar] [CrossRef]
- Liu, W.; Chen, D.Q.; Wang, Y.Z.; Wang, D.Y.; Qu, M.H. Char-forming mechanism of a novel polymeric flame retardant with char agent. Polym. Degrad. Stabil. 2007, 92, 1046–1052. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Lin, L.; He, B.H.; Liu, S.J.; Yang, P.K. X-ray photoelectron spectroscopic analysis of celluloses with different crystallization index. Chem. Ind. Forest Prod. 2009, 29, 30–34. [Google Scholar]
- Zhao, C.X.; Liu, Y.; Wang, D.Y.; Wang, D.L.; Wang, Y.Z. Synergistic effect of ammonium polyphosphate and layered double hydroxide on flame retardant properties of poly (vinyl alcohol). Polym. Degrad. Stabil. 2008, 93, 1323–1331. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Flame retardancy through carbon nanomaterials: Carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene in polypropylene. Polym. Degrad. Stabil. 2013, 98, 1495–1505. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Cheng, D.L.; Chen, L.; Wang, D.Y.; Wang, Y.Z. Flame-retardant effect of sepiolite on an intumescent flame-retardant polypropylene system. Ind. Eng. Chem. Res. 2011, 50, 2047–2054. [Google Scholar] [CrossRef]
- Zhou, Q.; Lv, J.; Ren, Y.; Chen, J.Y.; Gao, D.W.; Lu, Z.Q.; Wang, C.X. A green in situ synthesis of silver nanoparticles on cotton fabrics using Aloe vera leaf extraction for durable ultraviolet protection and antibacterial activity. Text. Res. J. 2016, 87, 2407–2419. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Chen, J.Y.; Zhou, T.C.; Shao, J.Z. In situ polymerization of polyaniline on cotton fabrics with phytic acid as a novel efficient dopant for flame retardancy and conductivity switching. New J. Chem. 2020, 44, 3504–3513. [Google Scholar] [CrossRef]
- Qian, X.D.; Song, L.; Hu, Y.; Yuen, R.K.; Chen, L.J.; Guo, Y.Q.; Hong, N.N.; Jiang, S.H. Combustion and thermal degradation mechanism of a novel intumescent flame retardant for epoxy acrylate containing phosphorus and nitrogen. Ind. Eng. Chem. Res. 2011, 50, 1881–1892. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Fei, B.; Wang, X.W.; Xin, J.H. A novel halogen-free and formaldehyde-free flame retardant for cotton fabrics. Fire Mater. 2012, 36, 31–39. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Chang, S.; Condon, B.; Slopek, R.; Graves, E.; Yoshioka-Tarver, M. Structural effect of phosphoramidate derivatives on the thermal and flame-retardant behaviors of treated cotton cellulose. Ind. Eng. Chem. Res. 2013, 52, 4715–4724. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Chang, S.; Condon, B. The comparison of differences in flammability and thermal degradation between cotton fabrics treated with phosphoramidate derivatives. Polym. Adv. Technol. 2014, 25, 665–672. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Z.S.; Li Rong Huang, D.; Ren, X.H. Flame-retardant treatment of cotton fabric with organophosphorus derivative containing nitrogen and silicon. J. Therm. Anal. Calorim. 2017, 128, 653–660. [Google Scholar]
- Wang, S.; Liu, Q.; Luo, Z.; Wen, L.; Cen, K. Mechanism study on cellulose pyrolysis using thermogravimetric analysis coupled with infrared spectroscopy. Front. Energy 2006, 1, 413–419. [Google Scholar] [CrossRef]
- Bai, X.Y.; Che, D.Y.; Jiang, W.Q.; Gao, L.; Sun, Y.X.; Sun, Y.P. TG-FTIR analysis of cellulose pyrolysis. Renew. Energy Resour. 2015, 33, 1582–1588. [Google Scholar]
- Lu, Q.; Wu, Y.T.; Hu, B.; Liu, J.; Liu, D.J.; Dong, C.Q.; Yang, Y.P. Insight into the mechanism of secondary reactions in cellulose pyrolysis: Interactions between levoglucosan and acetic acid. Cellulose 2019, 26, 8279–8290. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, B.; Zhang, Z.X.; Wu, Y.T.; Cui, M.S.; Liu, D.J.; Dong, C.Q.; Yang, Y.P. Mechanism of cellulose fast pyrolysis: The role of characteristic chain ends and dehydrated units. Combust. Flame 2018, 198, 267–277. [Google Scholar] [CrossRef]






| δ | Chemical Environment of Protons |
|---|---|
| 4.2–4.4 | (P-OH) |
| 5.59 | (-O-CH2-CH(OH)-CH2-O-C(=O)-C(CH3) = CH2) |
| 4.1 | (-O-CH2-CH(OH)-CH2-O-C(=O)-C(CH3) = CH2) |
| 3.5–3.9 | (-O-CH2-CH(OH)-CH2-O-C(=O)-C(CH3) = CH2) |
| 1.9–2.2 | (-O-CH2-CH(OH)-CH2-O-C(=O)-C(CH3) =CH2) |
| 6.15 | (-O-CH2-CH(OH)-CH2-O-C(=O)-C(CH3) =CH2) |
| 2.45 | (-O-CH2-CH(OH)-CH2-O-C(=O)-C(CH3) = CH2) |
| Samples | LOI/% | WRA/° | Wg/% | BS/N | TS/N |
|---|---|---|---|---|---|
| O-C | 18 | 142 | 97.9 | 293.1 | 17.2 |
| GPA-C | 28.6 | 265 | 75.62 | 270.6 | 7.9 |
| GPA-C-1 | 23.5 | 248 | 77.46 | 265.4 | 7.1 |
| GPA-C-5 | 19.2 | 221 | 78.84 | 253.8 | 6. 5 |
| Cotton Fabrics | Ti/°C | Te/°C | Tmax/°C | CR at 700 °C/% |
|---|---|---|---|---|
| O-C | 256 | 345 | 380 | 10 |
| GPA-C | 200 | 320 | 344 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Zhou, Q.; Chen, J.; Zhang, X.; Zhu, C.; Wu, M. Vinylated Modification of Biophytic Acid and Flame-Retardant/Crease-Proofing Finishing of Cotton Fabrics via In Situ Copolymerization. Materials 2023, 16, 286. https://doi.org/10.3390/ma16010286
Cheng B, Zhou Q, Chen J, Zhang X, Zhu C, Wu M. Vinylated Modification of Biophytic Acid and Flame-Retardant/Crease-Proofing Finishing of Cotton Fabrics via In Situ Copolymerization. Materials. 2023; 16(1):286. https://doi.org/10.3390/ma16010286
Chicago/Turabian StyleCheng, Bingying, Qingqing Zhou, Jiayi Chen, Xu Zhang, Chenglei Zhu, and Minghao Wu. 2023. "Vinylated Modification of Biophytic Acid and Flame-Retardant/Crease-Proofing Finishing of Cotton Fabrics via In Situ Copolymerization" Materials 16, no. 1: 286. https://doi.org/10.3390/ma16010286




